Effect of Static and Dynamic Stretching on Corneal Fibroblast Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Stretching Experiments
2.3. Live Cell Imaging
2.4. Cell Imaging Analysis
2.5. Theoretical Analysis of Stress Concentration
2.6. Finite Element Simulation of Strain Distribution
2.7. Statistical Analysis
3. Results
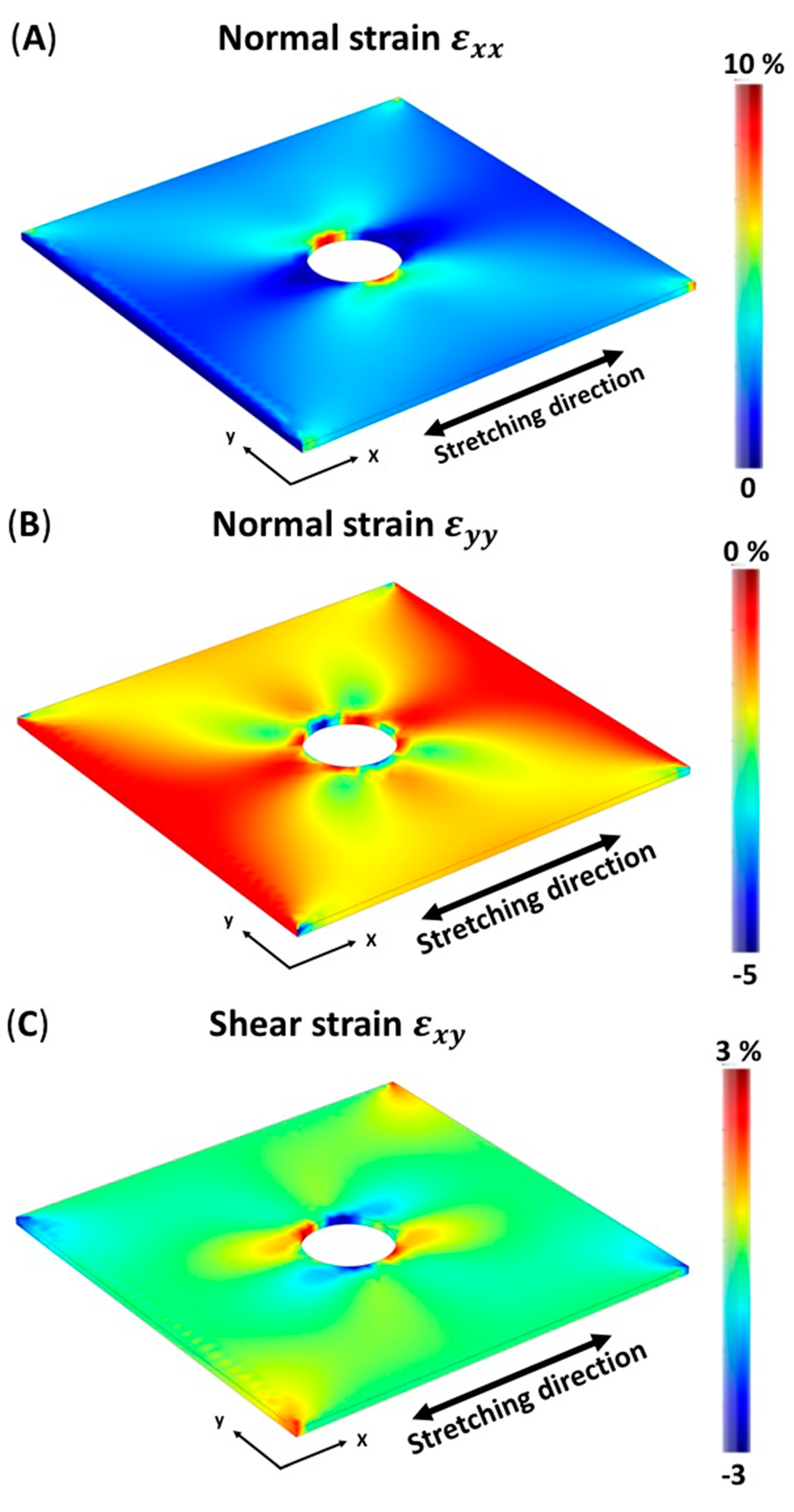
3.1. Finite Element Simulation of Membrane
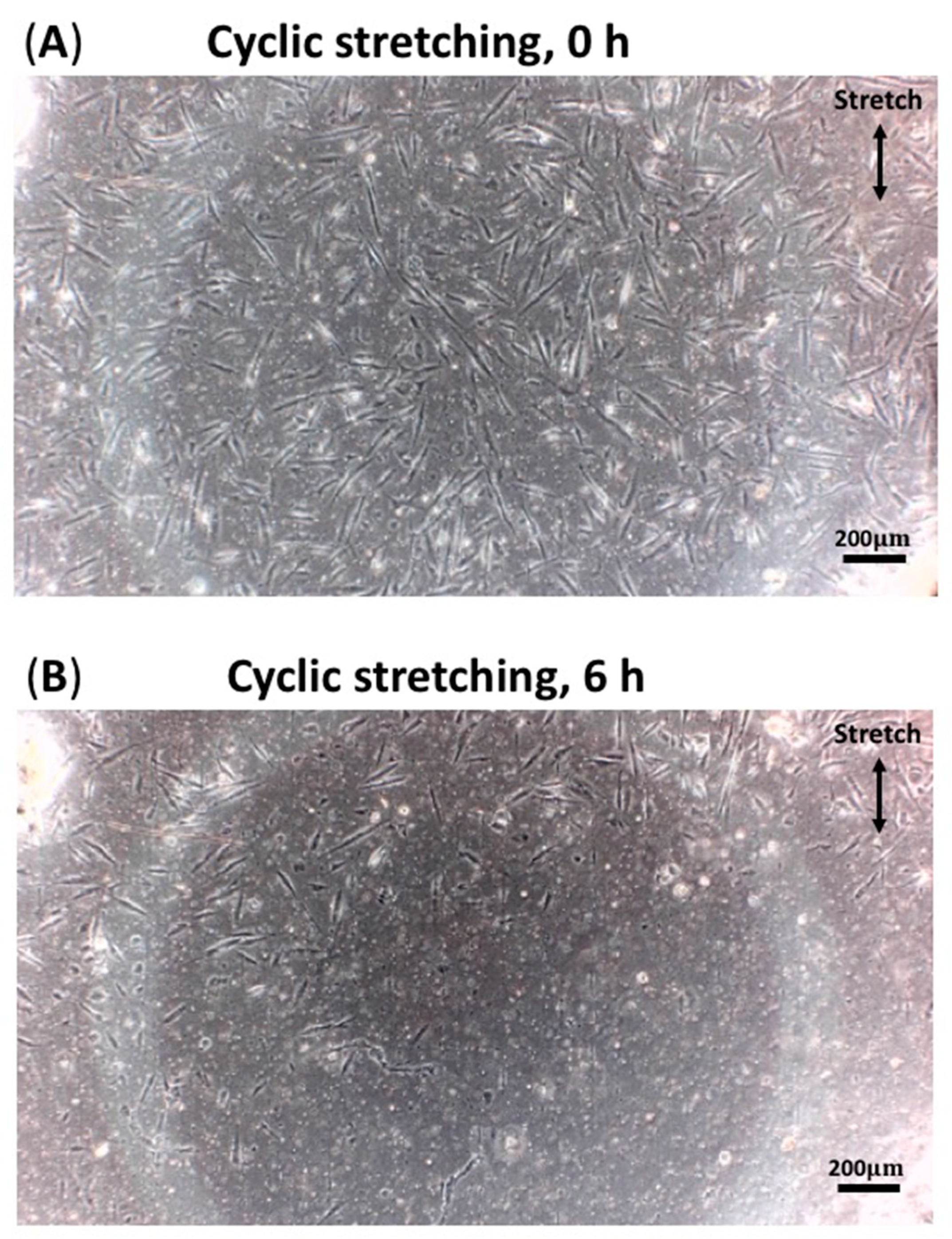
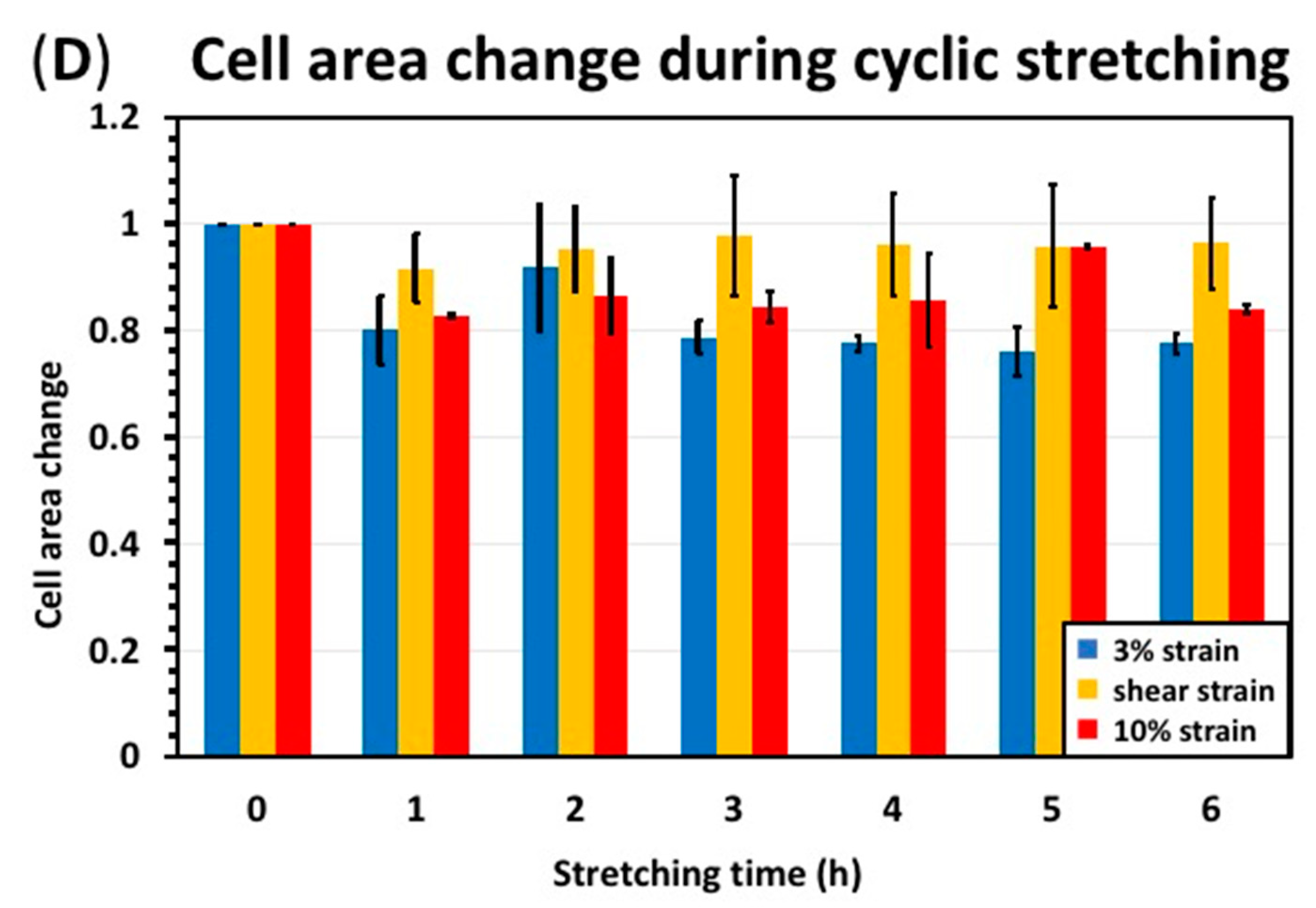
3.2. Cyclic Stretching Test Results
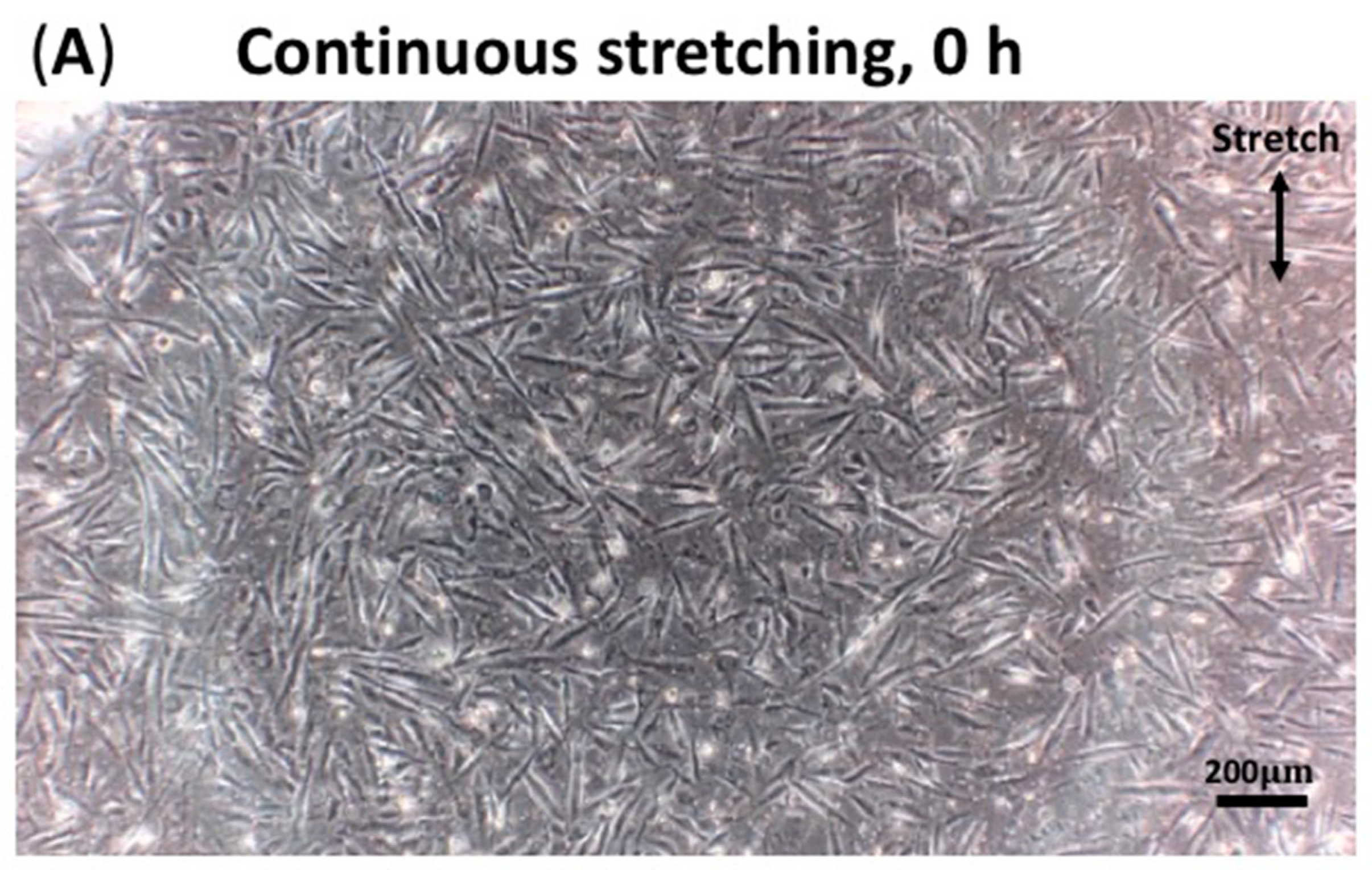
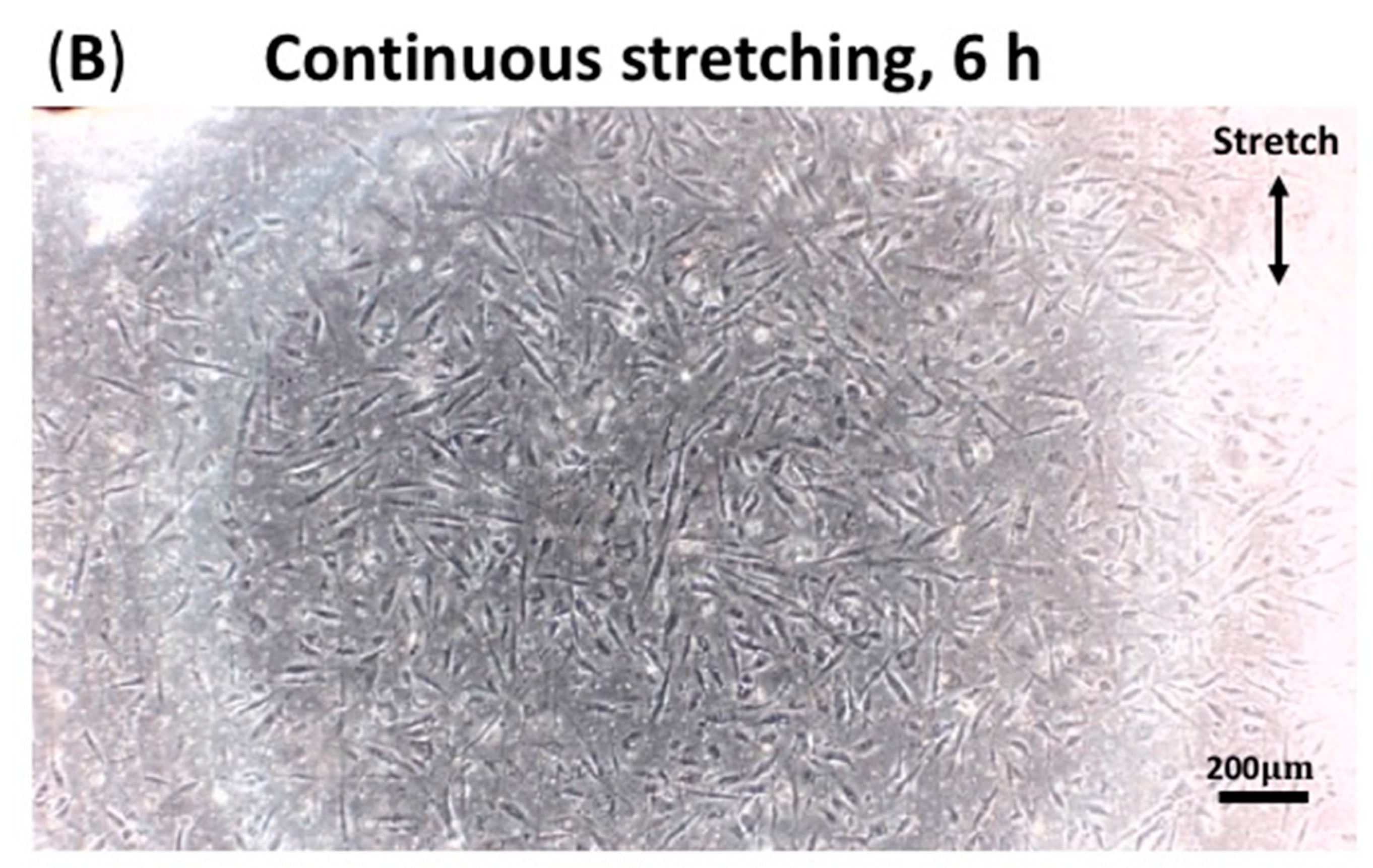
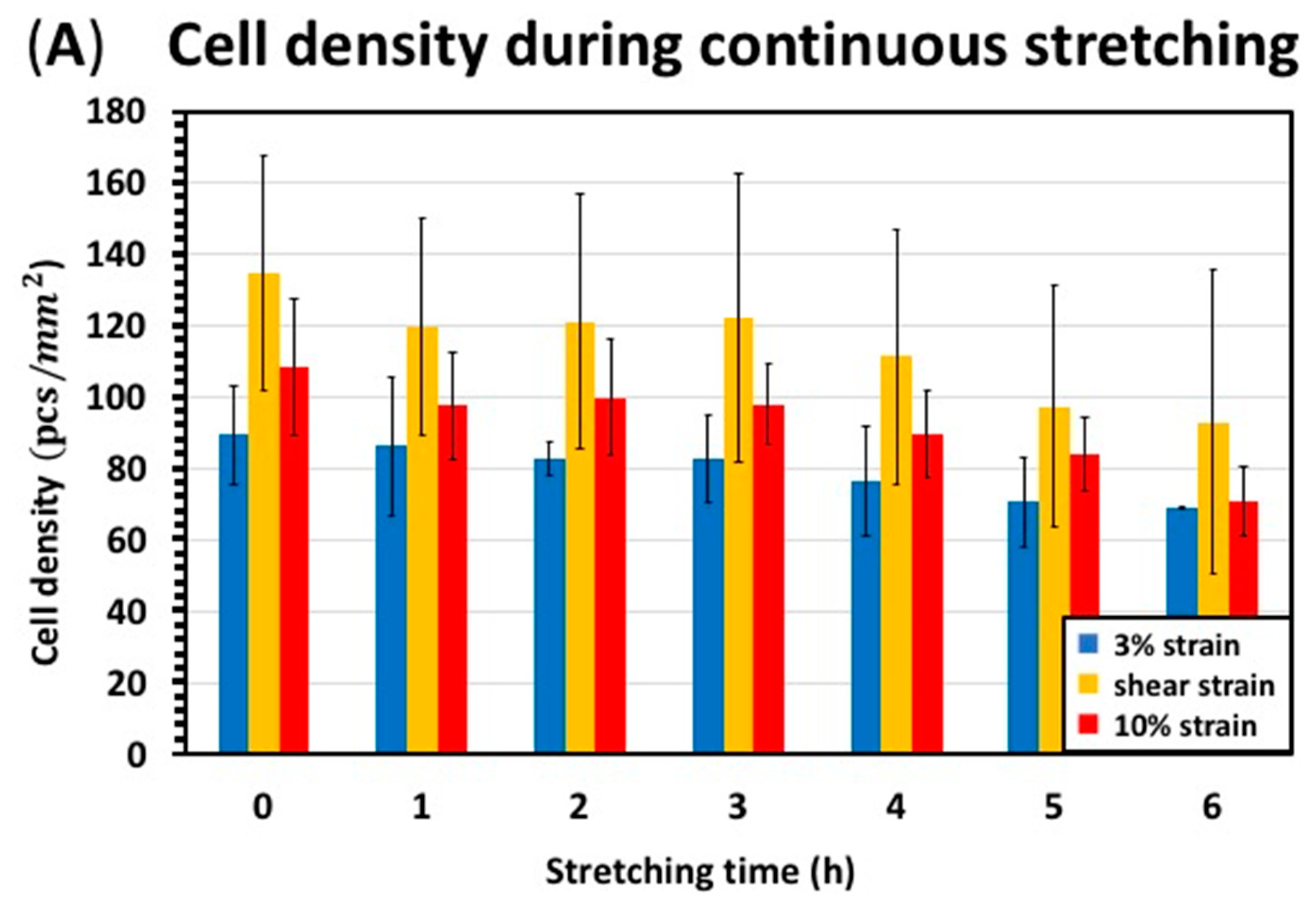
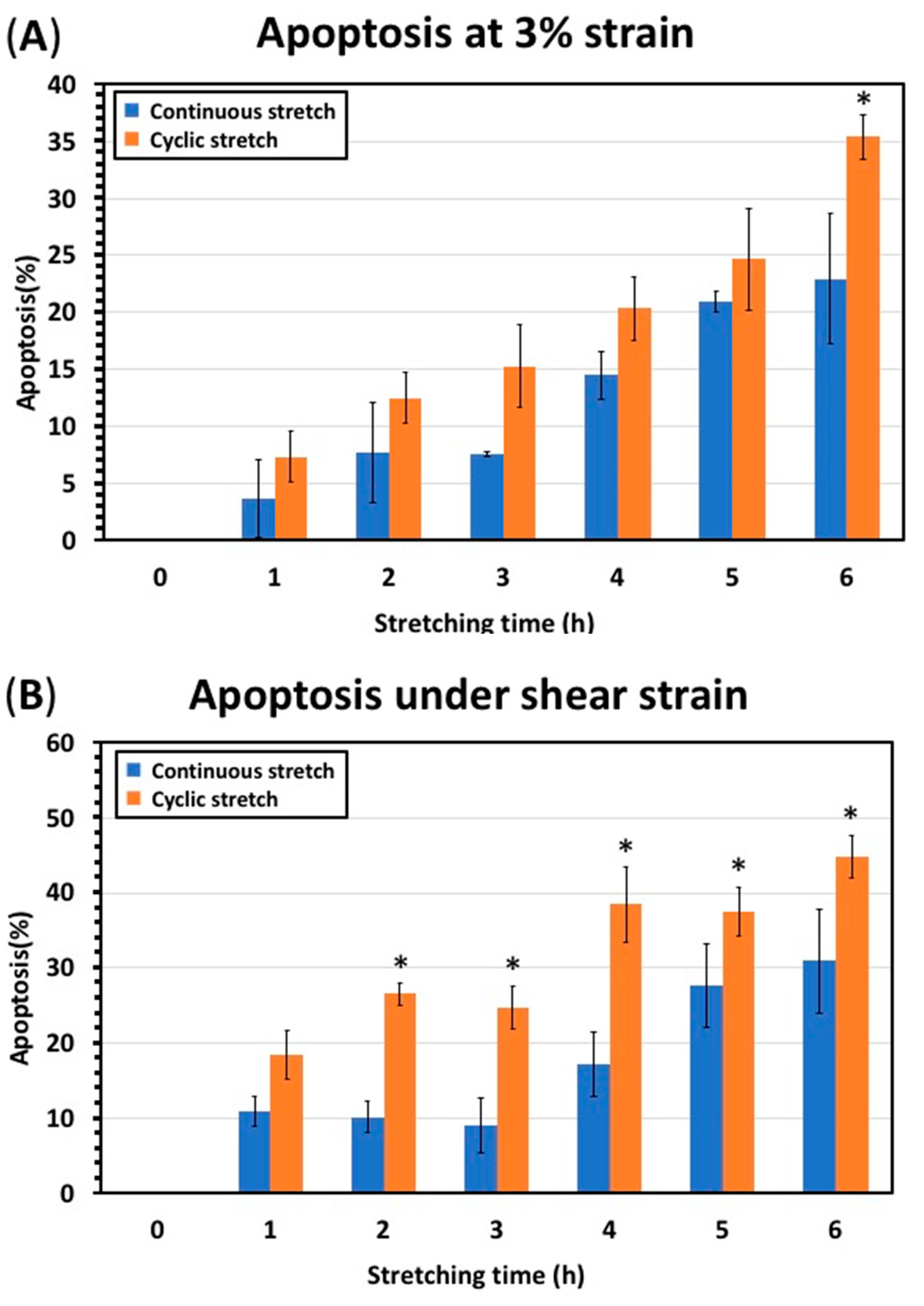
3.3. Continuous Stretching Test Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in mechanotransduction pathways that control cell morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Weyts, F.; Bosmans, B.; Niesing, R.; Leeuwen, J.; Weinans, H. Mechanical control of human osteoblast apoptosis and proliferation in relation to differentiation. Calcif. Tissue Int. 2003, 72, 505–512. [Google Scholar] [CrossRef]
- Nagatomi, J.; Arulanandam, B.P.; Metzger, D.W.; Meunier, A.; Bizios, R. Frequency-and duration-dependent effects of cyclic pressure on select bone cell functions. Tissue Eng. 2001, 7, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, A.-L.; Quiroga, X.; Walani, N.; Arroyo, M.; Roca-Cusachs, P. The plasma membrane as a mechanochemical transducer. Philos. Trans. R. Soc. B 2019, 374, 20180221. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Nagy, T.L.; Weiner, O.D. Joining forces: Crosstalk between biochemical signalling and physical forces orchestrates cellular polarity and dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170145. [Google Scholar] [CrossRef] [Green Version]
- Diz-Muñoz, A.; Fletcher, D.A.; Weiner, O.D. Use the force: Membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 2013, 23, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Neidlinger-Wilke, C.; Wilke, H.J.; Claes, L. Cyclic stretching of human osteoblasts affects proliferation and metabolism: A new experimental method and its application. J. Orthop. Res. 1994, 12, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chiu, C.-Y.; Yeh, J.-M.; Wang, S.-H. Effect of low intensity ultrasounds on the growth of osteoblasts. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 5833–5836. [Google Scholar]
- Salim, M.T.; Esmerats, J.F.; Arjunon, S.; Villa-Roel, N.; Nerem, R.M.; Jo, H.; Yoganathan, A.P. miR-214 is stretch-sensitive in aortic valve and inhibits aortic valve calcification. Ann. Biomed. Eng. 2019, 47, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Helms, F.; Lau, S.; Klingenberg, M.; Aper, T.; Haverich, A.; Wilhelmi, M.; Böer, U. Complete myogenic differentiation of adipogenic stem cells requires both biochemical and mechanical stimulation. Ann. Biomed. Eng. 2020, 48, 913–926. [Google Scholar] [CrossRef]
- Adams, S.; Wuescher, L.M.; Worth, R.; Yildirim-Ayan, E. Mechano-immunomodulation: Mechanoresponsive changes in macrophage activity and polarization. Ann. Biomed. Eng. 2019, 47, 2213–2231. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.J.; Buenzli, P.R.; Tambyah, T.A.; Thompson, E.W.; Hugo, H.J.; Baker, R.E.; Simpson, M.J. The role of mechanical interactions in EMT. Phys. Biol. 2021, 18, 046001. [Google Scholar] [CrossRef] [PubMed]
- Patteson, A.E.; Carroll, R.J.; Iwamoto, D.V.; Janmey, P.A. The vimentin cytoskeleton: When polymer physics meets cell biology. Phys. Biol. 2020, 18, 011001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Q.; Chen, Z.; Sun, J.; Xu, B.; Ju, Y.; Song, G. Cyclic mechanical stretching promotes migration but inhibits invasion of rat bone marrow stromal cells. Stem Cell Res. 2015, 14, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidovich, N.; DiPaolo, B.C.; Lawrence, G.G.; Chhour, P.; Yehya, N.; Margulies, S.S. Cyclic stretch–induced oxidative stress increases pulmonary alveolar epithelial permeability. Am. J. Respir. Cell Mol. Biol. 2013, 49, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Esteban, J.; Wang, Y.; Cicchiello, L.A.; Rubin, L.P. Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L448–L456. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-S.J.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.M.; Chen, H.; Spatz, J.P.; Kemkemer, R. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS ONE 2013, 8, e77328. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Adachi, T.; Matsuo, M.; Tomita, Y. Quantitative evaluation of threshold fiber strain that induces reorganization of cytoskeletal actin fiber structure in osteoblastic cells. J. Biomech. 2005, 38, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Shikata, Y.; Rios, A.; Kawkitinarong, K.; DePaola, N.; Garcia, J.G.; Birukov, K.G. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp. Cell Res. 2005, 304, 40–49. [Google Scholar] [CrossRef]
- Yoshigi, M.; Hoffman, L.M.; Jensen, C.C.; Yost, H.J.; Beckerle, M.C. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005, 171, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.-C.; Goldschmidt-Clermont, P.; Wille, J.; Yin, F.C.-P. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001, 34, 1563–1572. [Google Scholar] [CrossRef]
- Horiuchi, R.; Akimoto, T.; Hong, Z.; Ushida, T. Cyclic mechanical strain maintains Nanog expression through PI3K/Akt signaling in mouse embryonic stem cells. Exp. Cell Res. 2012, 318, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Zhao, Y.; Liu, H.; Li, Z.; Chen, S.; Liu, D. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp. Cell Res. 2021, 403, 112598. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Shi, Q.; Wang, W.-B.; Bao, H.; Yan, J.; Gao, S.; Liu, Z.; Jiang, Z.-L.; Qi, Y.-X. Endothelial microvesicles induced by physiological cyclic stretch inhibit ICAM1-Dependent leukocyte adhesion. Exp. Cell Res. 2020, 386, 111710. [Google Scholar] [CrossRef]
- Zmurchok, C.; Bhaskar, D.; Edelstein-Keshet, L. Coupling mechanical tension and GTPase signaling to generate cell and tissue dynamics. Phys. Biol. 2018, 15, 046004. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Kemkemer, R.; Deibler, M.; Spatz, J.; Gao, H. Cyclic stretch induces cell reorientation on substrates by destabilizing catch bonds in focal adhesions. PLoS ONE 2012, 7, e48346. [Google Scholar] [CrossRef] [Green Version]
- Livne, A.; Bouchbinder, E.; Geiger, B. Cell reorientation under cyclic stretching. Nat. Commun. 2014, 5, 3938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.Y.; Pingguan-Murphy, B.; Abbas, A.A.; Merican, A.M.; Kamarul, T. Uniaxial cyclic tensile stretching at 8% strain exclusively promotes tenogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Stem Cells Int. 2019, 2019, 9723025. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.-X.; Shih, P.-J.; Yen, J.-Y.; Wang, I.-J. Functional assistance for stress distribution in cell culture membrane under periodically stretching. J. Biomech. 2021, 125, 110564. [Google Scholar] [CrossRef] [PubMed]
- Orsola, A.; Adam, R.M.; Peters, C.A.; Freeman, M.R. The decision to undergo DNA or protein synthesis is determined by the degree of mechanical deformation in human bladder muscle cells. Urology 2002, 59, 779–783. [Google Scholar] [CrossRef]
- Dai, Y.; Tian, Y.; Luo, D.-Y.; Wazir, R.; Yue, X.; Li, H.; Wang, K.-J. Cyclic stretch induces human bladder smooth muscle cell proliferation in vitro through muscarinic receptors. Mol. Med. Rep. 2015, 11, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Wedgwood, S.; Lakshminrusimha, S.; Schumacker, P.T.; Steinhorn, R.H. Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L196–L203. [Google Scholar] [CrossRef] [Green Version]
- Dan, P.; Velot, É.; Decot, V.; Menu, P. The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. J. Cell Sci. 2015, 128, 2415–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohberger, B.; Kaltenegger, H.; Stuendl, N.; Payer, M.; Rinner, B.; Leithner, A. Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. BioMed Res. Int. 2014, 2014, 189516. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, X.; Chen, W.; Liu, C.; Rong, S.; Wang, X.; Du, G. Combined effects of interleukin-1β and cyclic stretching on metalloproteinase expression in corneal fibroblasts in vitro. Biomed. Eng. Online 2016, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barron, V.; Brougham, C.; Coghlan, K.; McLucas, E.; O’Mahoney, D.; Stenson-Cox, C.; McHugh, P.E. The effect of physiological cyclic stretch on the cell morphology, cell orientation and protein expression of endothelial cells. J. Mater. Sci. Mater. Med. 2007, 18, 1973–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, M.Y.; Cheng, T.; Pakstis, L.; Dunkers, J. Solutions for determining equibiaxial substrate strain for dynamic cell culture. J. Biomech. 2010, 43, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Weinhold, P.; Banes, A.; Link, G.; Jones, G. Strain profiles for circular cell culture plates containing flexible surfaces employed to mechanically deform cells in vitro. J. Biomech. 1994, 27, 1169–1177. [Google Scholar] [CrossRef]
- Huang, C.Y.C.; Hagar, K.L.; Frost, L.E.; Sun, Y.; Cheung, H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 2004, 22, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Takahashi, I.; Anada, T.; Arai, F.; Fukuda, T.; Takano-Yamamoto, T.; Suzuki, O. Development of a cell culture system loading cyclic mechanical strain to chondrogenic cells. J. Biotechnol. 2008, 133, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tata, U.; Xu, H.; Rao, S.; Chuong, C.-J.; Nguyen, K.T.; Chiao, J.-C. A novel multiwell device to study vascular smooth muscle cell responses under cyclic strain. J. Nanotechnol. Eng. Med. 2011, 2, 021007. [Google Scholar] [CrossRef]
- Xie, K.Y.; Yang, L.; Chen, K.; Li, Q. In vitro study of the effect of cyclic strains on the dermal fibroblast (GM3384) morphology—Mapping of cell responses to strain field. Med. Eng. Phys. 2012, 34, 826–831. [Google Scholar] [CrossRef]
- Józsa, T.I.; Padmos, R.M.; El-Bouri, W.K.; Hoekstra, A.G.; Payne, S.J. On the sensitivity analysis of porous finite element models for cerebral perfusion estimation. Ann. Biomed. Eng. 2021, 49, 3647–3665. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.; Britton, S.; Nematbakhsh, A.; Zandi, R.; Chen, W.; Alber, M. Role of combined cell membrane and wall mechanical properties regulated by polarity signals in cell budding. Phys. Biol. 2020, 17, 065011. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Kume, M.; Wyczalkowski, M.A.; Taber, L.A.; Pless, R.B.; Xia, Y.; Genin, G.M.; Thomopoulos, S. Simple and accurate methods for quantifying deformation, disruption, and development in biological tissues. J. R. Soc. Interface 2014, 11, 20140685. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mende, M.; Yang, X.; Körber, H.-F.; Schnittler, H.-J.; Weinert, S.; Heubach, J.; Werner, C.; Ravens, U. Design and validation of a bioreactor for simulating the cardiac niche: A system incorporating cyclic stretch, electrical stimulation, and constant perfusion. Tissue Eng. Part A 2013, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Sato, T.; Watanabe, S.; Ju, Y. Determination of precise optimal cyclic strain for tenogenic differentiation of mesenchymal stem cells using a non-uniform deformation field. Exp. Mech. 2015, 55, 635–640. [Google Scholar] [CrossRef]
- Shakiba, D.; Alisafaei, F.; Savadipour, A.; Rowe, R.A.; Liu, Z.; Pryse, K.M.; Shenoy, V.B.; Elson, E.L.; Genin, G.M. The balance between actomyosin contractility and microtubule polymerization regulates hierarchical protrusions that govern efficient fibroblast–collagen interactions. ACS Nano 2020, 14, 7868–7879. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lin, Z.; Li, Y.-m. Effects of different magnitudes of mechanical strain on osteoblasts in vitro. Biochem. Biophys. Res. Commun. 2006, 344, 122–128. [Google Scholar] [CrossRef] [PubMed]




| Authors | Cell | Stretch Type | Strain (%) | Frequency (Hz) | Time | Response |
|---|---|---|---|---|---|---|
| Neidlinger-Wilke [8] | Human osteoblasts | Biaxial cyclic stretch | 1.0, 2.4, 5.3, 8.8 | 1 | 15 min per day, 3 days | Proliferation |
| Dai [33] | HBSMC | Uniaxial cyclic stretch | 5, 10, 15, 20 | 0.1 | 6, 12 h | Proliferation |
| Tang [51] | MC3T3-E1 | Uniaxial cyclic stretch | 0, 6, 12, 18 | 0.1 | 24 h | Proliferation |
| This work | HK | Uniaxial cyclic/continuous stretch | 3, 10, Shear | 1 | 6 h | Apoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.-X.; Shih, P.-J.; Yen, J.-Y.; Wang, I.-J. Effect of Static and Dynamic Stretching on Corneal Fibroblast Cell. Processes 2022, 10, 605. https://doi.org/10.3390/pr10030605
Dai Z-X, Shih P-J, Yen J-Y, Wang I-J. Effect of Static and Dynamic Stretching on Corneal Fibroblast Cell. Processes. 2022; 10(3):605. https://doi.org/10.3390/pr10030605
Chicago/Turabian StyleDai, Zhi-Xuan, Po-Jen Shih, Jia-Yush Yen, and I-Jong Wang. 2022. "Effect of Static and Dynamic Stretching on Corneal Fibroblast Cell" Processes 10, no. 3: 605. https://doi.org/10.3390/pr10030605
APA StyleDai, Z.-X., Shih, P.-J., Yen, J.-Y., & Wang, I.-J. (2022). Effect of Static and Dynamic Stretching on Corneal Fibroblast Cell. Processes, 10(3), 605. https://doi.org/10.3390/pr10030605






