Study on Gas-Solid Heat Transfer and Decomposition Reaction of Calcination Process in an Annular Shaft Kiln Based on the Finite Volume Method
Abstract
:1. Introduction
2. Models Introduction and Operating Conditions
2.1. Mathematical Models
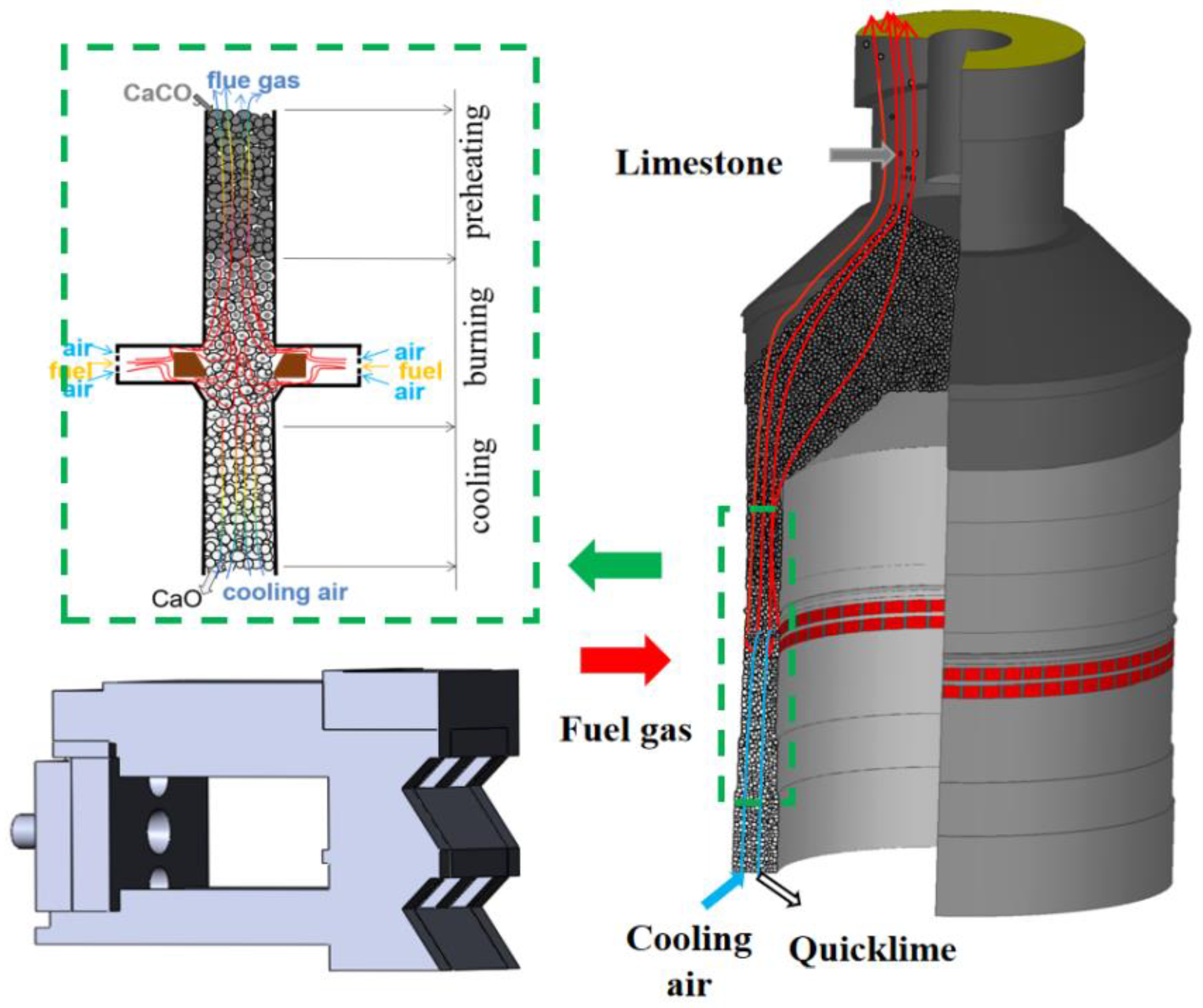
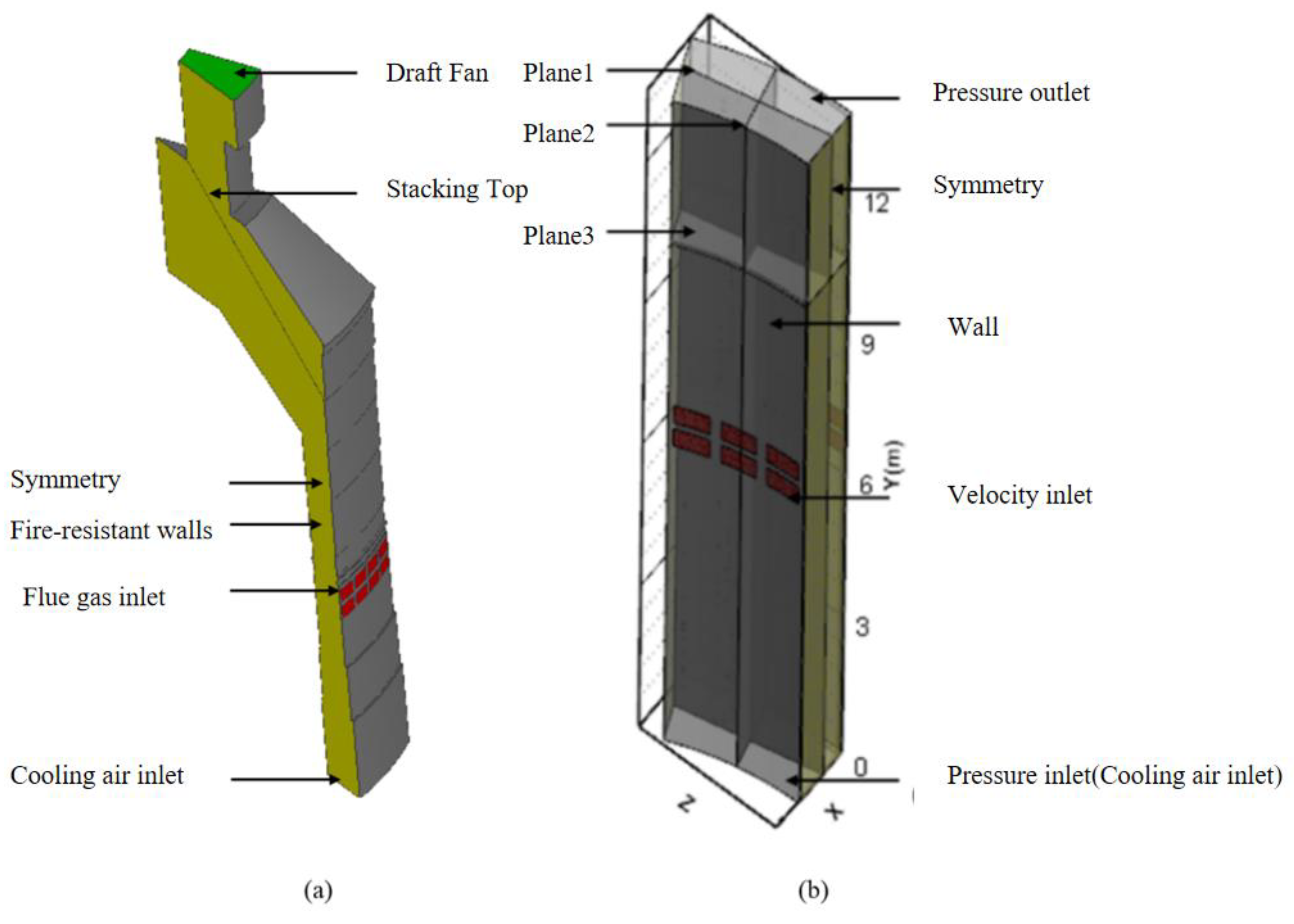
2.2. Physical Model and Boundary Conditions
3. Calcination Simulation Process
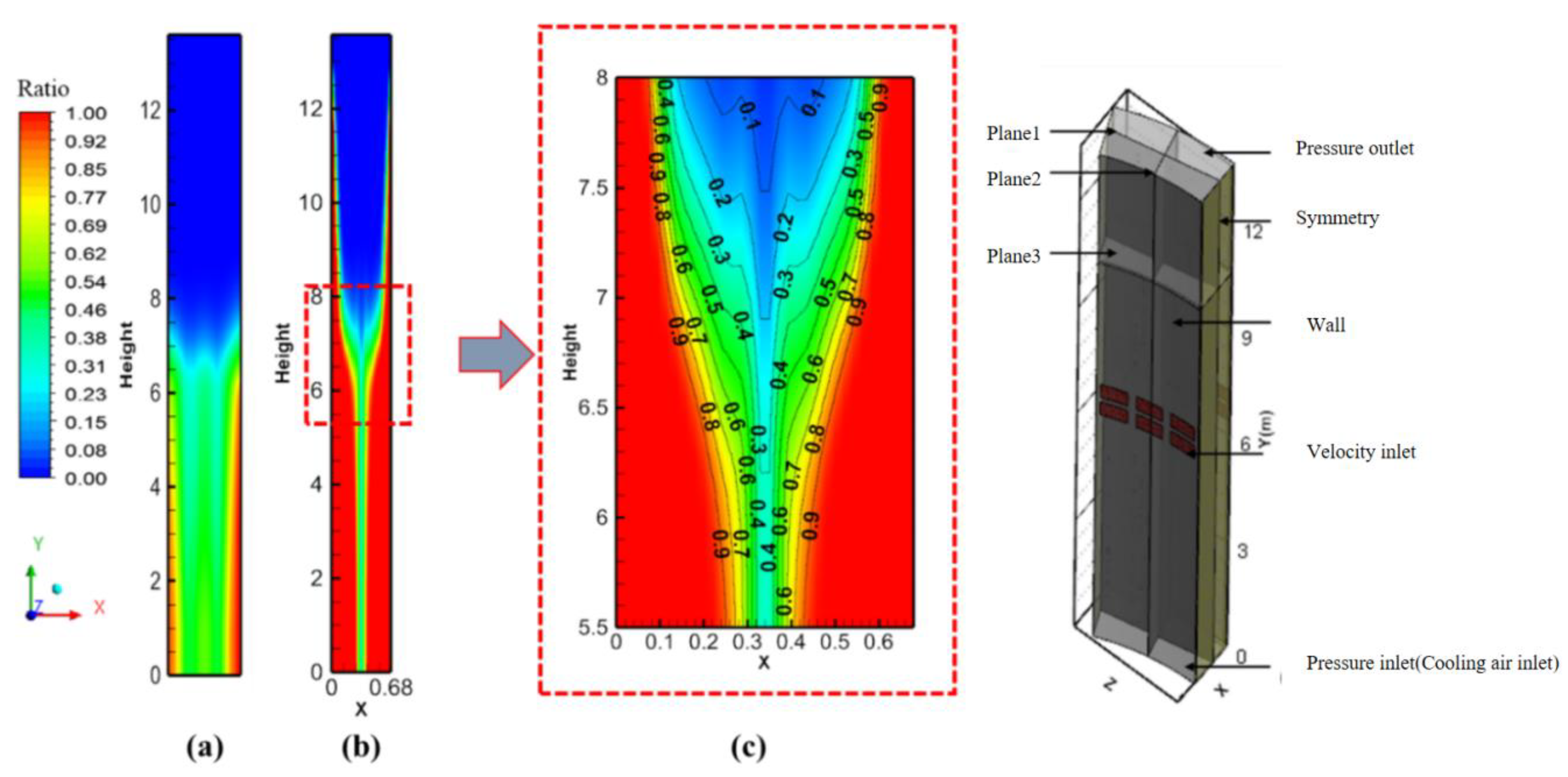
3.1. Flow Field and Temperature Field Analysis
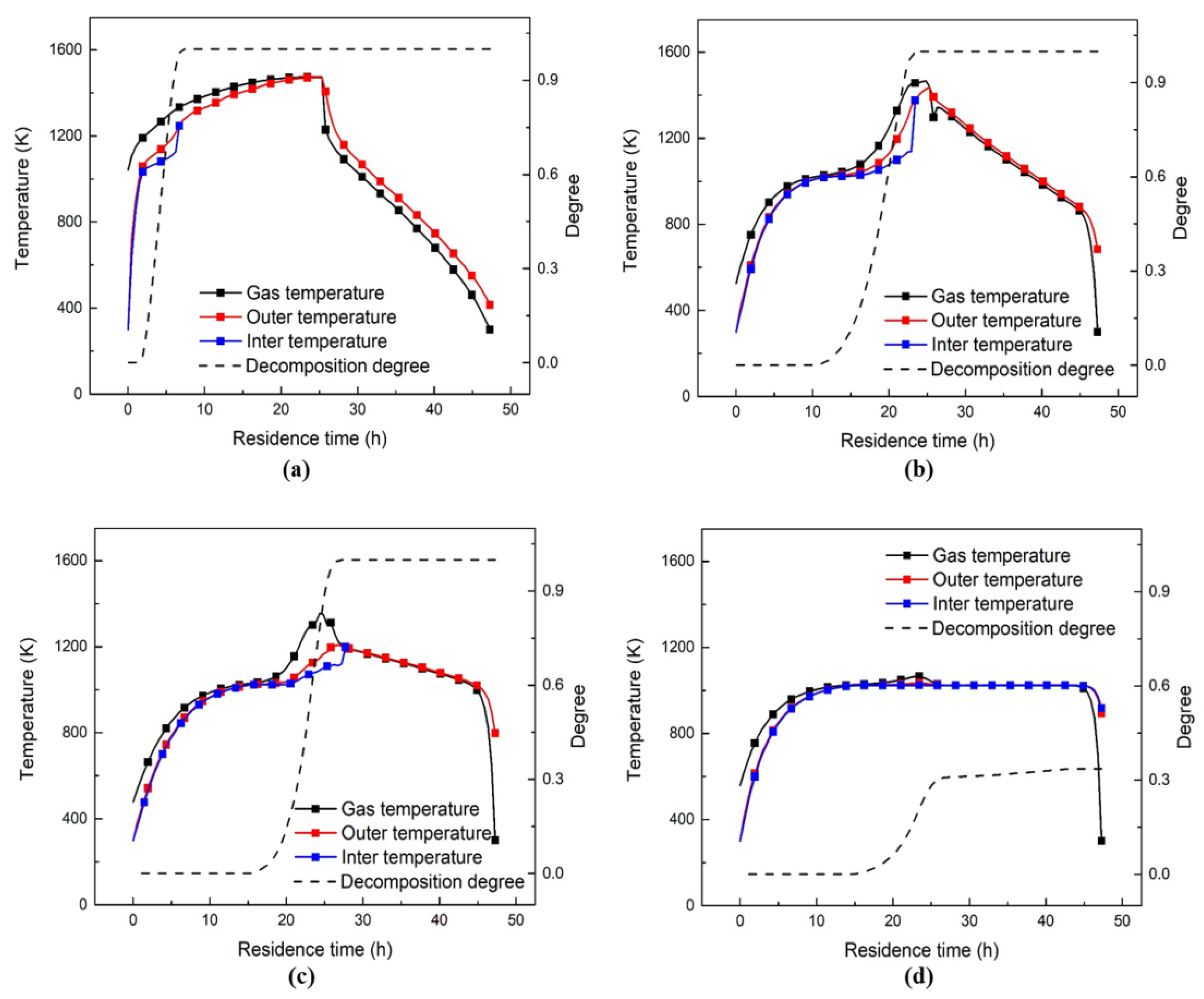
3.2. Decomposition Analysis
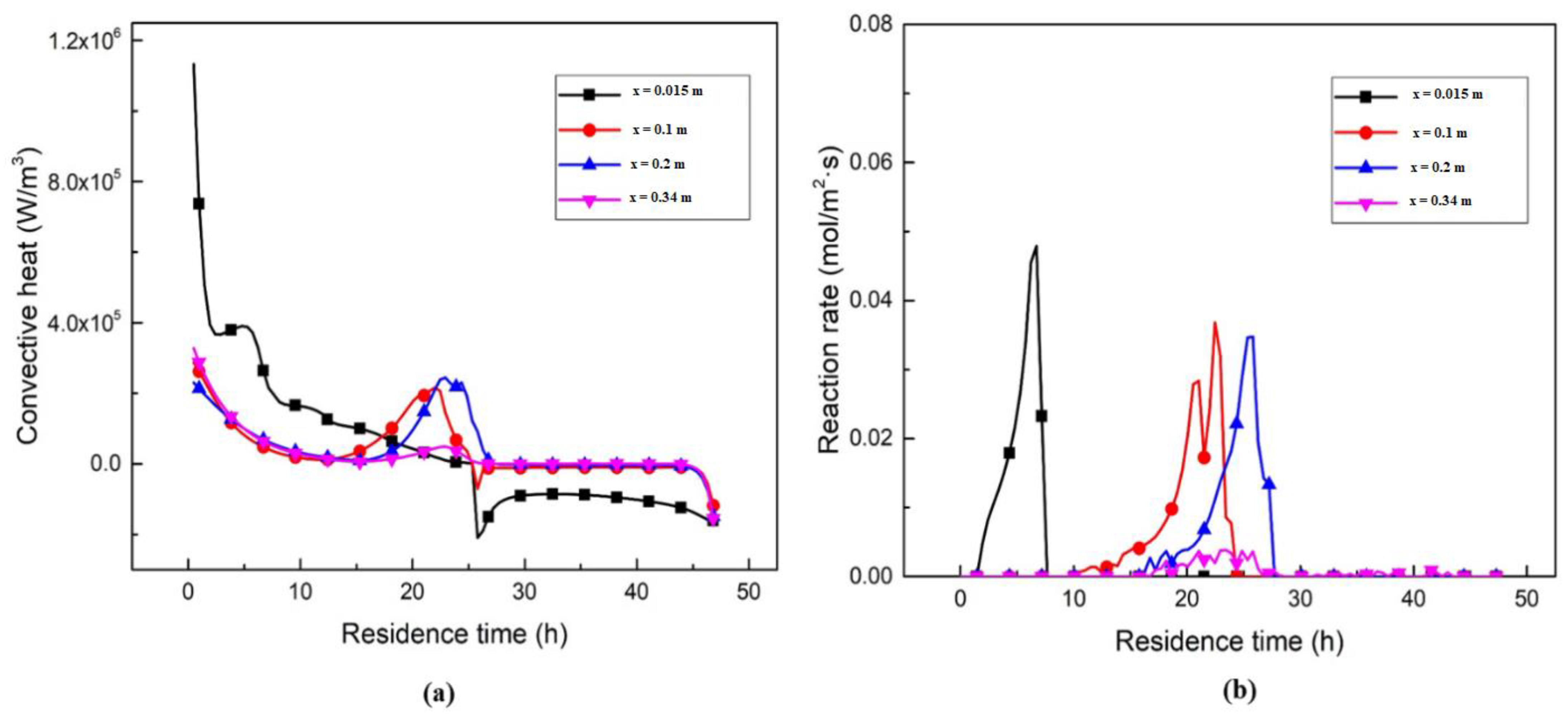
3.3. Heat and Mass Transfer Analysis inside ASK
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
References
- Song, Y.; Zhang, X. The production and application of active lime in metallurgy. J. Hebei Inst. Technol. 2006, 28, 5–11. [Google Scholar]
- Guo, S. Application of active lime in metallurgy. Refract. Lime 2007, 5, 4–5. [Google Scholar]
- Oates, T. Lime and limestone. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid-Reactions; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Hills, A.W.D. The mechanism of the thermal decomposition of calcium carbonate. Chem. Eng. Sci. 1986, 23, 297–320. [Google Scholar] [CrossRef]
- Escardino, A.; García-Ten, J.; Feliu, C. Kinetic study of the thermal decomposition process of calcite particles in air and CO2 atmosphere. J. Ind. Eng. Chem. 2013, 19, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Khinast, J.; Krammer, G.F.; Brunner, C. Decomposition of limestone: The influence of CO2 and particle size on the reaction rate. Chem. Eng. Sci. 1995, 51, 623–634. [Google Scholar] [CrossRef]
- Shangqing, L.; Sufang, W.U. Advances in calcium carbonate thermal decomposition. CIESC J. 2015, 66, 2895–2902. [Google Scholar]
- Chen, H.; Chen, J.; Wei, R.; Suo, X. Study of the decomposition characteristics of limestone and the law governing the migration of the microscopic structure. J. Eng. Therm. Energy Power. 2013, 1, 73–77, 112. [Google Scholar]
- Feng, Y.; Chen, Y. Development of research on calcium carbonate for decomposed kinetics. Bull. Chin. Ceram. Soc. 2006, 3, 140–145, 154. [Google Scholar]
- Senegačnik, A.; Oman, J.; Širok, B. Analysis of calcination parameters and the temperature profile in an annular shaft kiln. Part 1: Theoretical survey. Appl. Therm. Eng. 2007, 27, 1467–1472. [Google Scholar] [CrossRef]
- Senegačnik, A.; Oman, J.; Širok, B. Analysis of calcination parameters and the temperature profile in an annular shaft kiln. Part 2: Results of tests. Appl. Therm. Eng. 2007, 27, 1473–1482. [Google Scholar] [CrossRef]
- Li, J.; Niu, H. Optimization design measures of the structure and performance of the lime kiln. Constr. Des. Project. 2014, 2, 37–40. [Google Scholar]
- Senegačnik, A.; Oman, J.; Širok, B. Annual shaft kiln for lime burning with kiln gas recirculation. Appl. Therm. Eng. 2008, 28, 785–792. [Google Scholar] [CrossRef]
- Sagastume, A.; Vandecasteele, C. Exergy-based indicators to evaluate the possibilities to reduce fuel consumption in lime production. Energy 2011, 36, 2820–2827. [Google Scholar]
- Sagastume, A.; Cogollos, J.B.; Vandecasteele, C. Energy and exergy assessments of a lime shaft kiln. Appl. Therm. Eng. 2013, 51, 273–280. [Google Scholar] [CrossRef]
- Cheng, C.; Specht, E. Reaction rate coefficients in decomposition of lumpy limestone of different origin. Thermochim. Acta 2006, 449, 8–15. [Google Scholar] [CrossRef]
- Duc, H.; Specht, E. Determination of reaction coefficient, thermal conductivity and pore diffusivity in decomposition of limestone of different origin. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 19–21 October 2011; Volume 11, pp. 978–984. [Google Scholar]
- Bluhm-Drenhaus, T.; Simsek, E.; Wirtz, S. A couple fluid dynamic-discrete element simulation of heat and mass transfer in a lime shaft kiln. Chem. Eng. Sci. 2010, 65, 2821–2834. [Google Scholar] [CrossRef]
- Krause, B.; Liedmann, B.; Wiese, J. Coupled three dimensional DEM-CFD simulation of a lime shaft kiln Calcination, particle movement and gas phase flow field. Chem. Eng. Sci. 2015, 134, 834–849. [Google Scholar] [CrossRef]
- Ning, J.; Zhong, B.; Fu, W. Study on the calcination of fine limestone powder at high temperature. J. Combust. Sci. Technol. 2003, 3, 205–208. [Google Scholar]
- Mikulčić, H.; Vujanović, M.; Fidaros, D.K. The application of CFD modelling to support the reduction of CO2 emissions in cement industry. Energy 2012, 45, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Long, H.; Xu, M.; Yu, D.; Ding, Y.; Wang, K. Two-temperature model of water gas shift reaction in porous media based on fuel NT. Comput. Appl. Chem. 2012, 8, 981–985. [Google Scholar]
- Bu, S.; Yang, J.; Li, S.; Wang, Q. Effects of point contact treatment methods on flow and heat transfer of structured packed beds. J. Eng. Thermophys. 2013, 3, 534–537. [Google Scholar]
- Liu, G.; Cui, G.; Ma, S.; Han, N. Simulation of the temperature field in a lime kiln by using a local non-heat-equilibrium model and its optimized analysis. J. Eng. Therm. Energy Power. 2018, 2, 96–105. [Google Scholar]
- Zhongda, T.; Shujiang, L.; Yanhong, W.; Xiangdong, W. SVM predictive control for calcination zone temperature in lime rotary kiln with improved PSO algorithm. Trans. Inst. Meas. Control. 2018, 40, 3134–3146. [Google Scholar] [CrossRef]
- González Suárez, V.M.; García-Gonzalo, E.; Mayo Bayón, R.; García Nieto, P.J.; Álvarez Antón, J.C. Predictive model of gas consumption and air emissions of a lime kiln in a kraft process using the ABC/MARS-based technique. Int. J. Adv. Manuf. Technol. 2018, 100, 1549–1562. [Google Scholar] [CrossRef]
- Metz, J.C.; Maciel, E.F.; Garbin, M.; Modolo, R.C.E.; Moraes, C.A.M.; Gomes, L.B.; Brehm, F.A. Infuelnce of electric arc furnace dust and lime kiln waste in portland cement hydration. Ambiente Construído 2020, 20, 225–241. [Google Scholar] [CrossRef]
- Juneja, P.K.; Sunori, S.K.; Sharma, A.; Sharma, A.; Joshi, V. Modeling, control and instrumentation of lime kiln process: A review. In Proceedings of the 2020 International Conference on Advances in Computing, Communication & Materials, Dehradun, India, 21–22 August 2020. [Google Scholar]
- Yur’ev, B.; Dudko, V. Determination of key parameters required to optimize calcination process in ferrous metallurgy heating plants. Solid State Phenom. 2021, 316, 282–287. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Xia, D.; Jiang, Z.; Jiang, B.; Zhang, P. Novel lime calcination system for CO2 capture and its thermal-mass balance analysis. ACS Omega 2020, 5, 27413–27424. [Google Scholar] [CrossRef]
- Zhaohua, L. Calculation of effective thermal conductivity of foam porous media. J. Nanjing Univ. Sci. Technol. 2001, 3, 257–261. [Google Scholar]
- Lingli, Z.; Jie, B.; Bin, Q. Calculation of convective heat transfer for active limes pre-heater. Metall. Equip. 2012, 6, 6–8. [Google Scholar]





| Structure Numerical | Value | Particulate Materiality | Value |
|---|---|---|---|
| Height of flow field, m | 22 | CaO density, kg·m−3 | 3310 |
| Height of calcination zone, m | 13.7 | CaCO3 density, kg·m−3 | 2810 |
| ASK inner ring, m | 7.24 | ) | 0.07 |
| ASK outer ring, m | 9.08 | ) | 2.26 |
| Pairs of fuel gas inlet spacing, m | 0.68 | 1073 | |
| Lower fuel gas inlet height, m | 6.47 | ||
| Upper fuel gas inlet height, m | 6.958 |
| Material Name | Value |
|---|---|
| O2 | |
| N2 | |
| CO2 | |
| Vapor | |
| CaO | |
| CaCO3 |
| fuel gas composition | H2O | N2 | CO2 | O2 |
| Volume fraction% | 1.02 | 71.89 | 15.12 | 11.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; Li, B.; Rong, W. Study on Gas-Solid Heat Transfer and Decomposition Reaction of Calcination Process in an Annular Shaft Kiln Based on the Finite Volume Method. Processes 2022, 10, 648. https://doi.org/10.3390/pr10040648
Duan S, Li B, Rong W. Study on Gas-Solid Heat Transfer and Decomposition Reaction of Calcination Process in an Annular Shaft Kiln Based on the Finite Volume Method. Processes. 2022; 10(4):648. https://doi.org/10.3390/pr10040648
Chicago/Turabian StyleDuan, Shaopei, Baokuan Li, and Wenjie Rong. 2022. "Study on Gas-Solid Heat Transfer and Decomposition Reaction of Calcination Process in an Annular Shaft Kiln Based on the Finite Volume Method" Processes 10, no. 4: 648. https://doi.org/10.3390/pr10040648
APA StyleDuan, S., Li, B., & Rong, W. (2022). Study on Gas-Solid Heat Transfer and Decomposition Reaction of Calcination Process in an Annular Shaft Kiln Based on the Finite Volume Method. Processes, 10(4), 648. https://doi.org/10.3390/pr10040648





