Rationally Designed Ternary Deep Eutectic Solvent Enabling Higher Performance for Non-Aqueous Redox Flow Batteries
Abstract
:1. Introduction
2. Results
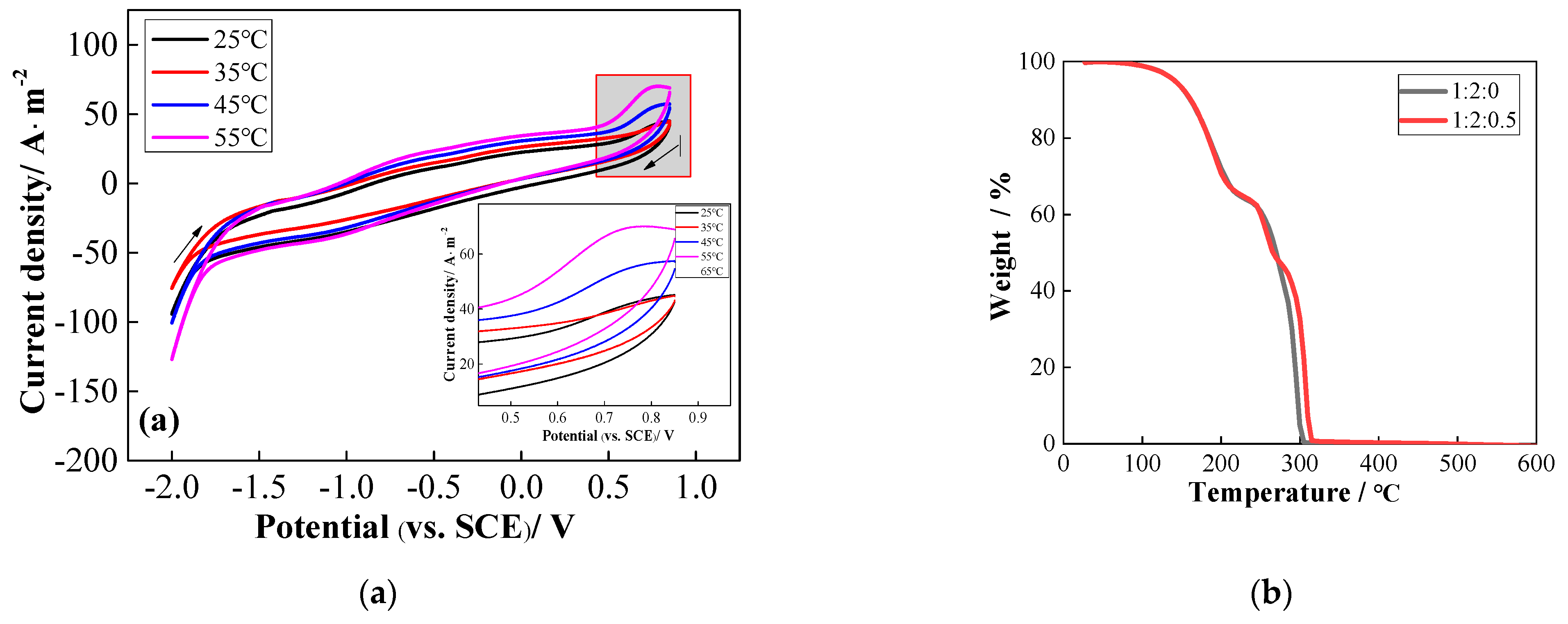
2.1. Physical and Electrochemical Properties of TDES
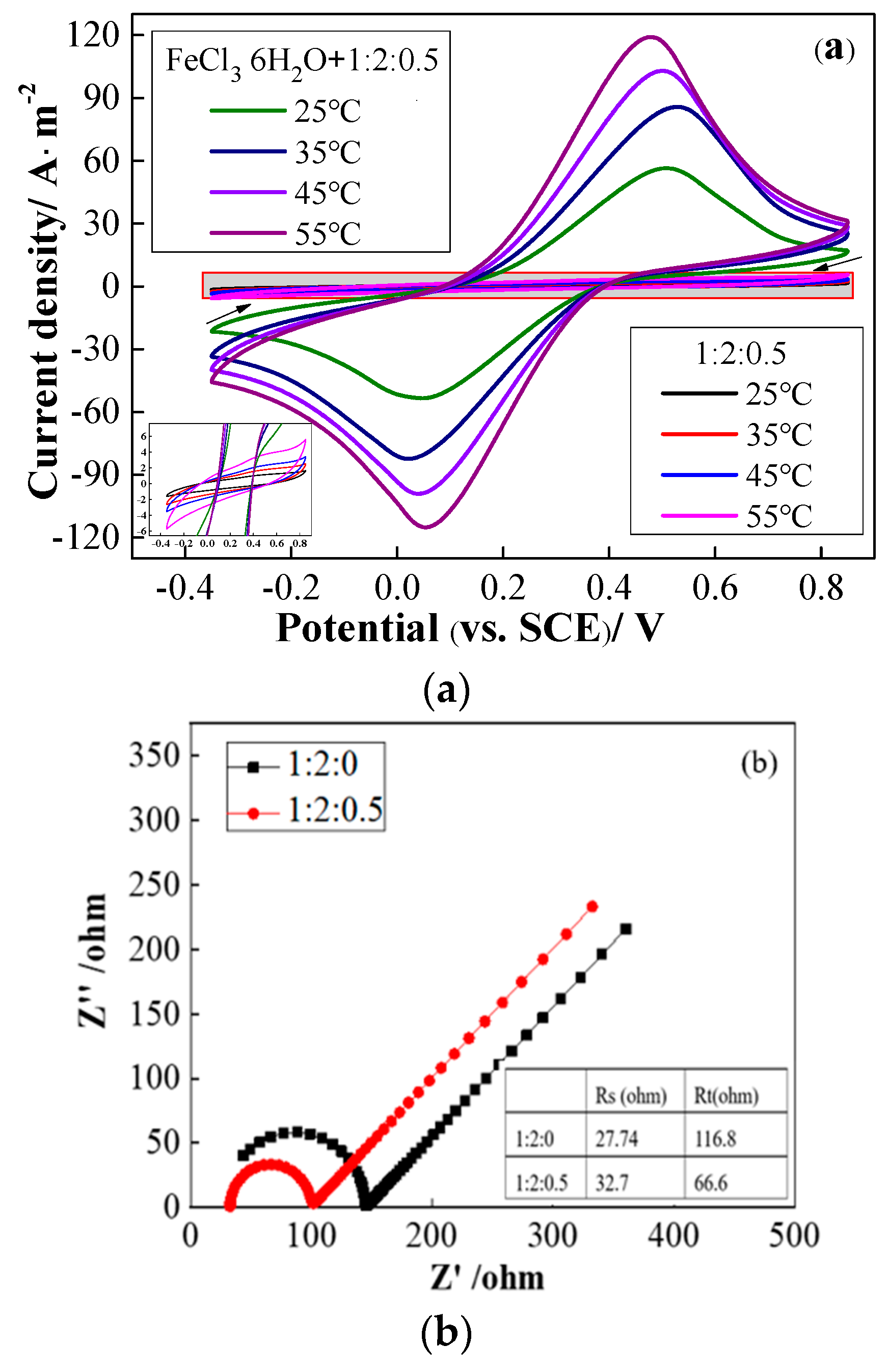
2.2. Effect of TDES on Fe (III)/Fe (II)
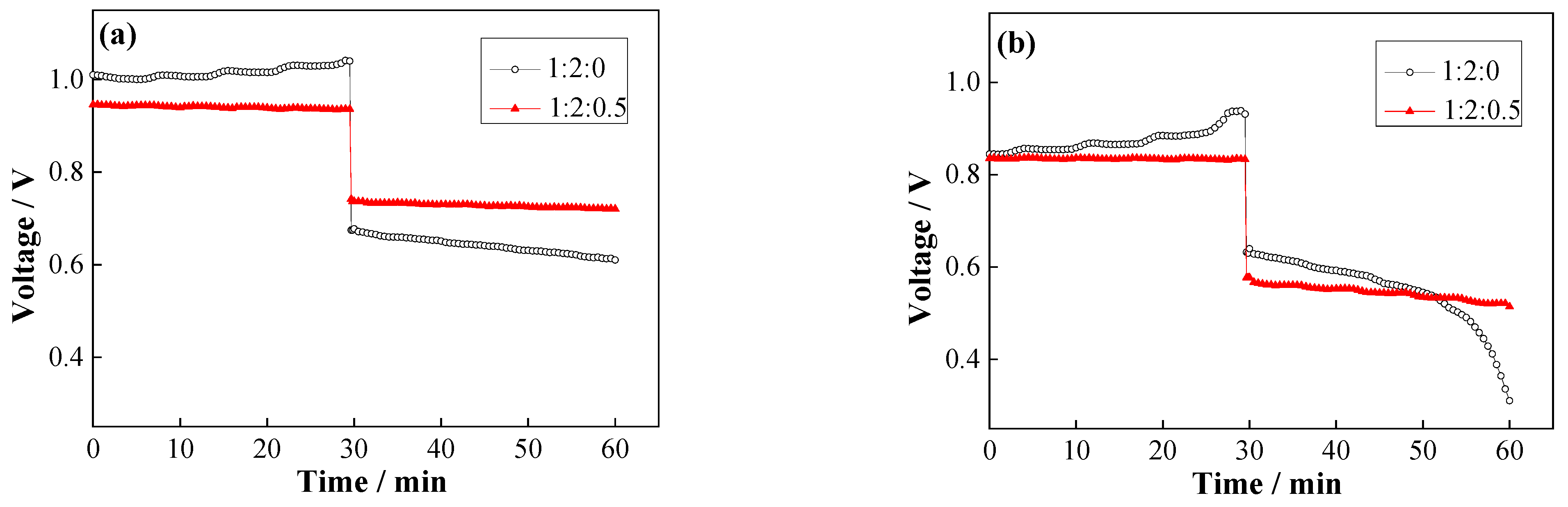
2.3. Influence of TDES on Fe–V Redox Flow Battery
2.4. FT-IR Spectroscopy
3. Materials and Methods
3.1. Preparation of Electrolyte
3.2. Electrochemical Measurement
4. Conclusions
- (1)
- The addition of glycerin has a positive effect on physical properties (viscosity and conductivity, etc.).
- (2)
- With 1:2:0.5-ternary deep eutectic solvents, the power density of the battery (9.01 mW·cm−2) is higher than that of the binary system (6.52 mW·cm−2). When glycerin is added, charging voltage decreases significantly, the discharging voltage increases, and the energy efficiency of the battery increases from 63.06% to 77.41%.
- (3)
- Combining the experimental results and FT-IR spectra, it can be inferred that the electron migration of metal cation is hindered by the formation of the chloride ion–metal cation–ethylene glycol supramolecular complex in the binary system.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, C.P.; Sharkh, S.M.; Li, X.; Walsh, F.C.; Zhang, C.N.; Jiang, J.C. The performance of a soluble lead-acid flow battery and its comparison to a static lead-acid battery. Energy Convers. Manag. 2011, 52, 3391–3398. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Battery Technologies for Large-Scale Stationary Energy Storage. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badwal, S.P.S.; Giddey, S.S.; Munnings, C.; Bhatt, A.I.; Hollenkamp, A.F. Emerging electrochemical energy conversion and storage technologies. Front. Chem. 2014, 2, 79. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Noack, J.; Roznyatovskaya, N.; Herr, T.; Fischer, P. The Chemistry of Redox-Flow Batteries. Angew. Chem. Int. Ed. 2015, 54, 9776–9809. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Xiao, M.; Meng, Y. Synthesis and properties of novel sulfonated poly(arylene ether sulfone) ionomers for vanadium redox flow battery. Energy Convers. Manag. 2010, 51, 2816–2824. [Google Scholar] [CrossRef]
- Rychcik, M.; Skyllas-Kazacos, M. Characteristics of a new all-vanadium redox flow battery. J. Power Sources 1988, 22, 59–67. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Case studies of operational failures of vanadium redox flow battery stacks, diagnoses and remedial actions. J. Energy Storage 2021, 33, 102078. [Google Scholar] [CrossRef]
- Viswanathan, V.; Crawford, A.; Stephenson, D.; Kim, S.; Wang, W.; Li, B.; Coffey, G.; Thomsen, E.; Graff, G.; Balducci, P.; et al. Cost and performance model for redox flow batteries. J. Power Sources 2014, 247, 1040–1051. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, Y.N.; Qin, L.Y.; Leung, P.K.; Qiao, F.; Li, Y.S.; Su, H.N. Evaluation of redox flow batteries goes beyond round-trip efficiency: A technical review. J. Energy Storage 2018, 16, 108–115. [Google Scholar] [CrossRef]
- Lu, P.; Qin, L.; Balakrishnan, P.; Ma, Q.; Su, H.; Yang, W.; Xu, Q. The effect of additive supporting electrolytes on transport and electrochemical properties of deep eutectic solvent (DES) applied in non-aqueous redox flow batteries. Ionics 2020, 26, 5029–5036. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, B.; Wei, X.; Li, L.; Yang, Z. Recent Progress in Redox Flow Battery Research and Development. Adv. Funct. Mater. 2013, 23, 970–986. [Google Scholar] [CrossRef]
- Bahadori, L.; Abdul Manan, N.S.; Chakrabarti, M.H.; Hashim, M.A.; Mjalli, F.S.; AlNashef, I.M.; Hussain, M.A.; Low, C.T.J. The electrochemical behaviour of ferrocene in deep eutectic solvents based on quaternary ammonium and phosphonium salts. Phys. Chem. Chem. Phys. 2013, 15, 1707–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Baby, J.N.; Sriram, B.; Wang, S.-F.; George, M. Effect of Various Deep Eutectic Solvents on the Sustainable Synthesis of MgFe2O4 Nanoparticles for Simultaneous Electrochemical Determination of Nitrofurantoin and 4-Nitrophenol. ACS Sustain. Chem. Eng. 2020, 8, 1479–1486. [Google Scholar] [CrossRef]
- Mou, H.; Wang, J.; Zhang, D.; Yu, D.; Chen, W.; Wang, D.; Mu, T. A one-step deep eutectic solvent assisted synthesis of carbon nitride/metal oxide composites for photocatalytic nitrogen fixation. J. Mater. Chem. A 2019, 7, 5719–5725. [Google Scholar] [CrossRef]
- Jaihindh, D.P.; Manikandan, A.; Chueh, Y.-L.; Fu, Y.-P. Deep Eutectic Solvent-Assisted Synthesis of Ternary Heterojunctions for the Oxygen Evolution Reaction and Photocatalysis. ChemSusChem 2020, 13, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jia, H.; Li, H.; Zhu, L.; Tao, R.; Zhu, W.; Li, H.; Dai, S. Boric acid-based ternary deep eutectic solvent for extraction and oxidative desulfurization of diesel fuel. Green Chem. 2019, 21, 3074–3080. [Google Scholar] [CrossRef]
- Sze, L.L.; Pandey, S.; Ravula, S.; Pandey, S.; Zhao, H.; Baker, G.A.; Baker, S.N. Ternary Deep Eutectic Solvents Tasked for Carbon Dioxide Capture. ACS Sustain. Chem. Eng. 2014, 2, 2117–2123. [Google Scholar] [CrossRef]
- Qin, H.; Owyeung, R.E.; Sonkusale, S.R.; Panzer, M.J. Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing. J. Mater. Chem. C 2019, 7, 601–608. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Ali, M.C.; Munyemana, J.C.; Qiu, H. Cadmium cobaltite nanosheets synthesized in basic deep eutectic solvents with oxidase-like, peroxidase-like, and catalase-like activities and application in the colorimetric assay of glucose. Microchim. Acta 2020, 187, 314. [Google Scholar] [CrossRef]
- Kandanelli, R.; Thulluri, C.; Mangala, R.; Rao, P.V.C.; Gandham, S.; Velankar, H.R. A novel ternary combination of deep eutectic solvent-alcohol (DES-OL) system for synergistic and efficient delignification of biomass. Bioresour. Technol. 2018, 265, 573–576. [Google Scholar] [CrossRef]
- Jiang, J.; Carrillo-Enríquez, N.C.; Oguzlu, H.; Han, X.; Bi, R.; Song, M.; Saddler, J.N.; Sun, R.-C.; Jiang, F. High Production Yield and More Thermally Stable Lignin-Containing Cellulose Nanocrystals Isolated Using a Ternary Acidic Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2020, 8, 7182–7191. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Q.; Xing, L.; Li, H.; Leung, P.; Yang, W.; Su, H.; Xu, Q. Modeling the effect of temperature on performance of an iron-vanadium redox flow battery with deep eutectic solvent (DES) electrolyte. J. Power Sources 2020, 449, 227491. [Google Scholar] [CrossRef]
- Zhong, M.; Tang, Q.F.; Qiu, Z.G.; Wang, W.P.; Chen, X.Y.; Zhang, Z.J. A novel electrolyte of ternary deep eutectic solvent for wide temperature region supercapacitor with superior performance. J. Energy Storage 2020, 32, 101904. [Google Scholar] [CrossRef]
- Cruz, H.; Jordao, N.; Pinto, A.L.; Dionisio, M.; Neves, L.A.; Branco, L.C. Alkaline Iodide-Based Deep Eutectic Solvents for Electrochemical Applications. ACS Sustain. Chem. Eng. 2020, 8, 10653–10663. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Y.; Zhang, L.; Wang, X.; Zhao, Y.; Zhang, X.; Yu, G. A Sustainable Redox-Flow Battery with an Aluminum-Based, Deep-Eutectic-Solvent Anolyte. Angew. Chem. Int. Ed. 2017, 56, 7454–7459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Ding, Y.; Ramirez-Meyers, K.; Yu, G. A Low-Cost and High-Energy Hybrid Iron-Aluminum Liquid Battery Achieved by Deep Eutectic Solvents. Joule 2017, 1, 623–633. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Design and evaluation of polarity controlled and recyclable deep eutectic solvent based biphasic system for the polarity driven extraction and separation of compounds. J. Clean. Prod. 2020, 268, 122306. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Q.; Su, H.; Qiao, F.; Leung, P.; Shah, A.; Xu, Q. Redox characteristics of iron ions in different deep eutectic solvents. Ionics 2020, 26, 483–492. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, L.; Wei, H.; Li, Y.; Yu, G. Insights into Hydrotropic Solubilization for Hybrid Ion Redox Flow Batteries. ACS Energy Lett. 2018, 3, 2641–2648. [Google Scholar] [CrossRef]
- Boisset, A.; Menne, S.; Jacquemin, J.; Balducci, A.; Anouti, M. Deep eutectic solvents based on N-methylacetamide and a lithium salt as suitable electrolytes for lithium-ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 20054–20063. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, T.S.; Xu, Q.; An, L.; Zhao, G. Effects of operating temperature on the performance of vanadium redox flow batteries. Appl. Energy 2015, 155, 349–353. [Google Scholar] [CrossRef]
- Shen, J.; Liu, S.; He, Z.; Shi, L. Influence of antimony ions in negative electrolyte on the electrochemical performance of vanadium redox flow batteries. Electrochim. Acta 2015, 151, 297–305. [Google Scholar] [CrossRef]
- Liu, H.; Chaudhary, D.; Yusa, S.-i.; Tadé, M.O. Glycerol/starch/Na+-montmorillonite nanocomposites: A XRD, FTIR, DSC and 1H NMR study. Carbohydr. Polym. 2011, 83, 1591–1597. [Google Scholar] [CrossRef]





| Technology | Power Density (Wkg−1/kWm−3) | Energy Density (Wkg−1/kWhm−3) | Lifetime (Cycle) | Drawbacks | |
|---|---|---|---|---|---|
| Physical energy storage | /0.1–5000 | /0.2–80 | >5000 | site specific, high cost | |
| Supercapacitors | 0.1–100/40,000–120,000 | 0.1–15/10–12 | 5 × 105 | cryogenics, high cost | |
| Lithium-ion batteries | /1300–10,000 | /250–625 | 4 × 103 | safety, high cost | |
| Redox flow batteries | Zn-Br2 | 50–150/1–25 | 60–80/20–35 | >2000 | low energy density |
| Vanadium | /0.5–2 | /20–35 | 13 × 103 | ||
| n choline chloride:n ethylene glycol:n glycerol | Dre/cm2·s−1 |
|---|---|
| 1:2:0 | 5.54 × 10−7 |
| 1:2:0.1 | 7.36 × 10−7 |
| 1:2:0.5 | 8.29 × 10−7 |
| 1:2:1 | 3. 90 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Sun, P.; Ma, Q.; Su, H.; Leung, P.; Yang, W.; Xu, Q. Rationally Designed Ternary Deep Eutectic Solvent Enabling Higher Performance for Non-Aqueous Redox Flow Batteries. Processes 2022, 10, 649. https://doi.org/10.3390/pr10040649
Lu P, Sun P, Ma Q, Su H, Leung P, Yang W, Xu Q. Rationally Designed Ternary Deep Eutectic Solvent Enabling Higher Performance for Non-Aqueous Redox Flow Batteries. Processes. 2022; 10(4):649. https://doi.org/10.3390/pr10040649
Chicago/Turabian StyleLu, Ping, Peizhuo Sun, Qiang Ma, Huaneng Su, Puiki Leung, Weiwei Yang, and Qian Xu. 2022. "Rationally Designed Ternary Deep Eutectic Solvent Enabling Higher Performance for Non-Aqueous Redox Flow Batteries" Processes 10, no. 4: 649. https://doi.org/10.3390/pr10040649






