The Age-Related Macular Degeneration (AMD)-Preventing Mechanism of Natural Products
Abstract
:1. Introduction
1.1. Definition and Classification of Age-Related Macular Degeneration (AMD)
1.2. Statistics of AMD
1.3. Problems of Current Treatments
2. Pathogenesis of Age-Related Macular Degeneration (AMD)
2.1. The Basic Structure of the Eye
2.2. The Pathogenic Factors of AMD
2.3. The Relation of Age-Related Macular Degeneration (AMD) and Retinal Pigment Epithelial Cell Death (Apoptosis)
3. Three Stresses and the Mechanism of Natural Products to Prevent Retinal Pigment Epithelial Cell Death (Apoptosis)
3.1. Anti-Oxidative Stress and Natural Products
3.1.1. Nuclear Factor Erythroid-Derived-2-like (Nrf2)/Heme Oxygenase-1 (HO-1)-Antioxidant Responsive Element (ARE) System
3.1.2. Other Anti-Oxidative Stress Pathways
3.2. Anti-Inflammatory Effects of Natural Products
3.2.1. Inflammasome and Inflammation
3.2.2. Other Anti-Inflammatory Pathways
3.3. Anti-Carbonyl Stress Effects and Natural Products
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Blindness and Vision Impairment. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 13 March 2022).
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.; Jonas, J.B. Causes of blindness and vision impairment in 2020 and trends over 30 years: Evaluating the prevalence of avoidable blindness in relation to VISION 2020; the right to sight: An analysis for the global burden of disease study. Lancet Glob. Health 2021, 9, E144–E160. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cherry, J.; Lotery, A.J. Age-related macular degeneration. Adv. Exp. Med. Biol. 2012, 724, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, R.; Meuer, S.M.; Myers, C.E.; Buitendijk, G.H.S.; Rochtchina, E.; Choudhury, F.; Klein, B.E.K. Harmonizing the classification of age-related macular degeneration in the three continent AMD consortium. Ophthalmic Epidemiol. 2014, 21, 14–23. [Google Scholar] [CrossRef]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the epidemiology of age-related macular degeneration. Asia-Pac. J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- World Population Ageing 2019. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fwww.un.org%2Fen%2Fdevelopment%2Fdesa%2Fpopulation%2Fpublications%2Fpdf%2Fageing%2FWorldPopulationAgeing2019-Highlights.pdf&clen=5752163&chunk=true (accessed on 6 February 2022).
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systemic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.K. The prevalence of age-related eye diseases and visual impairment in aging: Current estimates. Investig. Opthalmology Vis. Sci. 2013, 54, ORSF5–ORSF13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.K.; Park, D.H.; Jeon, I.C. Medication trends for age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 11837. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A.; Reichal, E. Transpupillary thermotherapy for age-related macular degeneration: Long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg. Lasers 2000, 31, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Bresnick, G.H. Diabetic maculopathy. Acritical review highlighting diffuse macular edema. Ophthalmology 1983, 90, 1301–1317. [Google Scholar] [CrossRef]
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of antio-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, J.R.; Vollmer-Snarr, H.R.; Zhou, J.; Jang, Y.P.; Jockusch, S.; Itagaki, Y.; Nakanishi, K. A2E-epoxides damage DNA in retinal pigment epithelial cells. J. Bio. Chem. 2003, 278, 18207–18213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, W.T.; Fuffolo, J.J.; Mueller, H.A.; Clarke, A.M.; Moon, M.E. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Investig. Ophthalmol. Vis. Sci. 1978, 17, 1029–1035. [Google Scholar]
- Eldred, G.E. Lipofuscin fluorophore inhibits lysosomal protein degradation and may cause early stages of macular degeneration. Gerontology 1995, 41, 15–28. [Google Scholar] [CrossRef]
- Crough, R.K.; Koutalos, Y.; Kono, M.; Schey, K.; Ablonczy, Z. A2E and lipofuscin. Prog. Mol. Biol. Transl. Sci. 2015, 134, 449–463. [Google Scholar] [CrossRef]
- Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic. Biol. Med. 1996, 21, 871–888. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yanase, E.; Feng, X.; Siegel, M.M.; Sparrow, J.R. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7275–7280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppinen, A.; Niskanen, H.; Suuronen, T.; Kinnunen, K.; Salminene, A.; Kaarniranta, K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells-implications for age-related macular degeneration (AMD). Immunol. Lett. 2012, 147, 29–33. [Google Scholar] [CrossRef]
- Newsome, D.A.; Dobard, E.P.; Liles, M.R.; Oliver, P.D. Human retinal pigment epithelium contains two distinct species of superoxide dismutase. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2508–2513. [Google Scholar]
- Imamura, Y.; Noda, S.; Hashizume, K.; Shinoda, K.; Yamaguchi, M.; Uchiyama, S.; Shimizu, T.; Mizushima, Y.; Shirasawa, T.; Tsubota, K. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: A model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 11282–11287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justilien, V.; Pang, J.J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. SOD2 knockdwon mouse model of early AMD. Investig. Opthalmology Vis. Sci. 2007, 48, 4407–4420. [Google Scholar] [CrossRef] [Green Version]
- Tate, D.J., Jr.; Miceli, M.V.; Newsome, D.A. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Investig. Opthalmology Vis. Sci. 1995, 36, 1271–1279. [Google Scholar]
- Futterman, S.; Andrews, J.S. The fatty acid composition of human retinal vitamin A ester and the lipids of human retinal tissue. Investig. Ophthalmol. 1964, 3, 441–444. [Google Scholar]
- Khandhadia, S.; Lotery, A. Oxidation and age-related macular degeneration: Insights from molecular biology. Expert Rev. Mol. Med. 2010, 12, e34. [Google Scholar] [CrossRef]
- Evereklioglu, C.; Er, H.; Doganay, S.; Cekmen, M.; Turkoz, Y.; Otlu, B.; Ozerol, E. Nitric oxide and lipid peroxidation are increased and associated with decreased antioxidant enzyme activities in patients with age-related macular degeneration. Doc. Ophthalmol. 2003, 106, 129–136. [Google Scholar] [CrossRef]
- Totan, Y.; Cekic, O.; Borazan, M.; Uz, E.; Sogut, S.; Akyol, O. Plasma malondialdehyde and nitric oxide levels in age-related macular degeneration. Br. J. Ophthalmol. 2001, 85, 1426–1428. [Google Scholar] [CrossRef] [Green Version]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [Green Version]
- Bazan, N.G. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: Significance in retinal degenerations. Adv. Exp. Med. Biol. 2006, 572, 531–540. [Google Scholar] [CrossRef]
- Anderson, O.A.; Finkelstein, A.; Shima, D.T. A2E induces IL-1β production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS ONE 2013, 8, e67263. [Google Scholar] [CrossRef] [Green Version]
- Augustin, A.J.; Kirchhof, J. Inflammation and the pathogenesis of age-related macular degeneration. Expert Opin. Ther. Targets 2009, 13, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, S.S.; Li, Y.C.; Bae, C.S.; Park, K.M.; Park, D.H. Anti-inflammatory effect of Curcuma longa and Allium hookeri co-treatment via NF-κB and COX-2 pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Collin, T. Acute and Chronic Inflammation in Robbins Pathological Basis of Disease; Cotran, R.S., Kumar, V., Collins, T., Eds.; Saunders WB: Philadelphia, PA, USA, 1999; pp. 50–88. [Google Scholar]
- Zhang, J.M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musolino, C.; Allegra, A.; Innao, V.; Allegra, A.G.; Pioggia, G.; Gangemi, S. Inflammatory and anti-inflammatory equilibrium, proliferative and antiproliferative balance: The role of cytokines in multiple myeloma. Mediat. Inflamm. 2017, 2017, 1852517. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, T.P.; Brinkmann-Frye, E.; Thorpe, S.R.; Baynes, J.W. Role of carbonyl stress in aging and age-related disease. In The Maillard Reaction in Foods and Medicine; O’Brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2005; pp. 3–10. [Google Scholar]
- Liu, Y.; Zhang, D.; Hu, J.; Liu, G.; Chen, J.; Sun, L.; Jiang, Z.; Zhang, X.; Chen, Q.; Ji, B. Visible light-induced lipid peroxidation of unsaturated fatty acids in the retina and the inhibitory effects of blueberry polyphenols. J. Agric. Food Chem. 2015, 63, 9295–9305. [Google Scholar] [CrossRef] [PubMed]
- Long, E.K.; Picklo Sr., M.J. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: Make some room HNE. Free Radic. Biol. Med. 2010, 49, 1–8. [Google Scholar] [CrossRef]
- Lederman, M.; Hagbi-Levi, S.; Grunin, M.; Obolensky, A.; Berenshtein, E.; Bannin, E.; Chevion, M.; Chowers, I. Degeneration modulates retinal response to transient exogenous oxidative injury. PLoS ONE 2014, 9, e87751. [Google Scholar] [CrossRef]
- Zor, R.K.; Ersan, S.; Kucuk, E.; Yildirim, G.; Sari, I. Serum malondialdehyde, monocyte chemoattractant protein-1, and vitamin C levels in wet type age-related macular degeneration patients. Ther. Adv. Ophthalmol. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Lambros, M.L.; Plafker, S.M. Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap 1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1-Nrf2 system in cancer: Stress response and anabolic metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, T.; Lipton, S. Recent advances in understanding Nrf2 as a druggable target: Development of pro-electrophilic and non-covalent Nrf2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Research 2017, 6, 2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.D.F.; Proccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas, C., Jr.; Tarozzi, A. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules 2018, 23, 1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zou, Y.; Xu, H.; Fan, L.; Guo, H.; Li, X.; Li, G.; Zhang, X.; Dong, M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012, 1482, 13–21. [Google Scholar] [CrossRef]
- Cao, X.; Xiao, H.; Zhang, Y.; Zou, L.; Chu, Y.; Chu, X. 1,5-dicaffeoylquinic acid-mediated glutathione synthesis through activation of Nrf2 protects against 0GD/reperfusion-induced oxidative stress in astrocytees. Brain Res. 2010, 1347, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Son, T.G.; Camandola, S.; Arumugam, T.V.; Cutler, R.G.; Telljohann, R.S.; Mughal, M.R.; Moore, T.A.; Luo, W.; Yu, Q.S.; Johnson, D.A.; et al. Plumbagin, a novel Nrf2/ARE activator, protects against cerebra ischemia. J. Neurochem. 2010, 112, 1316–1326. [Google Scholar] [CrossRef] [Green Version]
- Guan, S.P.; Tee, W.; Ng, D.S.; Chan, T.K.; Peh, H.Y.; Ho, W.E.; Cheng, C.; Mak, J.C.; Wong, W.S.F. Andrographolide protects against cigarette smoke-induce doxidative lung injury via augmentation of Nrf2 activity. Br. J. Pharmacol. 2013, 168, 1707–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.W.; Chun, S.W.; Kim, S.H.; Lee, Y.; Kang, E.S.; Cha, B.S.; Lee, H.C. Lithospermic acid B protects β-cells from cytokine-induced apoptosis by alleviating apoptotic pathways and activating anti-apoptotic pathways of Nrf2-HO-1 and Sirt1. Toxicol. Appl. Pharmacol. 2011, 252, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Huang, T.; Chang, Y.Y.; Hsu, Y.W.; Pan, T.M. Effects of monascin on anti-inflammation mediated by Nrf2 activation in advanced glycation end product-treated THP-1 monocytes and methylglyoxal-treated wistart rats. J. Agric. Food Chem. 2013, 61, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, W.; Li, G.; Yuan, S.; Xu, D.; Hoi, M.P.M.; Lin, Z.; Dou, J.; Han, Y.; Lee, S.M.Y. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCα and PI3K/AKT signaling pathways. J. Agric. Food Chem. 2012, 60, 8171–8182. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lim, J.; Bang, Y.; Gal, J.; Lee, S.U.; Cho, Y.C.; Yoon, G.; Kang, B.Y.; Cheon, S.H.; Choi, H.J. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: Therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 2012, 23, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Dore, S. The flavanol (1)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. Cereb. Blood Flow Metab. 2020, 30, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Li, Z.; Wang, W.; Zhang, W.; Liu, S.; Zhang, X.; Fang, J.; Maeda, H.; Matsukura, M. Protective effect of canolol from oxidative stress-induced cell damage in ARPE-19 cells via an ERK mediated antioxidative pathway. Mol. Vis. 2011, 17, 2040–2048. [Google Scholar] [PubMed]
- Zhao, H.; Wang, R.; Ye, M.; Zhang, L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. Int. J. Mol. Med. 2019, 43, 936–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Dong, Y.; Liu, H.; Ren, H.; Cui, Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap-1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed. Pharmacother. 2016, 88, 124–133. [Google Scholar] [CrossRef]
- Chen, W.; Ye, Y.; Wu, Z.; Lin, J.; Wang, Y.; Ding, Q.; Yang, X.; Yang, W.; Lin, B.; Lin, B. Temporary upregulation of Nrf2 by naringenin alleviates oxidative damage in the retina and ARPE-19 cells. Oxidative Med. Cell. Longev. 2021, 2021, 4053276. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [Green Version]
- Mayne, S.T. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J. Nutr. 2003, 133, 933S–940S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Choi, Y.R.; Shim, J.; Choi, Y.S.; Kim, Y.T.; Kim, M.K.; Kim, M.J. Suppressive effect of Arctium lappa L. leaves on retinal damage against A2E-induced ARPE-19 cells and mice. Molecules 2020, 25, 1737. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Ni, T.; Yang, W.; Xing, Y. Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. BioSci. Rep. 2019, 39, BSR20190689. [Google Scholar] [CrossRef] [Green Version]
- Aung, K.H.; Liu, H.; Ke, Z.; Jiang, S.; Huang, J. Glabridin attenuates the retinal degeneration induced by sodium lodate in vitro and in vivo. Front. Pharmacol. 2020, 11, 566699. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signaling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Winsor, N.; Krustev, C.; Bruce, J.; Philpott, D.J.; Girardin, S.E. Canonical and noncanonical inflammasomes in intestinal epithelial cells. Cell. Microbiol. 2019, 21, e13079. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zheng, W.; Rock, K.L. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 2000, 97, 14590–14595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Rock, K.L. Cell death releases endogenous adjuvants that selectively enhance immune surveillance of particulate antigens. Eur. J. Immunol. 2002, 32, 155–162. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2020, 32, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.J.; Jin, X.M.; Xu, J.; Xiao, Q. Baicalin alleviates age-related macular degeneration via miR-223/NLRP3-regulated pyroptosis. Pharmacology 2019, 105, 28–38. [Google Scholar] [CrossRef]
- Yang, M.; So, K.F.; Lo, A.C.Y.; Lam, W.C. The effect of Lycium barbarum polysaccharides on pyroptosis-associated amyloid β1-40 oligomers-induced adult retinal pigment epithelium 19 cell damage. Int. J. Mol. Sci. 2020, 21, 4658. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, X.; Zhang, K.; Yao, Y.; Zhuang, M.; Tan, C.; Zhou, F.; Zhu, L. Puerarin inhibits amyloid β-induced NLRP3 inflammasome activation in retinal pigment epithelial cells via suppressing ROS-dependent oxidative and endoplasmic reticulum stresses. Exp. Cell Res. 2017, 357, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhu, W.; Wu, Y.; Long, Y.; Liu, H.; Wan, W.; Wan, G.; Yu, J. Grape seed proanthocyanidin extract moderated retinal pigment epithelium cellular senescence through NAMPT/SIRTI/NLRP3 pathway. J. Inflamm. Res. 2021, 14, 3129–3143. [Google Scholar] [CrossRef]
- Jin, X.; Wang, C.; Wu, W.; Liu, T.; Ji, B.; Zhou, F. Cyanidin-3-glucoside alleviates 4-hydroxyhexenal-induced NLRP3 inflammasome activation via JNK-c-Jun/AP-1 pathway in human retinal pigment epithelial cells. J. Immunol. Res. 2018, 2018, 5604610. [Google Scholar] [CrossRef] [Green Version]
- Franzone, F.; Nebbioso, M.; Pergolizzi, T.; Attanasio, G.; Musacchio, A.; Greco, A.; Limoli, P.G.; Artico, M.; Spandidos, D.A.; Taurone, S.; et al. Anti-inflammatory role of curcumin in retinal disorders (Review). Exp. Ther. Med. 2021, 22, 790. [Google Scholar] [CrossRef]
- Militao, G.C.; Albuquerque, M.R.; Pessoa, O.D.; Pessoa, C.; Moraes, M.E.; de Moraes, M.O.; Costa-Lotufo, L.V. Cytotoxic activity of nepetin, a flavonoid from Eupatorium ballotaefolium HBK. Die Pharm. 2004, 59, 965–966. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and phenolic compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef]
- Thitilertdecha, P.; Rowan, M.G.; Guy, R.H. Topical formulation and dermal delivery of active phenolic compounds in the Thai medicinal plant-Clerodendrum petasites S. Moore. Int. J. Pharm. 2015, 478, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Han, R.; Hao, P.; Wang, L.; Liu, M.; Jin, M.; Kong, D.; Li, X. Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. Biomed. Pharmacother. 2018, 101, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.A.; Chen, C.S.; Wang, Y.C.; Lin, E.S.; Chang, C.Y.; Chen, J.J.Y.; Wu, M.Y.; Lin, H.J.; Wan, L. Anti-inflammatory effects of resveratrol on human retinal pigment cells and a myopia animal model. Curr. Issues Mol. Biol. 2021, 43, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Heitmar, R.; Brwon, J.; Kyrou, I. Saffron (Crocus sativus L.) in ocular diseases: A narrative review of the existing evidence from clinical studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Albarral, J.A.; Martinez-Lopez, M.A.; Marco, E.M.; de Hoz, R.; Martin-Sanchez, B.; Felipe, D.S.; Salobrar-Garcia, E.; Lopez-Cuenca, I.; Pinazo-Duran, M.D.; Salazar, J.J.; et al. Is saffron able to prevent the dysregulation of retinal cytokines induced by ocular hypertension in mice? J. Clin. Med. 2021, 10, 4801. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, D.; Lu, G. Wogonin protects human retinal pigment epithelium cells from LPS-induced barrier dysfunction and inflammatory responses by regulating the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2017, 15, 2289–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, G.; Zhang, Y.F.; Wei, W.; Li, L.; Zhang, Y.; Yang, J.; Xing, Y. Allicin attenuates H2O2-induced cytotoxicity in retinal pigmented epithelial cells by regulating the levels of reactive oxygen species. Mol. Med. Rep. 2016, 13, 2320–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.C.; Nandrot, E.F.; Dun, Y.; Finnemann, S.C. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking ανβ5 integrin. Free Radic. Biol. Med. 2012, 52, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Marti, R.; Rosello, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Chichili, G.R.; Nohr, D.; Frank, J.; Flaccus, A.; Fraser, P.D.; Enfissi, E.M.A.; Biesalski, H.K. Protective effects of tomato extract with elevated β-carotene levels on oxidative stress in ARPE-19 cells. Br. J. Nutr. 2006, 96, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Takayama, K.; Kaneko, H.; Ye, F.; Fukukita, H.; Tsunekawa, T.; Kataoka, K.; Hwang, S.J.; Nagasaka, Y.; Ito, Y.; et al. Nutritional supplementation inhibits the increase in serum malondialdehyde in patients with wet age-related macular degeneration. Oxidative Med. Cell. Longev. 2017, 2017, 9548767. [Google Scholar] [CrossRef]
- Khurana, R.N.; Hoang, C.; Khanani, A.M.; Steklov, N.; Singerman, L.J. A smart mobile application to monitor viual function in diabetic retinopathy and age-related macular degeneration: The clear study. Am. J. Ophthalmol. 2021, 227, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Oeverhaus, M.; Hirche, H.; Esser, J.; Eckstein, A.; Schaperdoth-Gerlings, B. Evaluation of the medical treatment situation of the visually impaired: Significant differences between young and old. Ophthalmologe 2019, 116, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bressler, S.B. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmologe 2009, 116, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Keunen, J.E.; Bird, A.C.; vanKuijk, F.J. Fruits and vegetables that are sources for lutein and zeaxanthin: The macular pigment in human eyes. Br. J. Ophthalmol. 1998, 82, 907–910. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analysis of the effects of lutein/zeaxanthin on age-related macular degeneration progression. JAMA Ophthalmol 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Tan, J.S.L.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration. The blue mountains eye study. Ophthalmologe 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, W.; Zhou, X.; Long, C.; Kuang, X.; Hu, J.; Tang, Y.; Liu, L.; He, J.; Huang, Z.; et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol. Med. Rep. 2017, 16, 2069–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawkat, A.M. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 2004, 39, 581–590. [Google Scholar] [CrossRef]
- Warden, C.; Barnett, J.M.; Brantley, M.A., Jr. Taurocholic acid inhibits features of age-related macular degeneration in vitro. Exp. Eye Res. 2020, 193, 107974. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Kang, J.H.; Kim, H.M.; Jeong, S.H.; Jang, D.S.; Jang, Y.P.; Choung, S.Y. Polyphenol-enriched Vaccinium uliginosum L. fractions reduce retinal damage induced by blue light in A2E-laden ARPE19 cell cultures and mice. Nutr. Res. 2016, 36, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- De Guimaraes, T.A.C.; Varela, M.D.; Georgiou, M.; Michaelides, M. Treatments for dry age-related macular degeneration: Therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, R.D.; Wang, S.; Klimanskaya, I.; Holmes, T.; Ramos-Kelsey, R.; Lu, B.; Girman, S.; Bischoff, N.; Sauve, Y.; Lanza, R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 2006, 8, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef]
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Morizur, L.; Herardot, E.; Monville, C.; M’Barek, K.B. Human pluripotent stem cells: A toolbox to understand and treat retinal degeneration. Mol. Cell. Neurosci. 2020, 107, 103523. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Absenger, Y.; Klein, H.; Addicks, K.; Schraermeyer, U. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 414–422. [Google Scholar] [CrossRef]
- Mu, Y.; Zhao, M.; Su, G. Stem cell-based therapies for age-related macular degeneration: Current status and prospects. Int. J. Clin. Exp. Med. 2014, 7, 3843–3852. [Google Scholar] [PubMed]
- Idelson, M.; Alper, R.; Obolensky, A.; Yachimovich-Cohen, N.; Rachmilewitz, J.; Ejzenberg, A.; Beider, E.; Banin, E.; Reubinoff, B. Immunological properties of human embryonic stem cell-derived retinal pigment epithelial cells. Stem Cell Rep. 2018, 11, 681–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, H.C.; Limnios, I.J.; Barnett, N.L. Advancing a stem cell therapy for age-related macular degeneration. Curr. Stem Cell Res Ther. 2020, 15, 89–97. [Google Scholar] [CrossRef]
 : carbohydrate.
: carbohydrate.
 : carbohydrate.
: carbohydrate.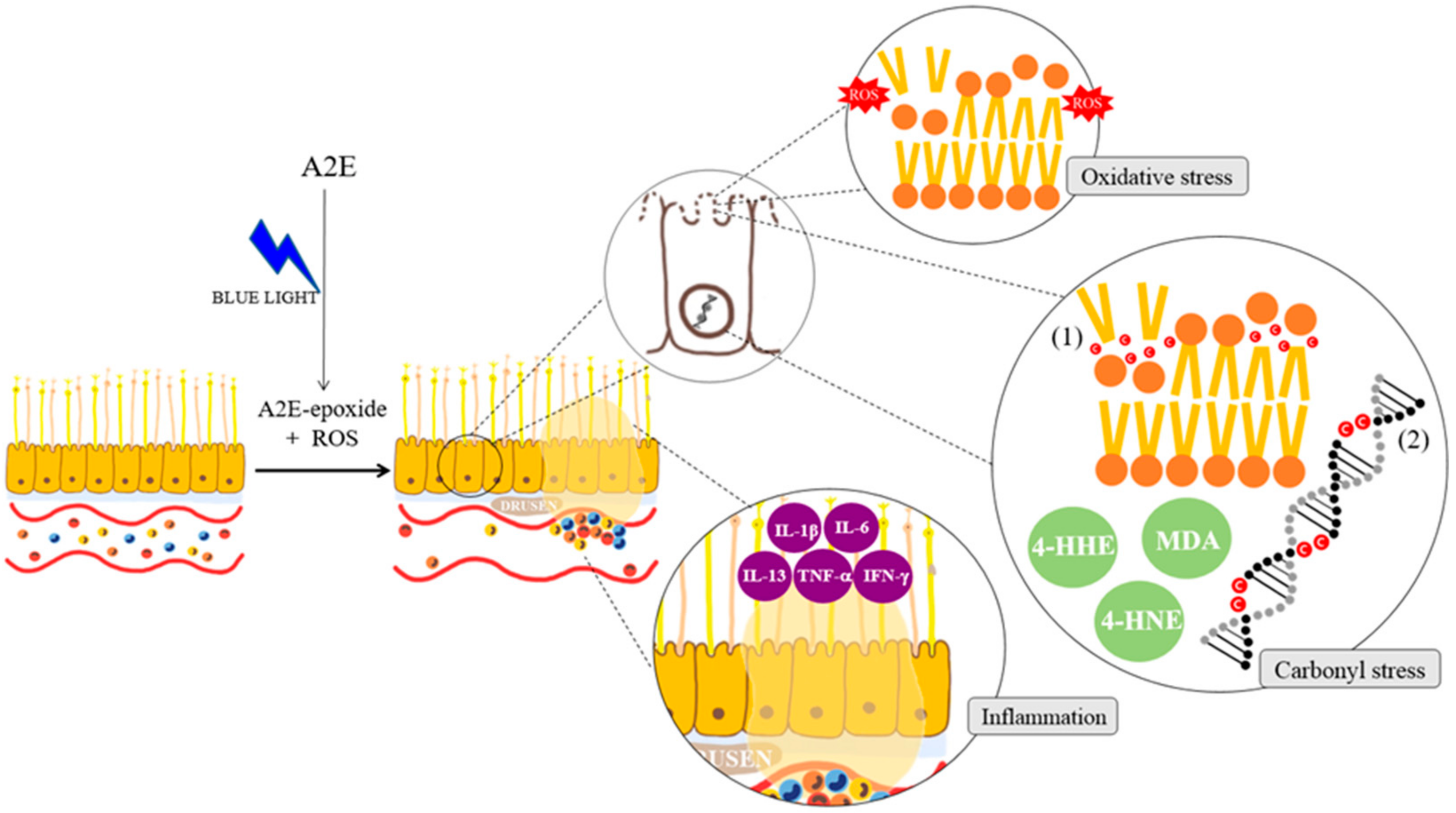


| Treatment | Principles of Treatment | Adverse Effects | References |
|---|---|---|---|
| Device-based treatment | (1) To eliminate the drusen (2) To inhibit the choroidal vascularization | Macular edema, retinovascular disease | [12] [13] |
| Anti-inflammatory drug treatment | (1) To block the inflammatory pathway (2) To inactivate cyclo-oxygenase or/and lipoxygenase | Hypertension, insulin resistance, insomnia, skin thinning, gastric ulceration | [14] |
| Anti-vascular endothelial growth factor (anti-VEGF) treatment | (1) To prohibit VEGF activation (2) To bind to the VEGF receptor (3) To block the tyrosine kinase pathway | Bleeding and infection risk by intravitreal injection | [15] |
| Category/Name | Clinical Trial ID (NCT #) | Study Phase | Route of Delivery | Status | Sponsor |
|---|---|---|---|---|---|
| Antioxidative | |||||
| Age-related eye disease study (AREDS) | 00000145 | III | Oral | Completed | National Eye Institute |
| AREDS2 | 00345176 | III | Oral | Completed | National Eye Institute |
| OT-551 | 00306488 | II | Topical | Completed | National Institutes of Health Clinical Center |
| Reduction in toxic byproducts | |||||
| GSK933776 | 01342926 | II | IV | Completed | GlaxoSmithKline |
| RN6G | 01577381 | II | IV | Terminated | Pfizer |
| Visual cycle modulators | |||||
| ACU-4429 | 01802866 | IIb/III | Oral | Completed | Kutoba Vision, Inc. |
| Fenretinide | 00429936 | II | Oral | Completed | Sirion Therapeutics, Inc. |
| C20-D3-vitamin A (ALK-001) | 03845582 | III | Oral | Ongoing/recruiting | Aleus Pharmaceuticals, Inc. |
| Anti-inflammatory and complement inhibition | |||||
| Eculizumab | 00935883 | II | IV | Completed | Philip J. Rosenfeld, MD |
| Lampalizumab | 02247531 | III | Intravitreal | Terminated | Hoffman-La Roche |
| Sirolimus (rapamycin) | 00766649 | I/II | Subconjunctival | Completed | National Eye Institute |
| Avacincaptad pegol (Zimura) | 02686658 | II/III | Intravitreal | Completed | IVERIC bio, Inc. |
| Pegcetacoplan (APL-2) | 03525613 | III | Intravitreal | Completed/not recruiting | Apellis Pharmaceuticals, Inc. |
| Tedisolumab (LFG316) | 01527500 | II | Intravitreal | Completed | Novartis Pharmaceuticals, Inc. |
| Risuteganib | 03626636 | I | Intravitreal | Completed | Allegro Ophthalmics |
| Neuroprotection | |||||
| Ciliary nerve trophic factor | 00447954 | II | Intravitreal | Completed | National Eye Institute |
| Brimonidine tartrate | 02087085 | IIB | Intravitreal | Terminated | Allergan |
| Gene therapy | |||||
| AAVCAGsCD59 | 03144999 | I | Intravitreal | Completed | Hemera Biosciences |
| GT005 | 03846193 | I/II | Subretinal | Ongoing/recruiting | Gyroscope Therapeutics |
| Cell-based therapies | |||||
| Palucorcel (CNTO-2476) | 01226628 | I/II | Subretinal | Completed | Janssen Research & Development, LLC |
| MA09-hRPE | 01344993 | I/II | Subretinal | Completed | Astelas Institute for Regenerative Medicine |
| CPCB-RPE1 | 02590692 | I/IIa | Subretinal | Ongoing/not recruiting | Regenerative Patch Technologies |
| Mitochondrial enhancers | |||||
| Elamipretide | 03891875 | II | Subcutaneous | Ongoing/recruiting | Stealth Biotechnologies, Inc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-K.; Lee, S.-M.; Kang, Y.-J.; Kang, Y.-M.; Jeon, I.-C.; Park, D.-H. The Age-Related Macular Degeneration (AMD)-Preventing Mechanism of Natural Products. Processes 2022, 10, 678. https://doi.org/10.3390/pr10040678
Cho Y-K, Lee S-M, Kang Y-J, Kang Y-M, Jeon I-C, Park D-H. The Age-Related Macular Degeneration (AMD)-Preventing Mechanism of Natural Products. Processes. 2022; 10(4):678. https://doi.org/10.3390/pr10040678
Chicago/Turabian StyleCho, Yeon-Kyoung, Seung-Min Lee, Yeong-Ji Kang, Yeong-Mo Kang, In-Chul Jeon, and Dae-Hun Park. 2022. "The Age-Related Macular Degeneration (AMD)-Preventing Mechanism of Natural Products" Processes 10, no. 4: 678. https://doi.org/10.3390/pr10040678






