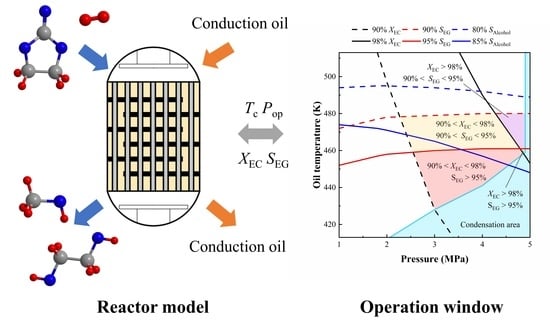
Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors
Abstract
Share and Cite
Huang, H.; Cao, C.; Wang, Y.; Yang, Y.; Lv, J.; Xu, J. Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors. Processes 2022, 10, 688. https://doi.org/10.3390/pr10040688
Huang H, Cao C, Wang Y, Yang Y, Lv J, Xu J. Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors. Processes. 2022; 10(4):688. https://doi.org/10.3390/pr10040688
Chicago/Turabian StyleHuang, Hai, Chenxi Cao, Yue Wang, Youwei Yang, Jianning Lv, and Jing Xu. 2022. "Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors" Processes 10, no. 4: 688. https://doi.org/10.3390/pr10040688
APA StyleHuang, H., Cao, C., Wang, Y., Yang, Y., Lv, J., & Xu, J. (2022). Model-Based Analysis for Ethylene Carbonate Hydrogenation Operation in Industrial-Type Tubular Reactors. Processes, 10(4), 688. https://doi.org/10.3390/pr10040688