Transfer of Potentially Toxic Elements in the Soil-Plant System in Magnesite Mining and Processing Areas
Abstract
:1. Introduction
2. Materials and Methods
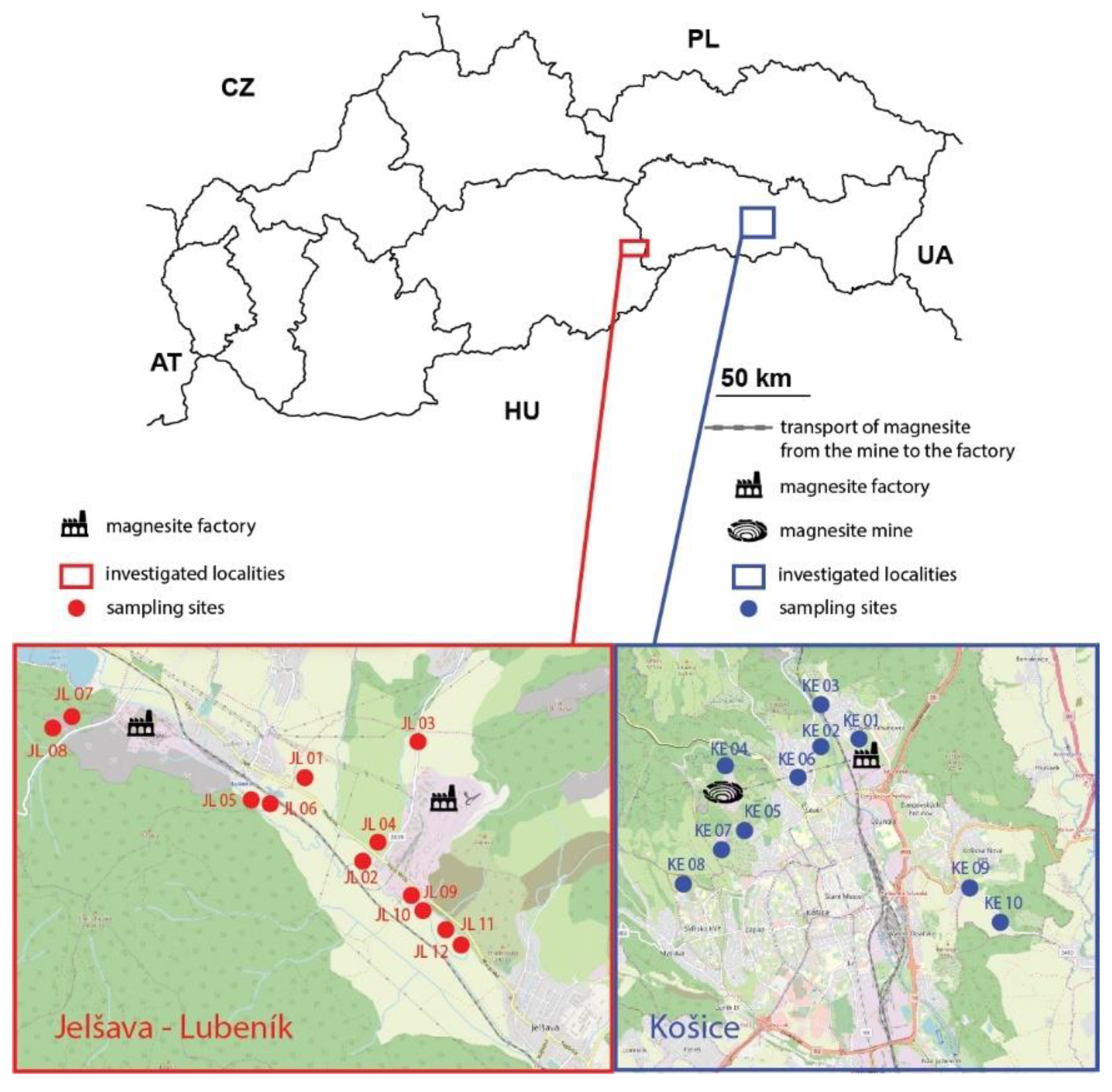
2.1. Study Area
2.2. Sampling Procedure
2.3. Analytical Methods
3. Results and Discussion
3.1. Soil Reaction, Soil Organic Matter, and Total Content of Potentially Toxic Elements in Soil
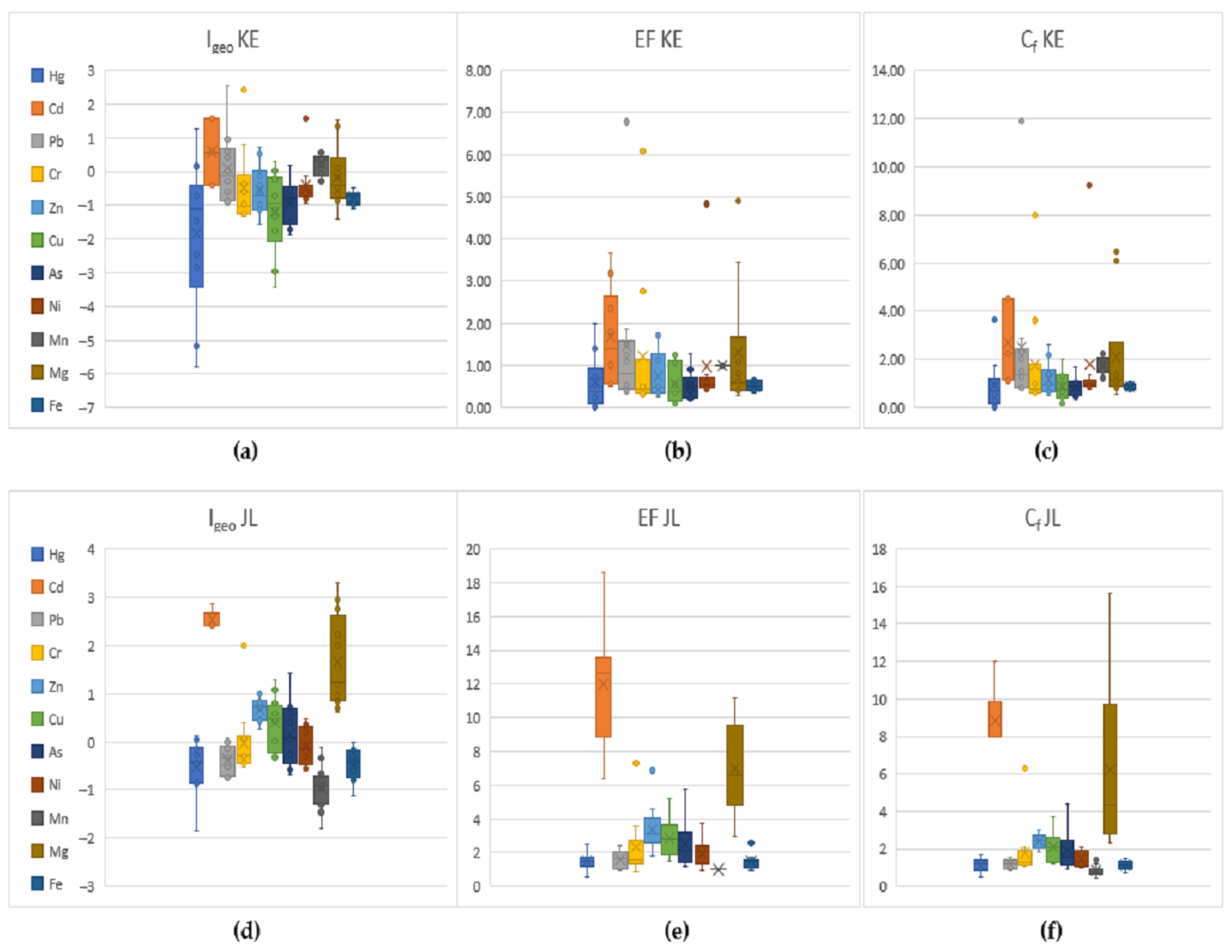
3.2. Assessment of Soil Pollution
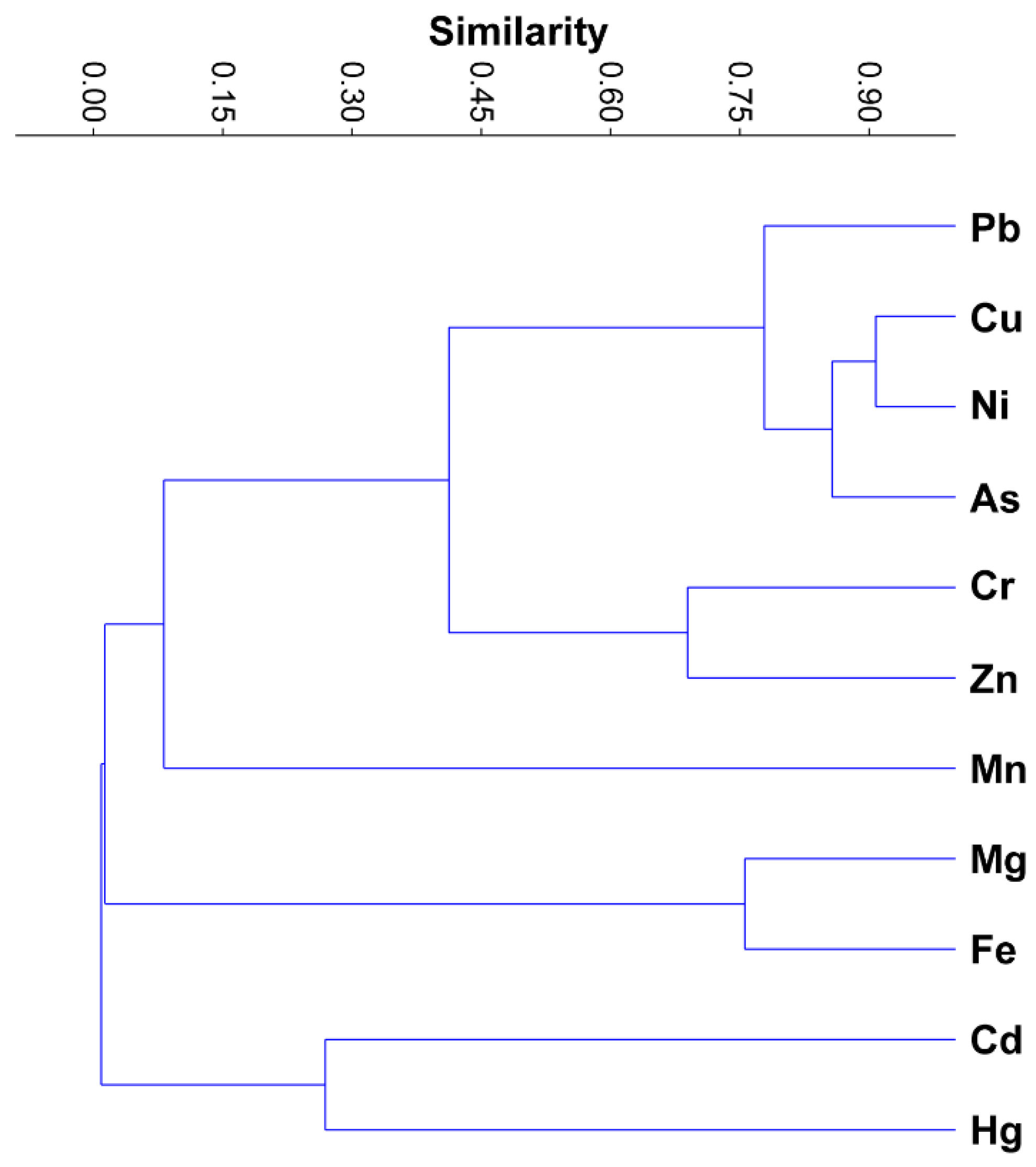
3.3. Correlation and Hierarchical Cluster Analyses
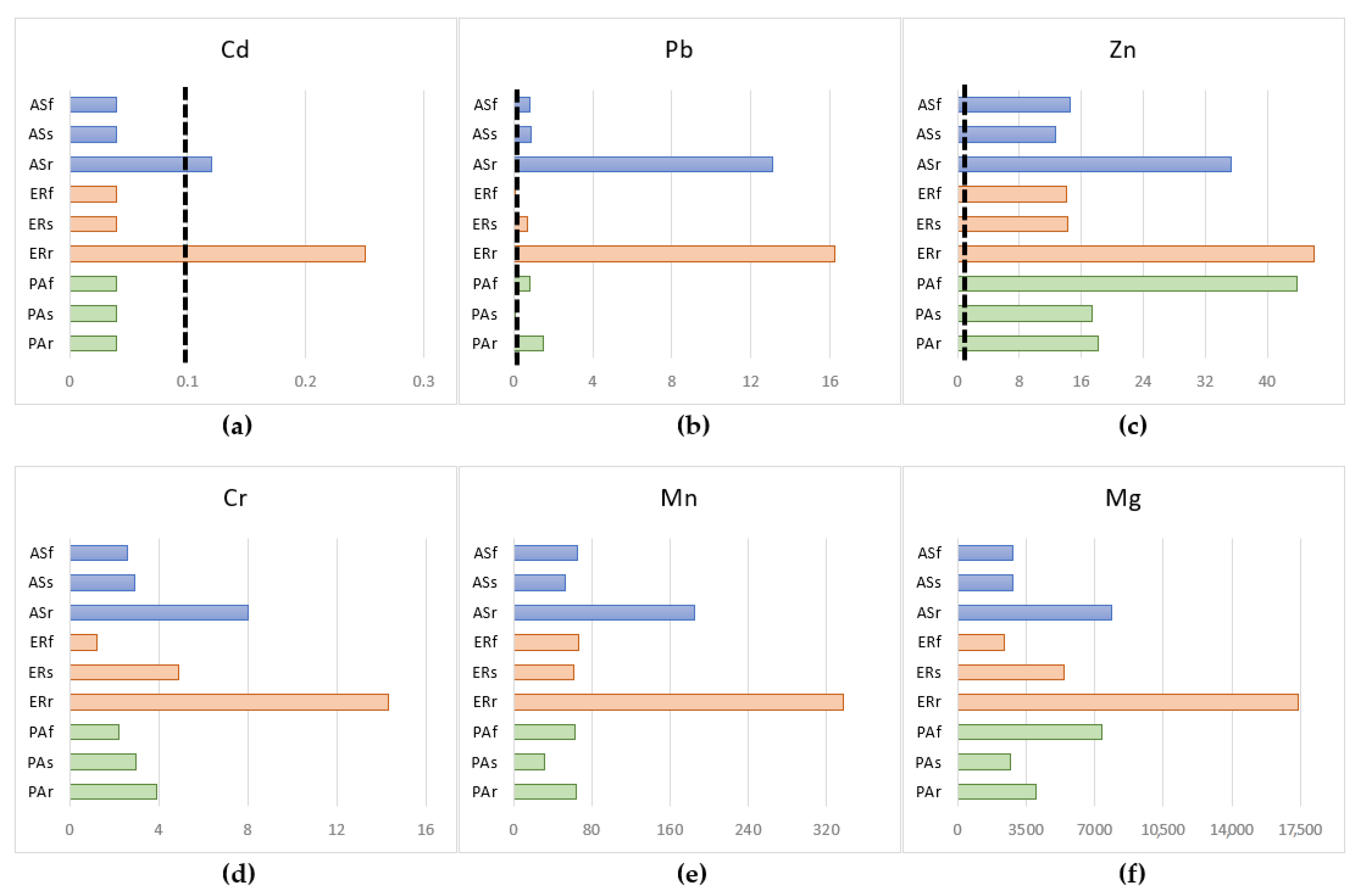
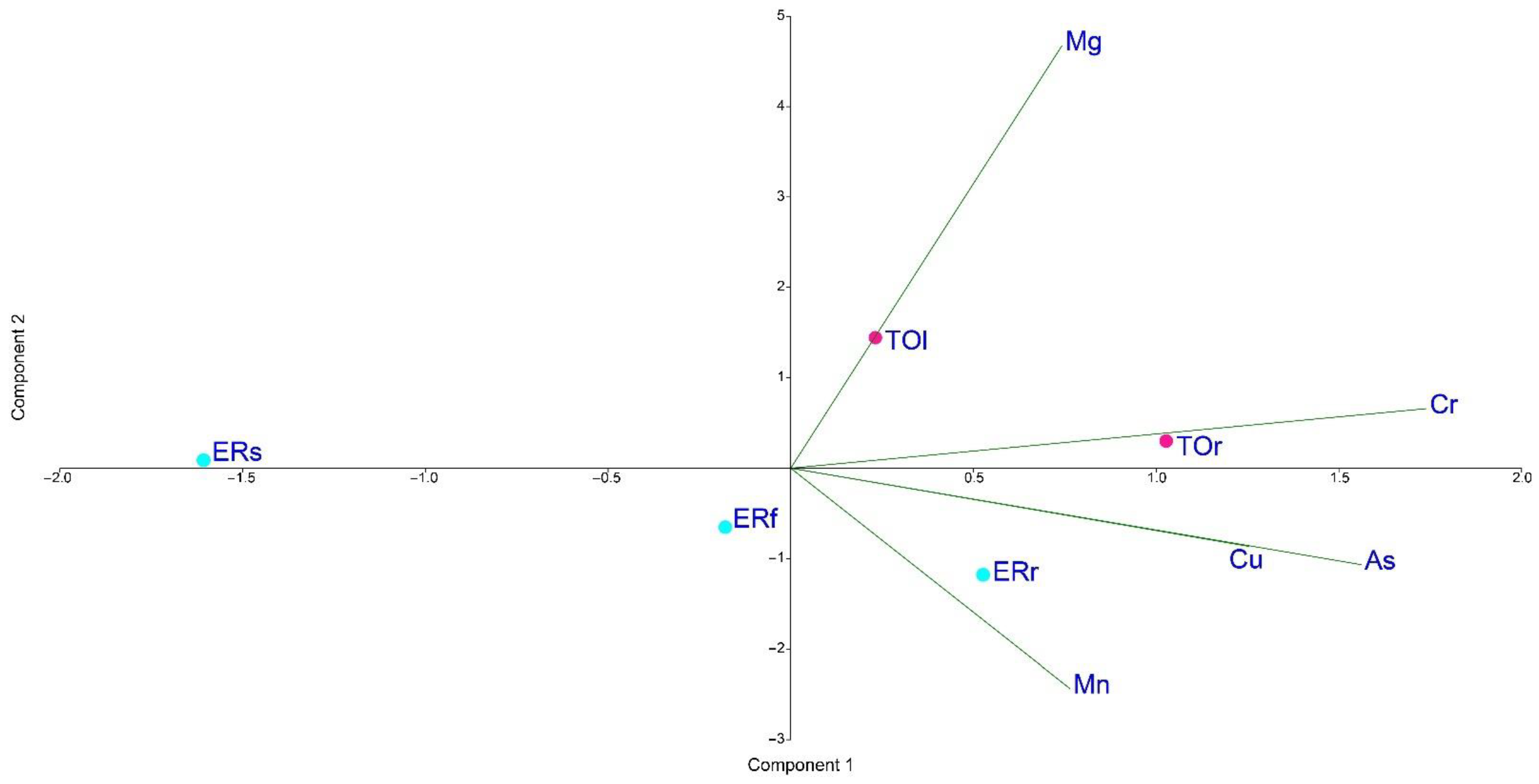
3.4. Total Content of Potentially Toxic Elements in Plants
4. Conclusions
- –
- The soil reaction in the studied areas indicates a strong alkalization of the soil;
- –
- Soils in the Jelšava-Lubeník area are significantly contaminated with Cr, As, Mn, and Mg. Based on the average Igeo values, the content of potentially toxic elements increases in the order of Mn < Hg < Fe < Pb < Ni < Cr < As < Cu < Zn < Mg < Cd. The most significant enrichment based on the average values of EF was found in the order of Cd > Mg > Zn > Cu > As > Cr > Ni > Pb > Fe > Hg > Mn. The observed Cf and Cd values indicate a high degree of soil contamination, mainly for Cd and Cr;
- –
- In the territory of Košice, soils are significantly contaminated with Cr, Mn, Mg, and Ni. Based on the average values of Igeo, the content of potentially toxic elements increases in the order of Hg < Cr < Fe < Cu < As < Mg < Zn < Pb < Ni < Cd < Mn. The most significant enrichment was found in the order of Cd > Mn > Ni > Pb > Zn > Mg > Cu > As > Fe > Cr > Hg. Very high Cf was found for Pb and Cr;
- –
- The results of correlation and hierarchical cluster analysis showed that high correlation coefficients and groups of associations between potentially toxic elements indicate a similar origin of pollution sources, which is caused by significant anthropogenic interventions due to magnesite mining and processing near industrial areas;
- –
- The examined dominant plant species in environmentally polluted areas can accumulate high concentrations of monitored potentially toxic elements without more serious burden or damage. The results of the bioconcentration factor (BCF) and translocation factor (TF) confirmed that P. australis and T. officinale appear to be suitable accumulators in the phytoextraction process, as they can accumulate potentially toxic elements in the roots and at the same time transport them to the above-ground parts, especially Cd, Cr, and Mg. In the case of E. repens and A. stolonifera, it was confirmed that these species accumulate and immobilize high concentrations of potentially toxic elements, especially in the roots, which confirms the suitability of their use in phytostabilization processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.C.; Zhang, T.; Stoffella, P.J. Heavy metal contamination of soils: Sources, indicators, and assessment. J. Environ. Indust. 2015, 9, 17–18. [Google Scholar]
- Xu, J.; Liu, C.; Hsu, P.C.; Zhao, J.; Wu, T.; Tang, J.; Liu, K.; Cui, Y. Remediation of heavy metal contaminated soil by asymmetrical alternating current electrochemistry. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef]
- Pelica, J.; Barbosa, S.; Reboredo, F.; Lidon, F.; Pessoa, F.; Calvão, T. The paradigm of high concentration of metals of natural or anthropogenic origin in soils—The case of Neves-Corvo mine area (Southern Portugal). J. Geochem. Explor. 2018, 186, 12–23. [Google Scholar] [CrossRef]
- Kronbauer, M.A.; Izquierdo, M.; Dai, S.; Waanders, F.B.; Wagner, N.J.; Mastalerz, M.; Hower, J.C.; Oliveira, M.L.S.; Taffarel, S.R.; Bizani, D.; et al. Geochemistry of ultra-fine and nano-compounds in coal gasification ashes: A synoptic view. Sci. Total Environ. 2012, 456, 95–103. [Google Scholar] [CrossRef]
- Yenilmez, F.; Kuter, N.; Emil, M.K.; Aksoy, A. Evaluation of pollution levels at an abandoned coal mine site in Turkey with the aid of GIS. Int. J. Coal Geol. 2011, 86, 12–19. [Google Scholar] [CrossRef]
- Bai, Z.K.; Fu, M.C.; Zhao, Z.Q. Soil environmental problems in mining area. Ecol. Environ. 2006, 15, 1122–1125. [Google Scholar]
- Liu, X.; Bai, Z.; Zhou, W.; Cao, Y.; Zhang, G. Changes in soil properties in the soil profile after mining and reclamation in an opencast coal mine on the Loess Plateau, China. Ecol. Eng. Times. 2017, 98, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Loupasakis, C.; Angelitsa, V.; Rozos, D.; Spanou, N. Mining geohazards-land subsidence caused by the dewatering of opencast coal mines: The case study of the Amyntaio coal mine, Florina, Greece. Nat. Hazards. 2014, 70, 675–691. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, W.; Yang, Q. The impacts of mining exploitation on the environment in the Changchun-Jilin-Tumen economic area, Northeast China. Nat. Hazards. 2015, 76, 1019–1038. [Google Scholar] [CrossRef]
- Navrátil, T.; Minařík, L. Trace Elements and Contaminants; Eolss Publishers: Oxford, UK, 2002. [Google Scholar]
- Bhaskar, C.V.; Kumar, K.; Nagendrappa, G. Assessment of heavy metals in water samples of certain locations situated around Tumkur, Karnataka, India. E-J. Chem. 2010, 7, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Keshavarzi, A.; Kumar, V. Spatial distribution and potential ecological risk assessment of heavy metals in agricultural soils of Northeastern Iran. Geol. Ecol. Landsc. 2020, 4, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Panuccio, M.R.; Sorgonà, A.; Rizzo, M.; Cacco, G. Cadmium adsorption on vermiculite, zeolite and pumice: Batch experimental studies. J. Environ. Manag. 2009, 90, 364–374. [Google Scholar] [CrossRef]
- Guala, S.D.; Vega, F.A.; Covelo, E.F. The dynamics of heavy metals in plant–soil interactions. Ecol. Modell. 2010, 221, 1148–1152. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Fasani, E.; Manara, A.; Martini, F.; Furini, A.; Dalcorso, G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018, 41, 1201–1232. [Google Scholar] [CrossRef]
- Naila, A.; Meerdink, G.; Jayasena, V.; Sulaiman, A.Z.; Ajit, A.B.; Berta, G. A review on global metal accumulators-mechanism, enhancement, commercial application, and research trend. Environ. Sci. Pollut. Res. 2019, 26, 26449–26471. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Badida, M.; Ladomerský, J.; Králiková, R.; Sobotová, L.; Bartko, L. Základy Environmentalistiky/Basics of Environmental Studies; Elfa s.r.o.: Košice, Slovakia, 2013. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Dalcorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheoran, V.; Sheoran, A.; Poonia, P. Role of hyperaccumulators in phytoextraction of metals from contaminated mining sites: A review. Crit. Rev. Env. Sci. Technol. 2011, 41, 168–214. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecol. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Inno. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Aken, B.V.; Correa, P.A.; Schnoor, J.L. Phytoremediation of polychlorinated biphenyls: New trends and promises. Environ. Sci. Technol. 2009, 44, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Garbisu, C.; Alkorta, I. Basic concepts on heavy metal soil bioremediation. European J. Miner. Process. Environ. Protect. 2003, 3, 58–66. [Google Scholar]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.D. Hyperaccumulation of trace elements by plants. In Phytoremediation of Metal-Contaminated Soils; Morel, J.L., Echevarria, G., Goncharova, N., Eds.; Springer: New York, NY, USA, 2006; pp. 1–25. [Google Scholar]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ent, A.; Baker, A.J.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil. 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Harrier, L.A.; Watson, C.A. The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest. Manag. Sci. 2004, 60, 149–157. [Google Scholar] [CrossRef]
- Janoušková, M.; Pavlíková, D.; Vosátka, M. Potential contribution of arbuscular mycorrhiza to cadmium immobilisation in soil. Chemosphere 2006, 65, 1959–1965. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kenelley, E.D. A fern that hyperaccumulates arsenic—A hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Schmidt, U. Enhancing phytoextraction: The effect of chemical soil manipulation on mobility, plant accumulation and leaching of heavy metals. J. Environ. Qual. 2003, 32, 1939–1954. [Google Scholar] [CrossRef] [Green Version]
- Scragg, A. Environmental Biotechnology; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Boguská, Z.; Fazekašová, D.; Angelovičová, L. Diversity of vegetation on contaminated substrates. In Proceedings of the 17th International Conference on Environment and Mineral Processing, VŠB TU, Ostrava, Czech Republic, 6–8 June 2013. [Google Scholar]
- Mazúr, E.; Lukniš, M. Regionálne Geomorfologické členenie Slovenska/Regional Division SSR; Geografický Ústav SAV: Bratislava, Slovakia, 1980. [Google Scholar]
- Klinda, J.; Mičík, T.; Némethová, M.; Slámková, M. Environmental Regionalisation of the Slovak Republic 2016; Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2016. [Google Scholar]
- Lapin, M.; Faško, P.; Melo, M.; Št’astný, P.; Tomlain, J. Climate Areas. Atlas of the Slovak Republic; Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2002. [Google Scholar]
- Significant Historical Milestones. Available online: http://www.slovmag.sk/ (accessed on 20 January 2022).
- It Used to be the Dirtiest City in Slovakia. IKEA was Supposed to Stand here Instead of Magnesite, but only a Black Hole Remained. Available online: https://www.startitup.sk/kedysi-to-bolo-najspinavejsie-mesto-na-slovensku-namiesto-magnezitky-tu-mala-stat-ikea-ostala-vsak-len-cierna-diera/ (accessed on 20 January 2022).
- One Hundred Years Ago, Glory, now Horror Ruins. The Magnesite Factory was Deserted, Ikea and other Plans Fell Through. Available online: https://kosice.korzar.sme.sk/c/22638309/pred-sto-rokmi-slava-teraz-hororove-ruiny-magnezitka-pustne-ikea-aj-ine-plany-padli.html (accessed on 20 January 2022).
- Slovak Technical Standard ISO 10381-6; Soil Quality—Sampling—Part 6: Guidance on the Collection, Handling and Storage of Soil Under Aerobic Conditions for the Assessment of Microbiological Processes, Biomass and Diversity in the Laboratory. Available online: https://www.iso.org/standard/43691.html (accessed on 20 November 2021).
- Ministry of Agriculture of the Slovak Republic. Ministry of Agriculture Decree No. 338/2005; Ministry of Agriculture of the Slovak Republic: Bratislava, Slovakia, 2006. [Google Scholar]
- Kobza, J.; Barančíková, G.; Čumová, L.; Dodok, R.; Hrivňaková, K.; Makovníková, J. Methods of Determining Indicators of Agrochemical Soil Properties; SSCRI: Bratislava, Slovakia, 2011. [Google Scholar]
- Act of the National Council of the Slovak Republic No. 220/2004 Coll. Available online: https://www.mpsr.sk/zakon-c-220-2004-z-z/27-23-27-8366/ (accessed on 20 November 2021).
- Müller, G. The heavy metal pollution of the sediments of Neckars and its tributary: A stocktaking. Chem. Zeit. 1981, 105, 157–164. [Google Scholar]
- Čurlík, J.; Šefčík, P. Geochemický Atlas Slovenskej Republiky/Geochemical Atlas of the Slovak Republic; SSCRI: Bratislava, Slovakia, 1999. [Google Scholar]
- Buat-Menard, P.; Chesselet, R. Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci. Lett. 1979, 42, 399–411. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and Excluders Strategies in Response of Plants to Heavy Metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization Potential of Jatropha Curcas L. in Polymetallic Acid Mine Tailings. Int. J. Phytoremediation 2011, 13, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Błońska, E.; Lasota, J.; Gruba, P. Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecol. Res. 2016, 31, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Gąsiorek, M.; Kowalska, J.; Mazurek, R.; Paja˛k, M. Comprehensive assessment of heavy metal pollution in topsoil of historical urban park on an example of the Planty Park in Krakow (Poland). Chemosphere 2017, 179, 148–158. [Google Scholar] [CrossRef]
- Weber, J.; Dradrach, A.; Karczewska, A.; Kocowicz, A. The distribution of sequentially extracted Cu, Pb, and Zn fractions in Podzol profiles under dwarf pine of different stages of degradation in subalpine zone of Karkonosze Mts (central Europe). J. Soils Sediments 2018, 18, 2387–2398. [Google Scholar] [CrossRef]
- Fuller, W.H. Movement of Selected Metals, Asbestos, and Cyanide in Soil: Applications to Waste Disposal Problems; EPA-600/2-77-020; U.S. Environmental Protection Agency, Office of Research and Development, Municipal Environmental Research Laboratory, Solid and Hazardous Waste Research Division: Cincinnati, OH, USA, 1977.
- Yang, D.; Zeng, D.H.; Zhang, J.; Li, L.J.; Mao, R. Chemical and microbial properties in contaminated soils around a magnesite mine in northeast China. Land Degrad. Dev. 2012, 23, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Štofejová, L.; Fazekaš, J.; Fazekašová, D. Analysis of heavy metal content in soil and plants in the dumping ground of Magnesite Mining Factory Jelšava-Lubeník (Slovakia). Sustainability 2021, 13, 4508. [Google Scholar] [CrossRef]
- Fazekaš, J.; Fazekašová, D.; Hronec, O.; Benková, E.; Boltižiar, M. Contamination of soil and vegetation at a magnesite mining area in Jelšava-Lubeník (Slovakia). Ekológia 2018, 37, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Hronec, O.; Adamišin, P. Management of Areas Revitalization with Innovative Processes. In Proceedings of the International Scientific Conference Sustainability-Environment-Safety, Bratislava, Slovakia, 27 November 2014. [Google Scholar]
- Tobiášová, E.; Barančíková, G.; Gömöryová, E. Pôdna Organická Hmota/Soil Organic Matter; Slovak University of Agriculture: Nitra, Slovakia, 2016. [Google Scholar]
- Fazekašová, D.; Barančíková, G.; Torma, S.; Ivanová, M.; Manko, P. Chemické a Environmentálne Aspekty Zložiek Životného Prostredia a Krajiny/Chemical and Environmental Aspects of the Environment and Landscape; University of Prešov: Prešov, Slovakia, 2014. [Google Scholar]
- Fazekašová, D.; Fazekaš, J. Soil Quality and Heavy Metal Pollution Assessment of Iron Ore Mines in Nizna Slana (Slovakia). Sustainability 2020, 12, 2549. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef] [PubMed]
- Stofejova, L.; Fazekasova, D.; Fazekas, J. Soil contamination in the area of magnesite mining in urban and agrarian land. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Prague, Czech Republic, 25–26 November 2021; Volume 900, p. 012043. [Google Scholar]
- Wang, N.; Han, J.; Wei, Y.; Li, G.; Sun, Y. Potential Ecological Risk and Health Risk Assessment of Heavy Metals and Metalloid in Soil around Xunyang Mining Areas. Sustainability 2019, 11, 4828. [Google Scholar] [CrossRef] [Green Version]
- Dolezalova Weissmannová, H.; Mihočová, S.; Chovanec, P.; Pavlovský, J. Potential Ecological Risk and Human Health Risk Assessment of Heavy Metal Pollution in Industrial Affected Soils by Coal Mining and Metallurgy in Ostrava, Czech Republic. Int. J. Environ. Res. Public Health 2019, 16, 4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazekašová, D.; Petrovič, F.; Fazekaš, J.; Štofejová, L.; Baláž, I.; Tulis, F.; Tóth, T. Soil contamination in the problem areas of agrarian Slovakia. Land 2021, 10, 1248. [Google Scholar] [CrossRef]
- Ma, Y.; Egodawatta, P.; McGree, J.; Liu, A.; Goonetilleke, A. Human health risk assessment of heavy metals in urban stormwater. Sci. Total Environ. 2016, 557, 764–772. [Google Scholar] [CrossRef]
- Siedlecka, A.; Tukendorf, A.; Skórzyńska-Polit, E.; Maksymiec, W.; Wójcik, M.; Baszyński, T.; Krupa, Z. Angiosperms (Asteraceae, Convolvulaceae, Fabaceae and Poaceae; other than Brassicaceae). In Metals in the Environment. Analysis by Biodiversity; Prasad, M.N.V., Ed.; Marcel Dekket, Inc.: New York, NY, USA, 2001; pp. 171–217. [Google Scholar]
- Minkina, T.M.; Mandzhieva, S.S.; Chaplygin, V.A.; Bauer, T.V.; Burachevskaya, M.V.; Nevidomskaya, D.G.; Sushkova, S.N.; Sherstnev, A.K.; Zamulina, I.V. Content and distribution of heavy metals in herbaceous plants under the effect of industrial aerosol emissions. J. Geochem. Explor. 2017, 174, 113–120. [Google Scholar] [CrossRef]
- Barančíková, G.; Fazekašová, D. Environmentálna Chémia/Environmental Chemistry; University of Prešov: Prešov, Slovakia, 2014. [Google Scholar]
- Allen, S.E. Chemical Analysis of Ecological Material, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1989; p. 368. [Google Scholar]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements in Soils and Plants; Springer: Berlin, Germany, 2007. [Google Scholar]
- Zeleňáková, E.; Hajdúk, J.; Holub, Z. Obsah horčíka a iných prvkov v rastlinách (Agropyrum repens P. Beauv., Secale cereale L., Triticum aestivum L.) ovplyvnených magnezitovými exhalačnými splodinami/Content of magnesium and other elements in plants (Agropyrum repens P. Beauv., Secale cereale L., Triticum aestivum L.) affected by magnesite exhalation fumes. Biológia 1966, 21, 365–375. [Google Scholar]
- Hronec, O.; Hajdúk, J. Remarkable resistance of Phragmites australis (Cav.) Trin. growing on soils intoxicated by magnesium immissions. Ekologia 1998, 17, 117–124. [Google Scholar]






| Study Area | Parameter | Mean | Median | Minimum | Maximum | Standard Deviation | Limit Value * |
|---|---|---|---|---|---|---|---|
| Jelšava-Lubeník | Cr | 157.08 | 104.50 | 78.00 | 793.00 | 141.99 | 70.00 |
| As | 25.81 | 20.50 | 11.00 | 84.00 | 15.36 | 25.00 | |
| Mn | 1348.94 | 1307.50 | 400.00 | 2817.00 | 478.76 | 2.10–95.27 ** | |
| Mg | 30,411.94 | 18,605.00 | 8400.00 | 113,400.00 | 24,493.51 | 200.00–400.00 ** | |
| Hg | 0.07 | 0.06 | 0.01 | 0.14 | 0.03 | 0.50 | |
| Cd | 0.44 | 0.40 | 0.40 | 1.00 | 0.12 | 0.70 | |
| Pb | 29.61 | 30.50 | 16.00 | 45.00 | 6.77 | 70.00 | |
| Zn | 82.61 | 82.00 | 52.00 | 113.00 | 14.76 | 150.00 | |
| Cu | 27.64 | 26.50 | 13.00 | 64.00 | 11.41 | 60.00 | |
| Ni | 31.92 | 29.50 | 19.00 | 54.00 | 9.14 | 50.00 | |
| Fe (%) | 4.17 | 4.32 | 2.20 | 6.40 | 0.92 | 2.64 ** | |
| pH/KCl | 8.20 | 7.90 | 7.00 | 9.30 | 0.91 | - | |
| Eh (mV) | 147.00 | 133.00 | 38.00 | 230.00 | 52.40 | - | |
| SOM (%) | 3.69 | 3.33 | 1.27 | 6.28 | 1.66 | - | |
| Košice | Cr | 155.70 | 82.00 | 59.00 | 682.00 | 181.60 | 70.00 |
| As | 19.80 | 19.50 | 8.00 | 32.00 | 7.58 | 25.00 | |
| Mn | 994.85 | 947.00 | 512.00 | 1500.00 | 286.09 | 2.10–95.27 ** | |
| Mg | 16,828.35 | 9580.00 | 3855.00 | 90,300.00 | 23,827.98 | 200.00–400.00 ** | |
| Hg | 0.29 | 0.16 | 0.08 | 0.98 | 0.28 | 0.50 | |
| Cd | 0.45 | 0.45 | 0.40 | 0.50 | 0.05 | 0.70 | |
| Pb | 42.45 | 32.00 | 23.00 | 239.00 | 46.63 | 70.00 | |
| Zn | 91.45 | 89.50 | 51.00 | 219.00 | 38.03 | 150.00 | |
| Cu | 41.95 | 31.00 | 16.00 | 108.00 | 27.12 | 60.00 | |
| Ni | 56.80 | 31.00 | 14.00 | 573.00 | 121.65 | 50.00 | |
| Fe (%) | 2.89 | 2.91 | 1.50 | 5.04 | 0.75 | 2.64 ** | |
| pH/KCl | 6.90 | 7.40 | 5.30 | 8.10 | 0.95 | - | |
| Eh (mV) | 167.00 | 174.00 | −6.00 | 274.00 | 80.75 | - | |
| SOM (%) | 1.88 | 1.91 | 0.83 | 2.96 | 0.57 | - |
| Area | Hg | Cd | Pb | Cr | Zn | Cu | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | |
| JL 01 | −0.29 | 1.52 | 2.42 | 9.94 | −0.34 | 1.45 | −0.45 | 1.36 | 0.44 | 2.50 | −0.33 | 1.52 |
| JL 02 | −0.46 | 2.52 | 2.42 | 18.58 | −0.53 | 2.39 | −0.31 | 2.79 | 0.26 | 4.15 | −0.30 | 2.81 |
| JL 03 | −0.87 | 1.32 | 2.42 | 12.52 | −0.01 | 2.34 | −0.43 | 1.74 | 0.50 | 3.33 | 0.44 | 3.18 |
| JL 04 | 0.04 | 2.06 | 2.68 | 12.82 | 0.06 | 2.05 | −0.44 | 1.45 | 0.45 | 2.68 | 0.42 | 2.73 |
| JL 05 | −0.76 | 1.46 | 2.42 | 13.19 | −0.26 | 2.06 | 0.08 | 2.61 | 0.88 | 4.57 | −0.32 | 1.98 |
| JL 06 | −0.09 | 1.77 | 2.68 | 13.03 | −0.06 | 1.81 | 0.40 | 2.48 | 0.99 | 3.74 | 0.01 | 1.90 |
| JL 07 | −0.85 | 1.04 | 2.86 | 13.75 | −0.70 | 1.14 | 2.00 | 7.32 | 0.82 | 3.28 | 0.80 | 3.57 |
| JL 08 | −0.87 | 1.66 | 2.42 | 17.41 | −0.75 | 1.93 | 0.15 | 3.61 | 1.01 | 6.87 | 0.53 | 5.18 |
| JL 09 | −1.87 | 0.59 | 2.61 | 10.88 | −0.77 | 1.05 | −0.36 | 1.36 | 0.75 | 3.00 | 1.07 | 3.69 |
| JL 10 | −0.37 | 1.46 | 2.42 | 8.52 | −0.73 | 0.97 | −0.23 | 1.37 | 0.75 | 2.69 | 1.28 | 3.93 |
| JL 11 | −0.15 | 1.16 | 2.42 | 6.69 | −0.32 | 1.02 | −0.53 | 0.88 | 0.54 | 1.84 | 0.60 | 1.91 |
| JL 12 | 0.14 | 1.20 | 2.52 | 6.39 | −0.14 | 0.99 | −0.22 | 0.95 | 0.68 | 1.77 | 0.50 | 1.60 |
| Area | As | Ni | Mn | Mg | Fe | |||||||
| Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | |||
| JL 01 | −0.58 | 1.24 | −0.54 | 1.27 | −0.88 | 1 | 1.18 | 7.01 | −0.78 | 1.10 | ||
| JL 02 | −0.69 | 2.14 | −0.53 | 2.39 | −1.79 | 1 | 0.69 | 5.61 | −1.11 | 1.59 | ||
| JL 03 | −0.16 | 2.10 | −0.08 | 2.21 | −1.22 | 1 | 0.82 | 4.13 | −0.45 | 1.71 | ||
| JL 04 | 0.60 | 3.22 | −0.05 | 1.92 | −0.97 | 1 | 0.61 | 2.99 | −0.43 | 1.48 | ||
| JL 05 | −0.52 | 1.73 | −0.61 | 1.62 | −1.30 | 1 | 1.18 | 5.60 | −0.79 | 1.42 | ||
| JL 06 | −0.27 | 1.56 | −0.31 | 1.52 | −0.91 | 1 | 1.27 | 4.56 | −0.60 | 1.24 | ||
| JL 07 | 1.44 | 5.71 | 0.35 | 2.42 | −0.86 | 1 | 1.99 | 7.24 | −0.30 | 1.53 | ||
| JL 08 | 0.41 | 4.43 | 0.19 | 3.72 | −1.46 | 1 | 0.97 | 6.16 | −0.43 | 2.55 | ||
| JL 09 | 0.72 | 3.02 | 0.48 | 2.45 | −0.79 | 1 | 2.22 | 8.32 | −0.17 | 1.56 | ||
| JL 10 | 0.76 | 2.71 | 0.37 | 2.08 | −0.67 | 1 | 2.75 | 11.18 | −0.02 | 1.57 | ||
| JL 11 | 0.10 | 1.35 | −0.23 | 1.08 | −0.33 | 1 | 2.94 | 10.00 | −0.20 | 1.09 | ||
| JL 12 | 0.04 | 1.14 | −0.27 | 0.92 | −0.12 | 1 | 3.31 | 10.92 | −0.18 | 0.97 | ||
| Area | Hg | Cd | Pb | Cr | Zn | Cu | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | |
| 1KE | −5.79 | 0.01 | −0.42 | 0.57 | −0.97 | 0.39 | −1.19 | 0.33 | −1.58 | 0.25 | −3.41 | 0.07 |
| 2KE | −2.84 | 0.12 | −0.42 | 0.64 | −0.89 | 0.46 | −1.33 | 0.34 | −1.12 | 0.39 | −1.34 | 0.34 |
| 3KE | −2.45 | 0.12 | −0.42 | 0.50 | −0.87 | 0.37 | −1.25 | 0.28 | −0.97 | 0.34 | −1.76 | 0.19 |
| 4KE | −0.66 | 0.73 | 1.57 | 3.18 | 2.54 | 6.78 | 2.40 | 6.07 | 0.72 | 1.72 | 0.30 | 1.29 |
| 5KE | 0.15 | 1.41 | 1.57 | 3.67 | 0.59 | 1.86 | 0.80 | 2.77 | −0.12 | 1.13 | −0.22 | 1.06 |
| 6KE | −5.16 | 0.02 | −0.42 | 0.56 | −0.60 | 0.49 | −1.08 | 0.36 | −1.15 | 0.33 | −2.95 | 0.09 |
| 7KE | 1.26 | 2.00 | 1.57 | 2.45 | 0.41 | 1.09 | −0.60 | 0.53 | −0.60 | 0.54 | −0.58 | 0.54 |
| 8KE | −0.59 | 0.54 | 1.57 | 2.34 | 0.93 | 1.49 | −0.36 | 0.62 | −0.85 | 0.43 | −1.15 | 0.35 |
| 9KE | −0.70 | 0.75 | 0.57 | 1.81 | 0.03 | 1.24 | −1.24 | 0.51 | 0.53 | 1.77 | 0.02 | 1.24 |
| 10KE | −1.47 | 0.24 | 0.57 | 1.01 | −0.28 | 0.54 | −0.96 | 0.34 | −0.39 | 0.51 | −0.71 | 0.41 |
| Area | As | Ni | Mn | Mg | Fe | |||||||
| Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | Igeo | EF | |||
| 1KE | −1.86 | 0.22 | −0.63 | 0.50 | 0.37 | 1 | 0.09 | 0.82 | −0.64 | 0.49 | ||
| 2KE | −1.46 | 0.31 | −0.63 | 0.56 | 0.20 | 1 | −0.47 | 0.62 | −0.72 | 0.52 | ||
| 3KE | −1.50 | 0.23 | −0.52 | 0.47 | 0.56 | 1 | −0.31 | 0.54 | −0.47 | 0.48 | ||
| 4KE | 0.19 | 1.28 | 1.55 | 4.82 | −0.05 | 1 | 1.51 | 3.43 | −0.67 | 0.65 | ||
| 5KE | −0.53 | 0.89 | −0.71 | 0.77 | −0.30 | 1 | 1.33 | 4.89 | −0.95 | 0.65 | ||
| 6KE | −1.72 | 0.22 | −0.72 | 0.45 | 0.41 | 1 | −0.85 | 0.41 | −0.61 | 0.49 | ||
| 7KE | −0.45 | 0.60 | −0.65 | 0.52 | 0.28 | 1 | −0.78 | 0.47 | −1 | 0.41 | ||
| 8KE | −0.45 | 0.56 | −0.95 | 0.40 | 0.35 | 1 | −1.42 | 0.29 | −1.11 | 0.35 | ||
| 9KE | −0.87 | 0.66 | −0.80 | 0.69 | −0.28 | 1 | −0.17 | 1.09 | −0.84 | 0.67 | ||
| 10KE | −0.70 | 0.40 | −0.11 | 0.61 | 0.58 | 1 | −0.68 | 0.41 | −0.71 | 0.40 | ||
| Contamination Factor (Cf) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Very High | Considerable | Moderate | Low | |||||
| Locality | Cd | Cf ≥ 6 | 3 ≤ Cf < 6 | 1 ≤ Cf < 3 | Cf < 1 | |||
| Degree of contamination (Cd) | Very high | Cd ≥ 32 | ||||||
| Considerable | 16 ≤ Cd < 32 | JL 07 | 29.88 | Cd > Cr | As | Cu > Zn | Pb > Hg | |
| JL 04 | 20.72 | Cd | As > Cu > Zn > Pb > Hg > Cr | |||||
| JL 06 | 20.62 | Cd | Zn > Cr > Cu > Hg > Pb > As | |||||
| JL 10 | 20.44 | Cd | Cu | As > Zn >Hg > Cr | Pb | |||
| JL 09 | 20.07 | Cd | Cu | Zn > As > Cr | Pb > Hg | |||
| JL 08 | 19.26 | Cd | Zn | Cu > As > Cr | Pb > Hg | |||
| JL 12 | 19.15 | Cd | Zn > Cu > Hg > As > Cr > Pb | |||||
| JL 11 | 17.74 | Cd | Cu > Zn > As > Hg > Pb > Cr | |||||
| JL 03 | 16.97 | Cd | Zn > Cu > Pb > AS > Cr | Hg | ||||
| JL 05 | 16.78 | Cd | Zn > Cr > Pb > Cu > As | Hg | ||||
| Moderate | 8 ≤ Cd < 16 | JL 01 | 15.84 | Cd | Zn > Hg > Cu > Pb > Cr > As | |||
| JL 02 | 15.34 | Cd | Zn > Cu > Cr > Hg > Pb | As | ||||
| Low | Cd < 8 | |||||||
| Contamination Factor (Cf) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Very High | Considerable | Moderate | Low | |||||
| Locality | Cd | Cf ≥ 6 | 3 ≤ Cf < 6 | 1 ≤ Cf < 3 | Cf < 1 | |||
| Degree of contamination (Cd) | Very high | Cd ≥ 32 | ||||||
| Considerable | 16 ≤ Cd < 32 | KE 04 | 31.68 | Pb > Cr | Cd | Zn > Cu > As | Hg | |
| Moderate | 8 ≤ Cd < 16 | KE 05 | 15.97 | Cd > Cr | Pb > Hg > Zn > Cu > As | |||
| KE 07 | 14.20 | Cd > Hg | Pb > As > Cu | Cr > Zn | ||||
| KE 08 | 12.22 | Cd | Pb > Cr > As > Hg | Zn > Cu | ||||
| KE 09 | 9.90 | Cd > Zn > Pb > Cr | Hg > As > Cr | |||||
| Low | Cd < 8 | KE 10 | 7.79 | Cd > Pb > Zn | As > Cu > Cr > Hg | |||
| KE 03 | 4.58 | Cd | Pb > Zn > Cr > As > Cu > Hg | |||||
| KE 02 | 4.57 | Cd | Pb > Zn > Cr > As > Cu > Hg | |||||
| KE 06 | 4.21 | Cd | Pb > Cr > Zn > As > Cu > Hg | |||||
| KE 01 | 3.64 | Cd | Pb > Cr > Zn > As > Cu > Hg | |||||
| Parameter | As | Mn | Mg | Hg | Cd | Pb | Zn | Cu | Ni | Fe | pH | Eh | SOM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | 0.217 | −0.189 | 0.007 | −0.205 | 0.332 | −0.266 | 0.781 ** | 0.126 | 0.126 | 0.014 | −0.155 | 0.347 | −0.042 |
| As | 0.503 | 0.343 | −0.162 | 0.465 | −0.371 | 0.340 | 0.895 ** | 0.916 ** | 0.825 ** | 0.563 | −0.200 | 0.559 | |
| Mn | 0.902 ** | 0.349 | 0.262 | −0.126 | 0.028 | 0.625 * | 0.385 | 0.811 ** | 0.739 ** | −0.364 | 0.895 ** | ||
| Mg | 0.187 | 0.059 | −0.322 | 0.231 | 0.540 | 0.217 | 0.706 * | 0.627 * | −0.119 | 0.783 ** | |||
| Hg | 0.146 | 0.579 * | −0.223 | −0.188 | −0.335 | 0.039 | 0.291 | −0.592 * | 0.483 | ||||
| Cd | 0.164 | 0.186 | 0.233 | 0.336 | 0.227 | 0.232 | −0.337 | 0.387 | |||||
| Pb | −0.301 | −0.530 | −0.483 | −0.385 | −0.099 | −0.298 | −0.175 | ||||||
| Zn | 0.285 | 0.231 | 0.242 | −0.134 | 0.519 | 0.000 | |||||||
| Cu | 0.912 ** | 0.926 ** | 0.721 ** | −0.228 | 0.642 * | ||||||||
| Ni | 0.797 ** | 0.500 | −0.116 | 0.406 | |||||||||
| Fe | 0.768 ** | −0.231 | 0.769 ** | ||||||||||
| pH | −0.702 * | 0.838 ** | |||||||||||
| Eh | −0.609 * |
| Parameter | As | Mn | Mg | Hg | Cd | Pb | Zn | Cu | Ni | Fe | pH | Eh | SOM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | 0.417 | 0.249 | 0.621 ** | −0.357 | 0.347 | 0.329 | 0.393 | 0.350 | 0.530 * | 0.526 * | −0.142 | 0.648 * | −0.139 |
| As | −0.109 | 0.527 * | 0.192 | −0.252 | 0.118 | 0.462 * | 0.613 ** | 0.779 ** | 0.478 * | 0.028 | 0.207 | 0.335 | |
| Mn | −0.130 | −0.173 | 0.278 | 0.022 | −0.308 | −0.106 | 0.084 | 0.484 * | −0.288 | 0.315 | 0.012 | ||
| Mg | 0.041 | 0.225 | 0.211 | 0.721 ** | 0.591 ** | 0.602 ** | 0.415 | 0.634 * | 0.188 | −0.236 | |||
| Hg | −0.182 | −0.344 | 0.277 | 0.575** | 0.097 | 0.056 | 0.272 | −0.549 | 0.598 | ||||
| Cd | 0.150 | 0.165 | 0.096 | −0.043 | 0.104 | . | . | . | |||||
| Pb | 0.524 * | 0.073 | −0.035 | 0.018 | 0.012 | 0.286 | −0.012 | ||||||
| Zn | 0.745 ** | 0.439 | 0.345 | 0.431 | −0.055 | 0.236 | |||||||
| Cu | 0.574 ** | 0.488 * | 0.283 | −0.030 | 0.261 | ||||||||
| Ni | 0.699 ** | 0.160 | 0.224 | 0.285 | |||||||||
| Fe | −0.028 | 0.427 | 0.378 | ||||||||||
| pH | −0.234 | −0.258 | |||||||||||
| Eh | −0.382 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štofejová, L.; Fazekaš, J.; Fazekašová, D. Transfer of Potentially Toxic Elements in the Soil-Plant System in Magnesite Mining and Processing Areas. Processes 2022, 10, 720. https://doi.org/10.3390/pr10040720
Štofejová L, Fazekaš J, Fazekašová D. Transfer of Potentially Toxic Elements in the Soil-Plant System in Magnesite Mining and Processing Areas. Processes. 2022; 10(4):720. https://doi.org/10.3390/pr10040720
Chicago/Turabian StyleŠtofejová, Lenka, Juraj Fazekaš, and Danica Fazekašová. 2022. "Transfer of Potentially Toxic Elements in the Soil-Plant System in Magnesite Mining and Processing Areas" Processes 10, no. 4: 720. https://doi.org/10.3390/pr10040720
APA StyleŠtofejová, L., Fazekaš, J., & Fazekašová, D. (2022). Transfer of Potentially Toxic Elements in the Soil-Plant System in Magnesite Mining and Processing Areas. Processes, 10(4), 720. https://doi.org/10.3390/pr10040720






