Hydrometallurgical Recovery of Iron, Nickel, and Chromium from Stainless Steel Sludge with Emphasis on Solvent Extraction and Chemical Precipitation
Abstract
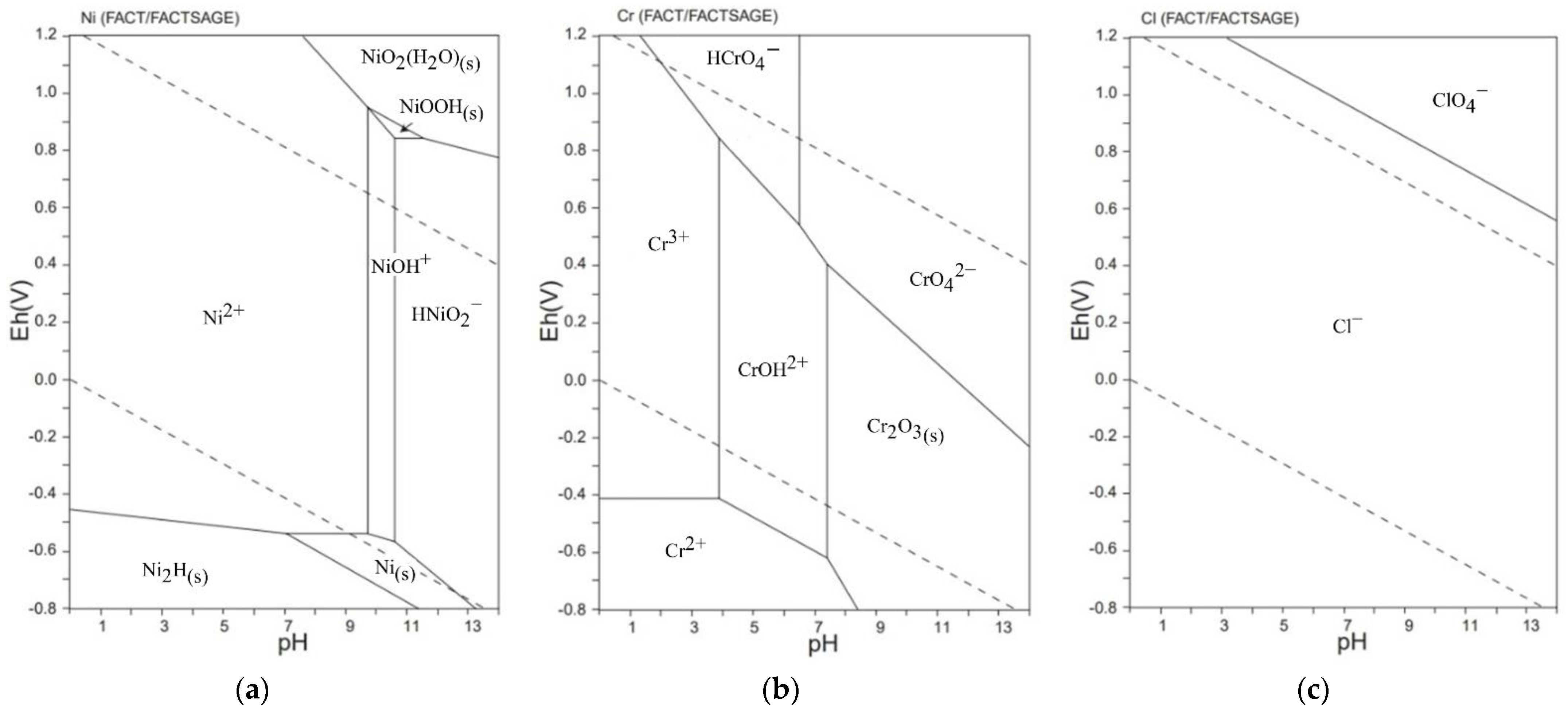
:1. Introduction
2. Materials and Methods
2.1. Materials, Reagents, and Instruments
2.2. Pre-Treatment
2.3. Acid Leaching
2.4. Solvent Extraction
2.5. Chemical Precipitation
3. Results and Discussion
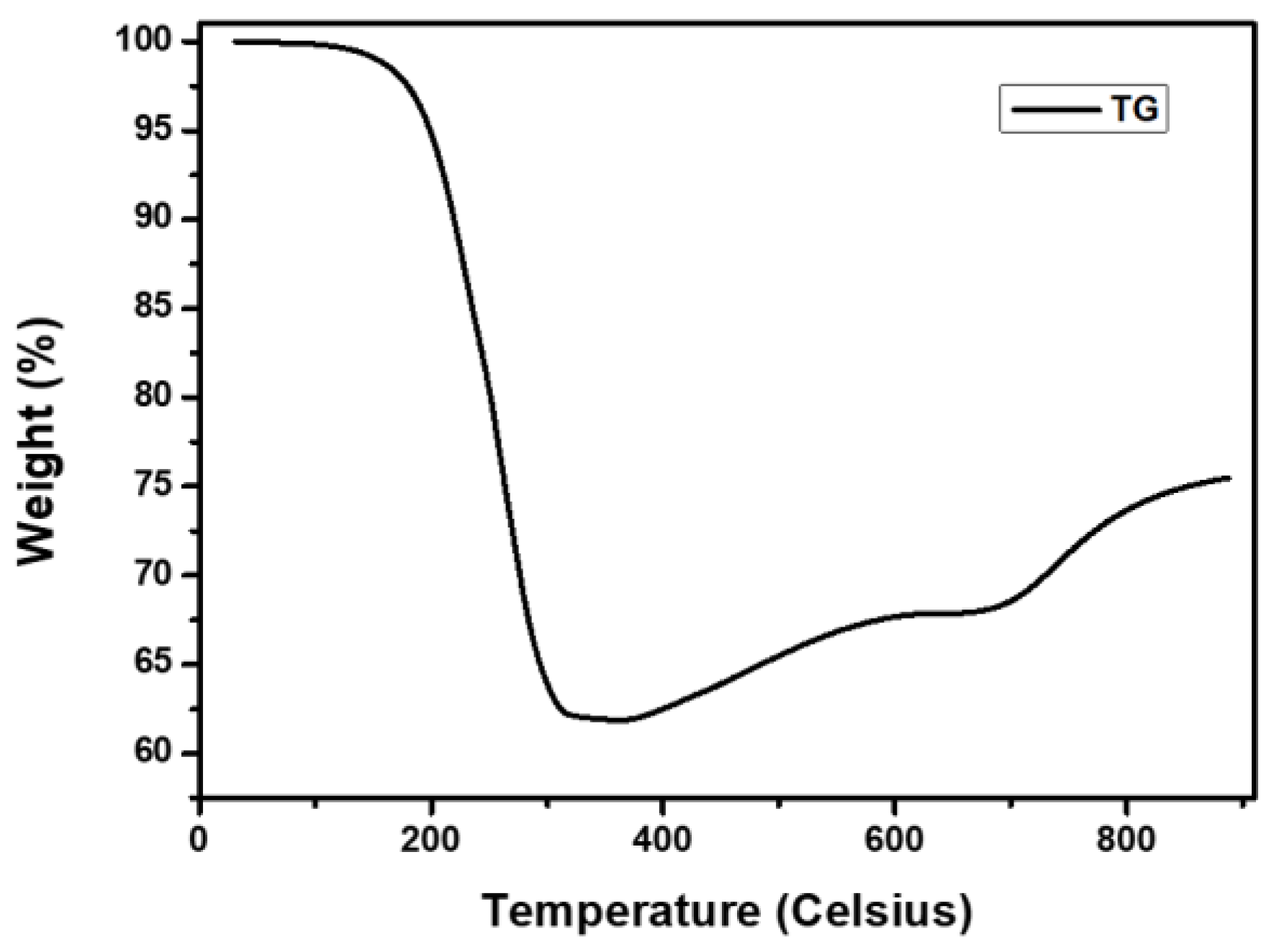
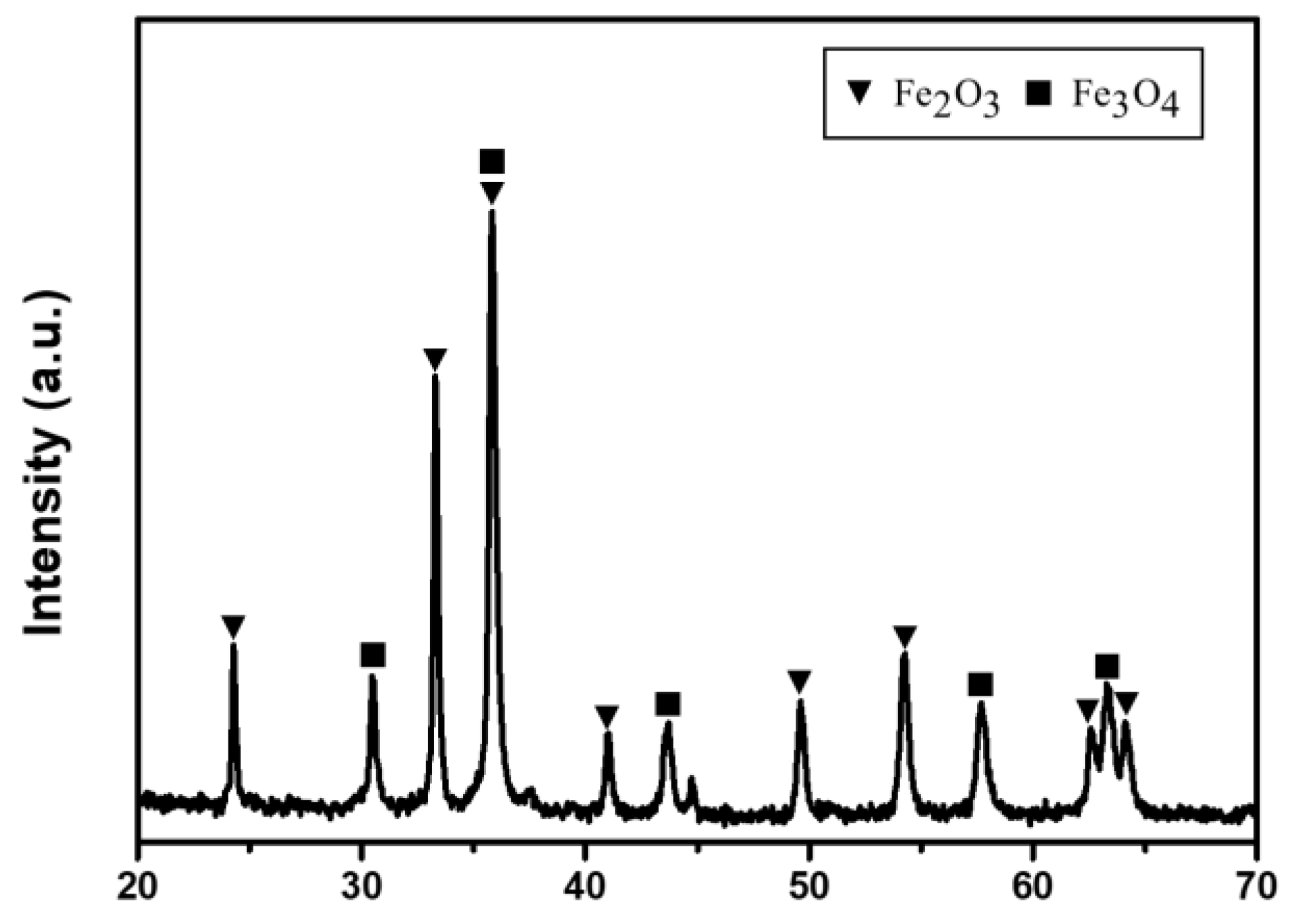
3.1. Pre-Treatment
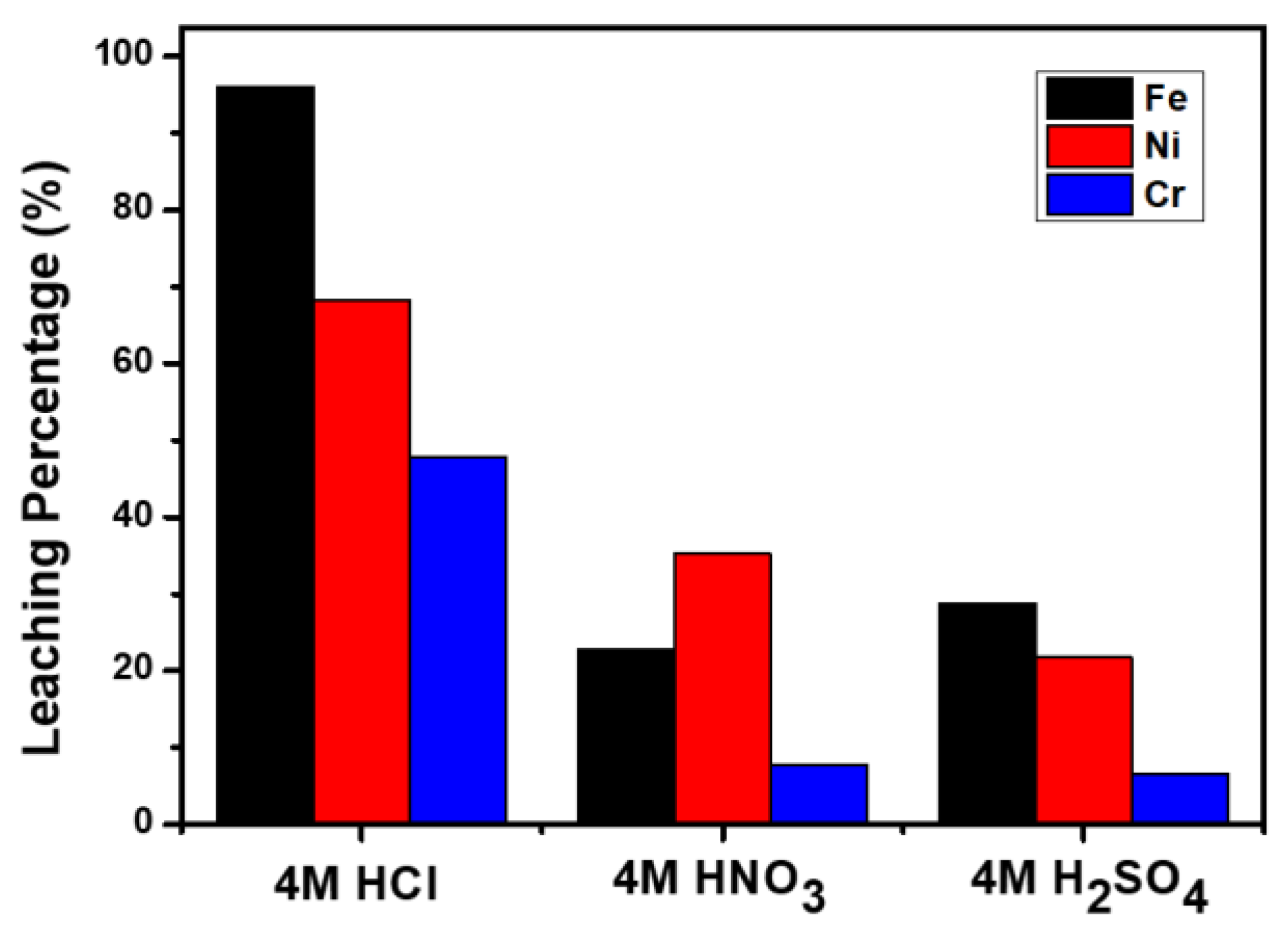
3.2. Acid Leaching
3.3. Solvent Extraction with D2EHPA
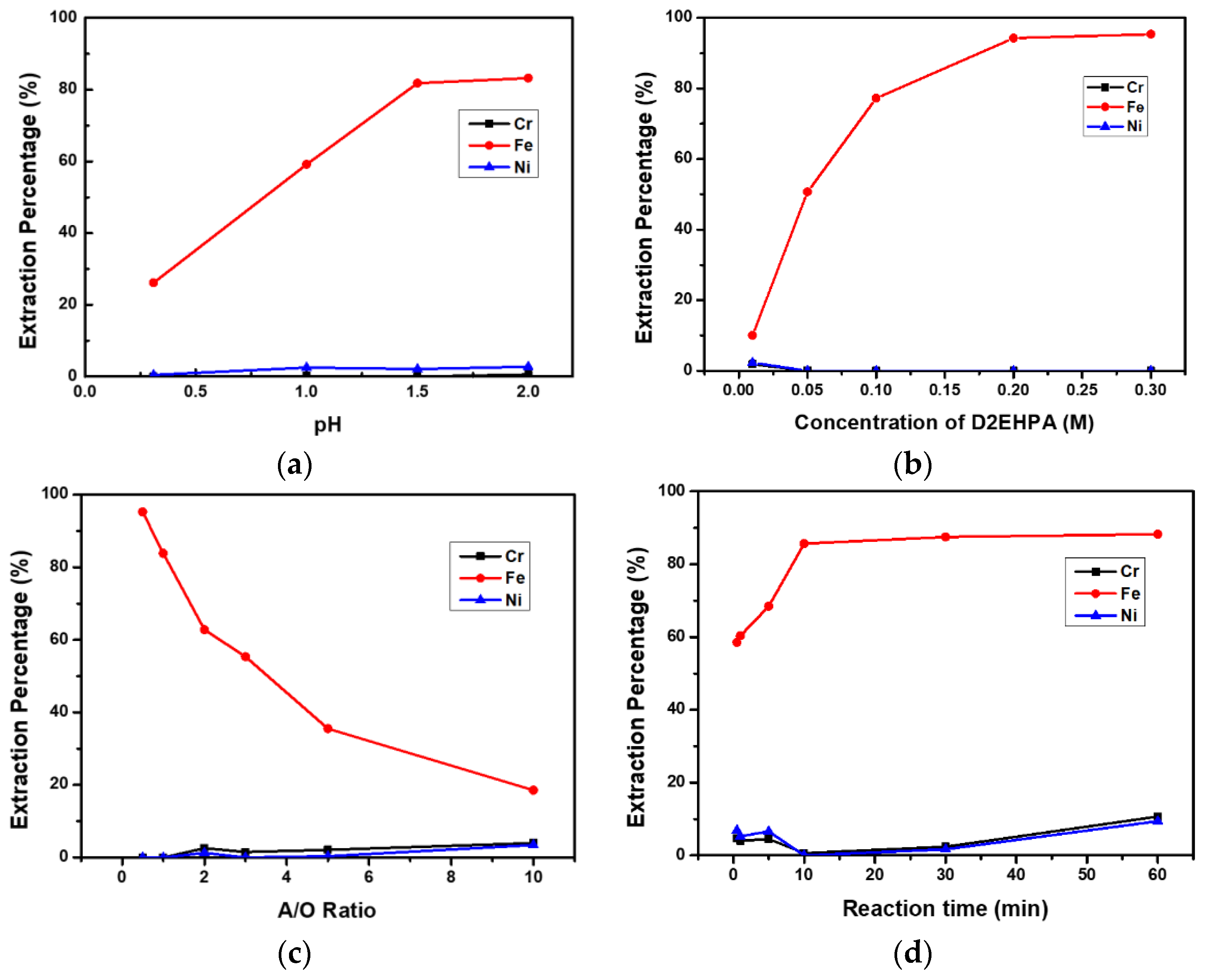
3.3.1. Effect of pH Value of the Aqueous Phase
3.3.2. Effect of D2EHPA Concentration
3.3.3. Effect of A/O Ratio
3.3.4. Effect of Reaction Time
3.4. Chemical Precipitation with NaOH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Association, W.S. The White Book of Steel; World Steel Association: Beijing, China, 2012. [Google Scholar]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Gunarathne, V.; Rajapaksha, A.U.; Vithanage, M.; Alessi, D.S.; Selvasembian, R.; Naushad, M.; You, S.; Oleszczuk, P.; Ok, Y.S. Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1022–1062. [Google Scholar] [CrossRef]
- Tang, Z.; Ding, X.; Yan, X.; Dong, Y.; Liu, C. Recovery of iron, chromium, and nickel from pickling sludge using smelting reduction. Metals 2018, 8, 936. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.T.; Li, Y.L.; Guo, Q.; Shao, D.W.; He, M.M.; Qi, T. Harmless treatment and resource utilization of stainless steel pickling sludge via direct reduction and magnetic separation. J. Clean. Prod. 2019, 240, 118187. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Yu, C.; Yang, F.; Wang, Z. Recovery Nickel-Ferrous Compound from Nickel-Bearing Secondary Resources; Chen, X., Zhong, Y., Zhang, L., Howarter, J.A., Baba, A.A., Wang, C., Sun, Z., Zhang, M., Olivetti, E., Luo, A., et al., Eds.; Springer: Berlin/Heidellberg, Germany, 2020. [Google Scholar]
- Costa, M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef]
- Delina, R.E.; Arcilla, C.; Otake, T.; Garcia, J.J.; Tan, M.; Ito, A. Chromium occurrence in a nickel laterite profile and its implications to surrounding surface waters. Chem. Geol. 2020, 558, 119863. [Google Scholar] [CrossRef]
- Chang, Y.; Zhai, X.; Li, B.; Fu, Y. Removal of iron from acidic leach liquor of lateritic nickel ore by goethite precipitate. Hydrometallurgy 2010, 101, 84–87. [Google Scholar] [CrossRef]
- Han, H.; Sun, W.; Hu, Y.; Cao, X.; Tang, H.; Liu, R.; Yue, T. Magnetite precipitation for iron removal from nickel-rich solutions in hydrometallurgy process. Hydrometallurgy 2016, 165, 318–322. [Google Scholar] [CrossRef]
- Hermoso, J.; Dufour, J.; Gálvez, J.L.; Negro, C.; López-Mateos, F. Nickel hydroxide recovery from stainless steel pickling liquors by selective precipitation. Ind. Eng. Chem. Res. 2005, 44, 5750–5756. [Google Scholar] [CrossRef]
- Rögener, F.; Sartor, M.; Bán, A.; Buchloh, D.; Reichardt, T. Metal recovery from spent stainless steel pickling solutions. Resour. Conserv. Recycl. 2012, 60, 72–77. [Google Scholar] [CrossRef]
- Rice, N.M. A hydrochloric acid process for nickeliferous laterites. Miner. Eng. 2016, 88, 28–52. [Google Scholar] [CrossRef]
- Panda, L.; Rao, D.S.; Mishra, B.K.; Das, B. Characterization and dissolution of low-grade ferruginous nickel lateritic ore by sulfuric acid. Miner. Metall. Process. 2014, 31, 57–65. [Google Scholar] [CrossRef]
- Sulaiman, R.N.R.; Othman, N. Solvent extraction of nickel ions from electroless nickel plating wastewater using synergistic green binary mixture of D2EHPA-octanol system. J. Environ. Chem. Eng. 2018, 6, 1814–1820. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Ramakrishnan, K.; Gayathri, R. Removal of nickel(II) from aqueous solutions by ceralite ir 120 cationic exchange resins. J. Eng. Sci. Technol. 2010, 5, 234–245. [Google Scholar]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Lanagan, M.D.; Ibana, D.C. The solvent extraction and stripping of chromium with Cyanex® 272. Miner. Eng. 2003, 16, 237–245. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini, S.L. Simultaneous solvent extraction of cobalt and nickel in the presence of manganese and magnesium from sulfate solutions by Cyanex 301. Hydrometallurgy 2004, 72, 269–278. [Google Scholar] [CrossRef]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Begum, N.; Bari, F.; Jamaludin, S.B.; Hussin, K. Solvent extraction of copper, nickel and zinc by Cyanex 272. Int. J. Phys. Sci. 2012, 7, 2905–2910. [Google Scholar]
- Guimarães, A.S.; Da Silva, P.S.; Mansur, M.B. Purification of nickel from multicomponent aqueous sulfuric solutions by synergistic solvent extraction using Cyanex 272 and Versatic 10. Hydrometallurgy 2014, 150, 173–177. [Google Scholar] [CrossRef]
- Kul, M.; Oskay, K.O. Separation and recovery of valuable metals from real mix electroplating wastewater by solvent extraction. Hydrometallurgy 2015, 155, 153–160. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Cobo, A. Solvent extraction equilibrium of nickel with LIX 54. Hydrometallurgy 1998, 48, 291–299. [Google Scholar] [CrossRef]
- Jung, M.J.; Venkateswaran, P.; Lee, Y.S. Solvent extraction of nickel(II) ions from aqueous solutions using triethylamine as extractant. J. Ind. Eng. Chem. 2008, 14, 110–115. [Google Scholar] [CrossRef]
- Biswas, R.K.; Begum, D.A. Solvent extraction of Fe3+ from chloride solution by D2EHPA in kerosene. Hydrometallurgy 1998, 50, 153–168. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Zhao, W.; Zhao, H.; Wang, L.; Wang, W.; Qi, T. A novel D2EHPA-based synergistic extraction system for the recovery of chromium (III). Chem. Eng. J. 2016, 302, 233–238. [Google Scholar] [CrossRef]
- Cheng, C.Y. Purification of synthetic laterite leach solution by solvent extraction using D2EHPA. Hydrometallurgy 2000, 56, 369–386. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Suzuki, T.M.; Inoue, K. The synergistic extraction of nickel and cobalt with a mixture of di(2-ethylhexyl) phosphoric acid and 5-dodecylsalicylaldoxime. Hydrometallurgy 2001, 61, 223–227. [Google Scholar] [CrossRef]
- Sole, K.C.; Hiskey, J.B. Solvent extraction characteristics of thiosubstituted organophosphinic acid extractants. Hydrometallurgy 1992, 30, 345–365. [Google Scholar] [CrossRef]
- Hu, G.; Wu, Y.; Chen, D.; Wang, Y.; Qi, T.; Wang, L. Selective removal of iron(III) from highly salted chloride acidic solutions by solvent extraction using di(2-ethylhexyl) phosphate. Front. Chem. Sci. Eng. 2021, 15, 528–537. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH diagrams. Geol. Surv. Jpn. Open File Rep. 2005, 419, 102. [Google Scholar]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, A.; Nunes, K.G.P.; Gutterres, M.; Marcílio, N.R. Production of basic chromium sulfate by using recovered chromium from ashes of thermally treated leather. J. Hazard. Mater. 2010, 176, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Z.; Chu, M.; Tang, J.; Gao, L.; Yan, R. Green and efficient utilization of stainless steel dust by direct reduction and self-pulverization. J. Hazard. Mater. 2021, 413, 125403. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Nishikata, A.; Tsuru, T. Pitting corrosion mechanism of Type 304 stainless steel under a droplet of chloride solutions. Corros. Sci. 2007, 49, 1394–1407. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetič, M.; Zalar, A. Oxidation of AISI 304L stainless steel surface with atomic oxygen. Appl. Surf. Sci. 2002, 200, 94–103. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Duan, F. Metal recovery and heavy metal migration characteristics of ferritic stainless steel pickling sludge reduced by municipal sludge. Waste Manag. 2022, 144, 57–66. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Z.; Chu, M.; Yan, R.; Li, F.; Tang, J. New understanding on metal recovery of Fe, Ni and Cr during carbon-thermal reduction of stainless steel dust. Adv. Powder Technol. 2021, 32, 4273–4285. [Google Scholar] [CrossRef]

| Composition | Oil | Water | Ash |
|---|---|---|---|
| Proportion (wt%) | 37–39 | 1–3 | 58–62 |
| Element | Fe | Ni | Cr |
|---|---|---|---|
| Concentration (mg/L) | 2380 | 300 | 307 |
| Element | Fe | Ni | Cr |
|---|---|---|---|
| 300 °C | 97.6% | 98.1% | 95.7% |
| 600 °C | 40.3% | 32.7% | 12.8% |
| Distribution Ratios | Separation Factors | ||
|---|---|---|---|
| 5.98 | 1616.22 | ||
| 0.0037 | 906.06 | ||
| 0.0066 | |||
| Element | Fe | Ni | Cr |
|---|---|---|---|
| Concentration (mg/L) | 0.004 | 293 | 292 |
| References | Materials | Recycling Technologies | Recovery Rate |
|---|---|---|---|
| Zhang et al. [38] | Pickling sludge | Reduction and magnetic separation | Fe 70.1%, Ni 60.3%, Cr 53.7% |
| Liu et al. [39] | Stainless steel dust | Carbon-thermal reduction | Fe 79.7%, Ni 83.6%, Cr 90.7% |
| Wu et al. [5] | Pickling sludge | Direct reduction and magnetic separation | Fe 95.3%, Ni 97.5%, Cr 88.7% |
| This study | Stainless steel sludge | Solvent extraction and chemical precipitation | Fe 99.9%, Ni 99.5%, Cr 75.1% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Chen, Y.-C.; Lee, C.-H. Hydrometallurgical Recovery of Iron, Nickel, and Chromium from Stainless Steel Sludge with Emphasis on Solvent Extraction and Chemical Precipitation. Processes 2022, 10, 748. https://doi.org/10.3390/pr10040748
Chen W-S, Chen Y-C, Lee C-H. Hydrometallurgical Recovery of Iron, Nickel, and Chromium from Stainless Steel Sludge with Emphasis on Solvent Extraction and Chemical Precipitation. Processes. 2022; 10(4):748. https://doi.org/10.3390/pr10040748
Chicago/Turabian StyleChen, Wei-Sheng, Yu-Chi Chen, and Cheng-Han Lee. 2022. "Hydrometallurgical Recovery of Iron, Nickel, and Chromium from Stainless Steel Sludge with Emphasis on Solvent Extraction and Chemical Precipitation" Processes 10, no. 4: 748. https://doi.org/10.3390/pr10040748
APA StyleChen, W.-S., Chen, Y.-C., & Lee, C.-H. (2022). Hydrometallurgical Recovery of Iron, Nickel, and Chromium from Stainless Steel Sludge with Emphasis on Solvent Extraction and Chemical Precipitation. Processes, 10(4), 748. https://doi.org/10.3390/pr10040748








