Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of Tb-PhPOSS (1)
2.1.2. Synthesis of TbEu-PhPOSS (2)
2.2. Analytical Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenz, V.; Gießmann, S.; Gun’Ko, Y.K.; Fischer, A.K.; Gilje, J.W.; Edelmann, F.T. Fully Metalated Silsesquioxanes: Building Blocks for the Construction of Catalyst Models. Angew. Chem. Int. Ed. 2004, 43, 4603–4606. [Google Scholar] [CrossRef] [PubMed]
- Feher, F.J.; Budzichowski, T.A.; Rahimian, K.; Ziller, J.W. Reactions of incompletely-condensed silsesquioxanes with pentamethylantimony: A new synthesis of metallasilsesquioxanes with important implications for the chemistry of silica surfaces. J. Am. Chem. Soc. 1992, 114, 3859–3866. [Google Scholar] [CrossRef]
- Crocker, M.; Herold, R.H.M.; Orpen, A.G.; Overgaag, M.T.A. Synthesis and characterisation of titanium silasesquioxane complexes: Soluble models for the active site in titanium silicate epoxidation catalysts. J. Chem. Soc. Dalton Trans. 1999, 3791–3804. [Google Scholar] [CrossRef]
- Wada, K.; Bundo, M.; Nakabayashi, D.; Itayama, N.; Kondo, T.; Mitsudo, T.-A. Synthesis of Novel Group 4 Metallocene-Containing Silsesquioxanes with a Vinyl Group. Chem. Lett. 2000, 29, 628–629. [Google Scholar] [CrossRef]
- Abbenhuis, H.C.L. Advances in Homogeneous and Heterogeneous Catalysis with Metal-Containing Silsesquioxanes. Chem. Eur. J. 2000, 6, 25–32. [Google Scholar] [CrossRef]
- Ward, A.J.; Masters, A.F.; Maschmeyer, T. Metallasilsesquioxanes: Molecular Analogues of Heterogeneous Catalysts. In Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C., Ed.; Advances in Silicon Science; Springer: Dordrecht, The Netherlands, 2011; pp. 135–166. ISBN 978-90-481-3787-9. [Google Scholar]
- Roesky, H.W.; Anantharaman, G.; Chandrasekhar, V.; Jancik, V.; Singh, S. Control of Molecular Topology and Metal Nuclearity in Multimetallic Assemblies: Designer Metallosiloxanes Derived from Silanetriols. Chem. A Eur. J. 2004, 10, 4106–4114. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N. Modern concepts and methods in the chemistry of polyhedral metallasiloxanes. Coord. Chem. Rev. 2016, 306, 235–269. [Google Scholar] [CrossRef]
- Harrison, P.G. Silicate cages: Precursors to new materials. J. Organomet. Chem. 1997, 542, 141–183. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Maschmeyer, T. Review: Oligomeric Silsesquioxanes: Synthesis, Characterization and Selected Applications. Aust. J. Chem. 2001, 54, 583–596. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Feher, F.J.; Newman, D.A.; Walzer, J.F. Silsesquioxanes as models for silica surfaces. J. Am. Chem. Soc. 1989, 111, 1741–1748. [Google Scholar] [CrossRef]
- Ye, M.; Wu, Y.; Zhang, W.; Yang, R. Synthesis of incompletely caged silsesquioxane (T7-POSS) compounds via a versatile three-step approach. Res. Chem. Intermed. 2018, 44, 4277–4294. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Wu, D. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Materials: Molecular Design, Solution Self-Assembly and Biomedical Applications. Chin. J. Chem. 2021, 39, 757–774. [Google Scholar] [CrossRef]
- Imoto, H.; Ueda, Y.; Sato, Y.; Nakamura, M.; Mitamura, K.; Watase, S.; Naka, K. Corner- and Side-Opened Cage Silsesquioxanes: Structural Effects on the Materials Properties. Eur. J. Inorg. Chem. 2020, 2020, 737–742. [Google Scholar] [CrossRef]
- Pan, G. Polyhedral Oligomeric Silsesquioxane (POSS). In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 577–584. ISBN 978-0-387-69002-5. [Google Scholar]
- Henig, J.; Tóth, É.; Engelmann, J.; Gottschalk, S.; Mayer, H.A. Macrocyclic Gd3+ Chelates Attached to a Silsesquioxane Core as Potential Magnetic Resonance Imaging Contrast Agents: Synthesis, Physicochemical Characterization, and Stability Studies. Inorg. Chem. 2010, 49, 6124–6138. [Google Scholar] [CrossRef]
- Köytepe, S.; Demirel, M.H.; Gültek, A.; Seçkin, T. Metallo-supramolecular materials based on terpyridine-functionalized polyhedral silsesquioxane. Polym. Int. 2014, 63, 778–787. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W.; et al. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Lorenz, V.; Fischer, A.; Gießmann, S.; Gilje, J.W.; Gun’Ko, Y.; Jacob, K.; Edelmann, F.T. Disiloxanediolates and polyhedral metallasilsesquioxanes of the early transition metals and f-elements. Coord. Chem. Rev. 2000, 206–207, 321–368. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Wada, K.; Mitsudo, T.-A. Preparation of Novel Materials for Catalysts Utilizing Metal-Containing Silsesquioxanes. Catal. Surv. Asia 2005, 9, 229–241. [Google Scholar] [CrossRef]
- Lichtenhan, J.D. Polyhedral Oligomeric Silsesquioxanes: Building Blocks for Silsesquioxane-Based Polymers and Hybrid Materials. Comments Inorg. Chem. 1995, 17, 115–130. [Google Scholar] [CrossRef]
- Olivero, F.; Renò, F.; Carniato, F.; Rizzi, M.; Cannas, M.; Marchese, L. A novel luminescent bifunctional POSS as a molecular platform for biomedical applications. Dalton Trans. 2012, 41, 7467–7473. [Google Scholar] [CrossRef]
- Kaneshiro, T.L.; Jeong, E.-K.; Morrell, G.; Parker, D.L.; Lu, Z.-R. Synthesis and Evaluation of Globular Gd-DOTA-Monoamide Conjugates with Precisely Controlled Nanosizes for Magnetic Resonance Angiography. Biomacromolecules 2008, 9, 2742–2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A. A Nanocage for Nanomedicine: Polyhedral Oligomeric Silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Duchateau, R. Incompletely Condensed Silsesquioxanes: Versatile Tools in Developing Silica-Supported Olefin Polymerization Catalysts. Chem. Rev. 2002, 102, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Venegoni, I.; Carniato, F.; Olivero, F.; Bisio, C.; Pira, N.L.; Lambertini, V.G.; Marchese, L. A novel electroluminescent PPV copolymer and silsesquioxane nanocomposite film for the preparation of efficient PLED devices. Nanotechnology 2012, 23, 435702. [Google Scholar] [CrossRef] [PubMed]
- Carniato, F.; Bisio, C.; Gatti, G.; Boccaleri, E.; Bertinetti, L.; Coluccia, S.; Monticelli, O.; Marchese, L. Titanosilsesquioxanes Embedded in Synthetic Clay as a Hybrid Material for Polymer Science. Angew. Chem. Int. Ed. 2009, 48, 6059–6061. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Provatas, A.; Luft, M.; Mu, J.C.; White, A.H.; Matisons, J.G.; Skelton, B. Silsesquioxanes: Part I: A key intermediate in the building of molecular composite materials. J. Organomet. Chem. 1998, 565, 159–164. [Google Scholar] [CrossRef]
- Wang, M.; Chi, H.; Joshy, K.S.; Wang, F. Progress in the Synthesis of Bifunctionalized Polyhedral Oligomeric Silsesquioxane. Polymers 2019, 11, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeArmitt, C. Polyhedral Oligomeric Silsesquioxanes in Plastics. In Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C., Ed.; Advances in Silicon Science; Springer: Dordrecht, The Netherlands, 2011; pp. 209–228. ISBN 978-90-481-3787-9. [Google Scholar]
- Brandhorst, H.W. Polyhedral Oligomeric Silsesquioxanes in Space Applications. In Applications of Polyhedral Oligomeric Silsesquioxanes; Hartmann-Thompson, C., Ed.; Advances in Silicon Science; Springer: Dordrecht, The Netherlands, 2011; pp. 327–361. ISBN 978-90-481-3787-9. [Google Scholar]
- Marchesi, S.; Carniato, F.; Marchese, L.; Boccaleri, E. Luminescent Mesoporous Silica Built through Self-Assembly of Polyhedral Oligomeric Silsesquioxane and Europium(III) Ions. ChemPlusChem 2015, 80, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Edelmann, F.T. Metallasilsesquioxanes. In Advances in Organometallic Chemistry; West, R., Hill, A.F., Stone, F.G.A., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 53, pp. 101–153. [Google Scholar]
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero- and Metallasiloxanes Derived from Silanediols, Disilanols, Silanetriols, and Trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, R.W.J.M.; van Santen, R.A.; Abbenhuis, H.C.L. The Dynamic Status Quo of Polyhedral Silsesquioxane Coordination Chemistry. Eur. J. Inorg. Chem. 2004, 2004, 675–683. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Zhang, W.; Yang, R.; Li, J. Fabrication of eco-friendly and multifunctional sodium-containing polyhedral oligomeric silsesquioxane and its flame retardancy on epoxy resin. Compos. Part B Eng. 2020, 191, 107961. [Google Scholar] [CrossRef]
- Gießmann, S.; Lorenz, V.; Liebing, P.; Hilfert, L.; Fischer, A.; Edelmann, F.T. Synthesis and structural study of new metallasilsesquioxanes of potassium and uranium. Dalton Trans. 2017, 46, 2415–2419. [Google Scholar] [CrossRef]
- Prigyai, N.; Chanmungkalakul, S.; Ervithayasuporn, V.; Yodsin, N.; Jungsuttiwong, S.; Takeda, N.; Unno, M.; Boonmak, J.; Kiatkamjornwong, S. Lithium-Templated Formation of Polyhedral Oligomeric Silsesquioxanes (POSS). Inorg. Chem. 2019, 58, 15110–15117. [Google Scholar] [CrossRef]
- Lorenz, V.; Edelmann, F.T. Dimeric Silsesquioxanes and Metallasilsesquioxanes—En route to large, welldefined Si-O-assemblies. Z. Anorg. Allg. Chem. 2004, 630, 1147–1157. [Google Scholar] [CrossRef]
- Lorenz, V.; Fischer, A.; Edelmann, F.T. Silsesquioxane chemistry, 6: The first beryllium silsesquioxane: Synthesis and structure of [Cy7Si7O12BeLi]2·2THF. Inorg. Chem. Commun. 2000, 3, 292–295. [Google Scholar] [CrossRef]
- Maxim, N.; Magusin, P.; Kooyman, P.; van Wolput, J.H.; van Santen, A.R.A.; Abbenhuis, H.C.L. Microporous Mg−Si−O and Al−Si−O Materials Derived from Metal Silsesquioxanes. Chem. Mater. 2001, 13, 2958–2964. [Google Scholar] [CrossRef]
- Hanssen, R.W.J.M.; Meetsma, A.; van Santen, R.A.; Abbenhuis, H.C.L. Synthesis, Structural Characterization, and Transmetalation Reactions of a Tetranuclear Magnesium Silsesquioxane Complex. Inorg. Chem. 2001, 40, 4049–4052. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, G.S.; Levitsky, M.M.; Zubavichus, Y.V.; Khrustalev, V.N.; Titov, A.A.; Dorovatovskii, P.V.; Smol’Yakov, A.F.; Shubina, E.S.; Kirillova, M.V.; Kirillov, A.M.; et al. Cu-6- and Cu-8-Cage Sil- and Germsesquioxanes: Synthetic and Structural Features, Oxidative Rearrangements, and Catalytic Activity. Inorg. Chem. 2021, 60, 8062–8074. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Zhu, S.; Tian, Q.; Li, N.; Yan, M.; Henderson, M.J. Aggregated germanium saponite: Removal and retention of polymeric thorium and uranium complexes. Appl. Clay Sci. 2021, 216, 106382. [Google Scholar] [CrossRef]
- Grzelak, M.; Frąckowiak, D.; Marciniec, B. Vinyl-Functionalized Silsesquioxanes and Germasilsesquioxanes. Eur. J. Inorg. Chem. 2017, 2017, 3337–3342. [Google Scholar] [CrossRef]
- Gerritsen, G.; Duchateau, R.; van Santen, A.R.A.; Yap, G.P.A. Boron, Aluminum, and Gallium Silsesquioxane Compounds, Homogeneous Models for Group 13 Element-Containing Silicates and Zeolites. Organometallics 2003, 22, 100–110. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A.; Ziller, J.W. Synthesis, reactivity, and dynamic behavior of a boron-containing silsesquioxane. Inorg. Chem. 1992, 31, 5100–5105. [Google Scholar] [CrossRef]
- Feher, F.J.; Budzichowski, T.A. Heterosilsesquioxanes: Synthesis and characterization of Group 15 containing polyhedral oligosilsesquioxanes. Organometallics 1991, 10, 812–815. [Google Scholar] [CrossRef]
- Żak, P.; Frąckowiak, D.; Grzelak, M.; Bołt, M.; Kubicki, M.; Marciniec, B. Olefin Metathesis of Vinylgermanium Derivatives as Method for the Synthesis of Functionalized Cubic and Double-Decker Germasilsesquioxanes. Adv. Synth. Catal. 2016, 358, 3265–3276. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Zubavichus, Y.V.; Korlyukov, A.A.; Khrustalev, V.; Shubina, E.; Bilyachenko, A.N. Silicon and Germanium-Based Sesquioxanes as Versatile Building Blocks for Cage Metallacomplexes. A Review. J. Clust. Sci. 2019, 30, 1283–1316. [Google Scholar] [CrossRef]
- Kaźmierczak, J.; Kuciński, K.; Stachowiak, H.; Hreczycho, G. Introduction of Boron Functionalities into Silsesquioxanes: Novel Independent Methodologies. Chem. A Eur. J. 2018, 24, 2509–2514. [Google Scholar] [CrossRef]
- Besselink, R.; Venkatachalam, S.; Van Wüllen, L.; Elshof, J.E.T. Incorporation of niobium into bridged silsesquioxane based silica networks. J. Sol Gel Sci. Technol. 2014, 70, 473–481. [Google Scholar] [CrossRef]
- García, C.; Gómez, M.; Gómez-Sal, P.; Hernández, J.M. Monocyclopentadienyl(niobium) Compounds with Imido and Silsesquioxane Ligands: Synthetic, Structural and Reactivity Studies. Eur. J. Inorg. Chem. 2009, 2009, 4401–4415. [Google Scholar] [CrossRef]
- Feher, F.J.; Blanski, R.L. Olefin polymerization by vanadium-containing silsesquioxanes: Synthesis of a dialkyl-oxo-vanadium(V) complex that initiates ethylene polymerization. J. Am. Chem. Soc. 1992, 114, 5886–5887. [Google Scholar] [CrossRef]
- Feher, F.J.; Walzer, J.F. Synthesis and characterization of vanadium-containing silsesquioxanes. Inorg. Chem. 1991, 30, 1689–1694. [Google Scholar] [CrossRef]
- Annand, J.; Aspinall, H.C. Lanthanide silasesquioxanes: Monomeric and functionalised complexes. J. Chem. Soc. Dalton Trans. 2000, 1867–1871. [Google Scholar] [CrossRef]
- Annand, J.; Aspinall, H.C.; Steiner, A. Novel Heterometallic Lanthanide Silsesquioxane. Inorg. Chem. 1999, 38, 3941–3943. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, N. Highly transparent and luminescent lanthanide ion-containing bridged polysilsesquioxanes. Photochem. Photobiol. Sci. 2011, 10, 42–47. [Google Scholar] [CrossRef]
- Marchesi, S.; Carniato, F.; Boccaleri, E. Synthesis and characterisation of a novel europium(iii)-containing heptaisobutyl-POSS. New J. Chem. 2014, 38, 2480–2485. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Boccaleri, E.; Carniato, F. A Luminescent Polysilsesquioxane Obtained by Self-Condensation of Anionic Polyhedral Oligomeric Silsequioxanes (POSS) and Europium(III) Ions. ChemPlusChem 2020, 85, 176–182. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; Chen, M.; Li, H. Synthesis and luminescence of octacarboxy cubic polyhedral oligosilsesquioxanes coordinated with terbium. CrystEngComm 2016, 18, 177–182. [Google Scholar] [CrossRef]
- Kumar, B.P.; Kumar, A.P.; Bindu, P.H.; Mukherjee, K.; Patra, A.S. Red Light Emission of POSS Triol Chelated with Europium. Asian J. Nanosci. Mater. 2019, 2, 244–256. [Google Scholar]
- Lorenz, V.; Edelmann, A.; Gießmann, S.; Hrib, C.G.; Blaurock, S.; Edelmann, F.T. Disiloxanediolates and Metallasilsesquioxanes of the Rare Earth Elements. Z. Anorg. Allg. Chem. 2010, 636, 2172–2191. [Google Scholar] [CrossRef]
- Yi, S.S.; Jung, J.Y. Rare earth Doped organic–inorganic hybrid polyhedral oligomeric silsesquioxane phosphors applied for flexible sheet and anti-counterfeiting. Mater. Express 2021, 11, 1732–1738. [Google Scholar] [CrossRef]
- Lorenz, V.; Blaurock, S.; Hrib, C.G.; Edelmann, F.T. Coupling of Silsesquioxane Cages in the Coordination Sphere of Erbium. Eur. J. Inorg. Chem. 2010, 2010, 2605–2608. [Google Scholar] [CrossRef]
- Willauer, A.R.; Dabrowska, A.M.; Scopelliti, R.; Mazzanti, M. Structure and small molecule activation reactivity of a metallasilsesquioxane of divalent ytterbium. Chem. Commun. 2020, 56, 8936–8939. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, M.M.; Yalymov, A.I.; Kulakova, A.N.; Petrov, A.A.; Bilyachenko, N. Cage-like metallasilsesquioxanes in catalysis: A review. J. Mol. Catal. A Chem. 2017, 426, 297–304. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’Pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si- or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Quadrelli, E.A.; Basset, J.-M. On silsesquioxanes’ accuracy as molecular models for silica-grafted complexes in heterogeneous catalysis. Coord. Chem. Rev. 2010, 254, 707–728. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shubina, E.S.; Long, J.; Guari, Y.; Larionova, J. Magnetic cage-like metallasilsesquioxanes. Coord. Chem. Rev. 2019, 398, 213015. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Yalymov, A.; Dronova, M.; Korlyukov, A.A.; Vologzhanina, A.V.; Es’Kova, M.A.; Long, J.; Larionova, J.; Guari, Y.; Dorovatovskii, P.V.; et al. Family of Polynuclear Nickel Cagelike Phenylsilsesquioxanes; Features of Periodic Networks and Magnetic Properties. Inorg. Chem. 2017, 56, 12751–12763. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Hou, J.-L.; Wang, Z.; Gupta, R.K.; Jagličić, Z.; Jagodič, M.; Wang, W.-G.; Tung, C.-H.; Sun, D. An Octanuclear Cobalt Cluster Protected by Macrocyclic Ligand: In Situ Ligand-Transformation-Assisted Assembly and Single-Molecule Magnet Behavior. Inorg. Chem. 2020, 59, 5683–5693. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Vologzhanina, A.V.; Kozlov, Y.N.; Shul’Pina, L.S.; Nesterov, D.S.; Pombeiro, A.J.L.; Lamaty, F.; et al. A heterometallic (Fe6Na8) cage-like silsesquioxane: Synthesis, structure, spin glass behavior and high catalytic activity. RSC Adv. 2016, 6, 48165–48180. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Yalymov, A.I.; Korlyukov, A.A.; Khrustalev, V.N.; Vologzhanina, A.V.; Shul’Pina, L.S.; Ikonnikov, N.S.; Trigub, A.E.; Dorovatovskii, P.V.; et al. Cage-like Fe,Na-Germsesquioxanes: Structure, Magnetism, and Catalytic Activity. Angew. Chem. Int. Ed. 2016, 55, 15360–15363. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Yalymov, A.I.; Korlyukov, A.A.; Long, J.; Larionova, J.; Guari, Y.; Vologzhanina, A.V.; Es’Kova, M.A.; Shubina, E.S.; Levitsky, M.M. Unusual penta- and hexanuclear Ni(ii)-based silsesquioxane polynuclear complexes. Dalton Trans. 2016, 45, 7320–7327. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, H.; Wang, H.; Bei, Y.; Feng, S. Luminescent organo-polysiloxanes containing complexed lanthanide ions. Appl. Organomet. Chem. 2009, 23, 429–433. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Juris, A. Photochemistry and Photophysics: Concepts, Research, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 978-3-527-67104-5. [Google Scholar]
- Leonard, J.P.; Nolan, C.B.; Stomeo, F.; Gunnlaugsson, T. Photochemistry and Photophysics of Coordination Compounds: Lanthanides. In Photochemistry and Photophysics of Coordination Compounds II; Balzani, V., Campagna, S., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–43. ISBN 978-3-540-73349-2. [Google Scholar]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-infrared (NIR) lanthanide molecular probes for bioimaging and biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Carniato, F.; Tei, L.; Botta, M. Gd-Based Mesoporous Silica Nanoparticles as MRI Probes. Eur. J. Inorg. Chem. 2018, 2018, 4936–4954. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Pope, S.J.A. Using lanthanide ions in molecular bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Kitagawa, Y. Thermo-sensitive luminescence of lanthanide complexes, clusters, coordination polymers and metal–organic frameworks with organic photosensitizers. J. Mater. Chem. C 2019, 7, 7494–7511. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Chapter 281—Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Jean-Claude, B., Vitalij, K.P., Eds.; Including Actinides; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Andres, J.; Chauvin, A.-S. Colorimetry of Luminescent Lanthanide Complexes. Molecules 2020, 25, 4022. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273–274, 76–86. [Google Scholar] [CrossRef]
- Zucchi, C.; Shchegolikhina, O.; Borsari, M.; Cornia, A.; Gavioli, G.; Fabretti, A.; Rentschler, E.; Gatteschi, D.; Ugo, R.; Psaro, R.; et al. Cyclooligosiloxanolate cluster complexes of transition metals and lanthanides. J. Mol. Catal. A Chem. 1996, 107, 313–321. [Google Scholar] [CrossRef]
- Shchegolikhina, O.; Pozdniakova, Y.; Lindeman, S.; Zhdanov, A.; Psaro, R.; Ugo, R.; Gavioli, G.; Battistuzzi, R.; Borsari, M.; Rüffer, T.; et al. Cyclosiloxane sandwich complexes of a lanthanide metal: Na6{[(C6H5SiO2)8]2Nd4(μ4-O)}. J. Organomet. Chem. 1996, 514, 29–35. [Google Scholar] [CrossRef]
- Igonin, V.A.; Lindeman, S.V.; Struchkov, Y.T.; Molodtsova, Y.A.; Pozdnyakova, Y.A.; Shchegolikhina, O.I.; Zhdanov, A.A. Crystal structure of the Nd, Gd, and Dy sandwich complexes involving 8-membered macrocyclic phenylsiloxanolate ligands. Russ. Chem. Bull. 1993, 42, 176–181. [Google Scholar] [CrossRef]
- Igonin, V.A.; Lindeman, S.V.; Struchkov, Y.T.; Shchegolikhina, O.I.; Molodtsova, Y.A.; Pozdnyakova, Y.A.; Zhdanov, A.A. Crystal structure of the La3+ sandwich complex based on 8-membered macrocyclic siloxanolate ligands. Russ. Chem. Bull. 1993, 42, 168–173. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Shubina, E.S.; Felix, G.; Mamontova, E.; Long, J.; Guari, Y.; Larionova, J. New Luminescent Tetranuclear Lanthanide-Based Silsesquioxane Cage-Like Architectures. Chem. A Eur. J. 2020, 26, 16594–16598. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Esquivel, D.; LaForce, B.; Vincze, L.; Van Der Voort, P.; Romero-Salguero, F.J.; Van Deun, R. Luminescent thermometer based on Eu3+/Tb3+ -organic-functionalized mesoporous silica. Luminescence 2018, 33, 567–573. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Nigoghossian, K.; Félix, G.; Khrustalev, V.N.; Shubina, E.S.; Long, J.; Guari, Y.; Carlos, L.D.; Bilyachenko, A.N.; Larionova, J. New Magnetic and Luminescent Dy(III) and Dy(III)/Y(III) Based Tetranuclear Silsesquioxane Cages. Eur. J. Inorg. Chem. 2021, 2021, 2696–2701. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.; Zhang, D.; Liu, Y.; Huang, G. Synthesis and thermal properties of modified room temperature vulcanized (RTV) silicone rubber using polyhedral oligomeric silsesquioxane (POSS) as a cross linking agent. RSC Adv. 2014, 4, 41453–41460. [Google Scholar] [CrossRef]
- Dudziec, B.; Marciniec, B. Double-decker Silsesquioxanes: Current Chemistry and Applications. Curr. Org. Chem. 2018, 21. [Google Scholar] [CrossRef]
- Duszczak, J.; Mituła, K.; Santiago-Portillo, A.; Soumoy, L.; Rzonsowska, M.; Januszewski, R.; Fusaro, L.; Aprile, C.; Dudziec, B. Double-Decker Silsesquioxanes Self-Assembled in One-Dimensional Coordination Polymeric Nanofibers with Emission Properties. ACS Appl. Mater. Interfaces 2021, 13, 22806–22818. [Google Scholar] [CrossRef]
- Tanaka, T.; Hasegawa, Y.; Kawamori, T.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Double-Decker Silsesquioxanes from Substituted Difluorosilane. Organometallics 2019, 38, 743–747. [Google Scholar] [CrossRef]
- Barry, B.-D.; Dannatt, J.E.; King, A.K.; Lee, A.; Maleczka, R.E. A general diversity oriented synthesis of asymmetric double-decker shaped silsesquioxanes. Chem. Commun. 2019, 55, 8623–8626. [Google Scholar] [CrossRef] [PubMed]
- Kunthom, R.; Takeda, N.; Unno, M. Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage). Molecules 2019, 24, 4252. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-8493-2463-5. [Google Scholar]
- Carniato, F.; Boccaleri, E.; Marchese, L.; Fina, A.; Tabuani, D.; Camino, G. Synthesis and Characterisation of Metal Isobutylsilsesquioxanes and Their Role as Inorganic–Organic Nanoadditives for Enhancing Polymer Thermal Stability. Eur. J. Inorg. Chem. 2007, 2007, 585–591. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-68136-7. [Google Scholar]
- Li, Q.; Li, T.; Wu, J. Luminescence of Europium(III) and Terbium(III) Complexes Incorporated in Poly(Vinyl Pyrrolidone) Matrix. J. Phys. Chem. B 2001, 105, 12293–12296. [Google Scholar] [CrossRef]
- Marchesi, S.; Bisio, C.; Carniato, F. Novel light-emitting clays with structural Tb3+ and Eu3+ for chromate anion detection. RSC Adv. 2020, 10, 29765–29771. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, I.; Piccinelli, F.; Bettinelli, M. Luminescence of Tb-based materials doped with Eu3+: Case studies for energy transfer processes. J. Lumin. 2017, 189, 71–77. [Google Scholar] [CrossRef]
- Rodrigues, M.O.; Dutra, J.D.L.; Nunes, L.A.O.; de Sá, G.F.; de Azevedo, W.M.; Silva, P.; Paz, F.A.A.; Freire, R.O.; Júnior, S.A. Tb3+→Eu3+Energy Transfer in Mixed-Lanthanide-Organic Frameworks. J. Phys. Chem. C 2012, 116, 19951–19957. [Google Scholar] [CrossRef]
- Hou, Z.; Cheng, Z.; Li, G.; Wang, W.; Peng, C.; Li, C.; Ma, P.; Yang, D.; Kang, X.; Lin, J. Electrospinning-derived Tb2(WO4)3:Eu3+ nanowires: Energy transfer and tunable luminescence properties. Nanoscale 2011, 3, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K.; Görller-Walrand, C. Application of the Eu3+ Ion for Site Symmetry Determination. J. Rare Earth 1996, 14, 173–180. [Google Scholar]
- Tang, S.; Babai, A.; Mudring, A.-V. Europium-Based Ionic Liquids as Luminescent Soft Materials. Angew. Chem. Int. Ed. 2008, 47, 7631–7634. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Liu, H.; Chen, Y. Preparation and luminescence of europium(iii) terpyridine complex-bridged polysilsesquioxanes. J. Mater. Chem. 2011, 21, 18462–18466. [Google Scholar] [CrossRef]
- Yin, H.; Li, Y.; Bai, J.; Ma, M.; Liu, J. Effect of calcinations temperature on the luminescence intensity and fluorescent lifetime of Tb 3+ -doped hydroxyapatite (Tb-HA) nanocrystallines. J. Materiomics 2017, 3, 144–149. [Google Scholar] [CrossRef]
- Sahu, I.P.; Bisen, D.; Tamrakar, R.K.; Murthy, K.; Mohapatra, M. Luminescence studies on the europium doped strontium metasilicate phosphor prepared by solid state reaction method. J. Sci. Adv. Mater. Devices 2017, 2, 59–68. [Google Scholar] [CrossRef]
- Parker, D. Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 2000, 205, 109–130. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S.; Vyprachticky, D.; Furuya, H.; Abe, A.; Okamoto, Y. Ion Binding Properties of Polycarboxylates Using Terbium(III) as a Fluorescent Probe: Viscosities and Coordinated Water Molecules in Polycarboxylate−Terbium(III) Complexes in Aqueous Solutions. Macromolecules 1996, 29, 3511–3514. [Google Scholar] [CrossRef]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; de Sousa, A.S.; Williams, J.A.G.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 1999, 2, 493–504. [Google Scholar] [CrossRef]
- Supkowski, R.M.; Horrocks, W.D. On the determination of the number of water molecules, q, coordinated to europium(III) ions in solution from luminescence decay lifetimes. Inorg. Chim. Acta 2002, 340, 44–48. [Google Scholar] [CrossRef]
- Cotton, S.A. Scandium, Yttrium & the Lanthanides: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2011; p. eibc0195. ISBN 978-1-119-95143-8. [Google Scholar]
- Olivero, F.; Carniato, F.; Bisio, C.; Marchese, L. Promotion of Förster Resonance Energy Transfer in a Saponite Clay Containing Luminescent Polyhedral Oligomeric Silsesquioxane and Rhodamine Dye. Chem. Asian J. 2014, 9, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.; Carniato, F.; Devaux, A.; De Cola, L.; Marchese, L. Efficient Photoinduced Energy Transfer in a Newly Developed Hybrid SBA-15 Photonic Antenna. Chem. A Eur. J. 2012, 18, 15310–15315. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cheng, L.; Tang, H.; Wang, Z.; Sun, H.; Lu, L.; Mi, X.; Liu, Q.; Zhang, X. Wide range color tunability and efficient energy transfer of novel NaCaGd(WO4)3:Tb3+,Eu3+ phosphors with excellent thermal stability for pc-WLEDs. Inorg. Chem. Front. 2021, 8, 4517–4527. [Google Scholar] [CrossRef]
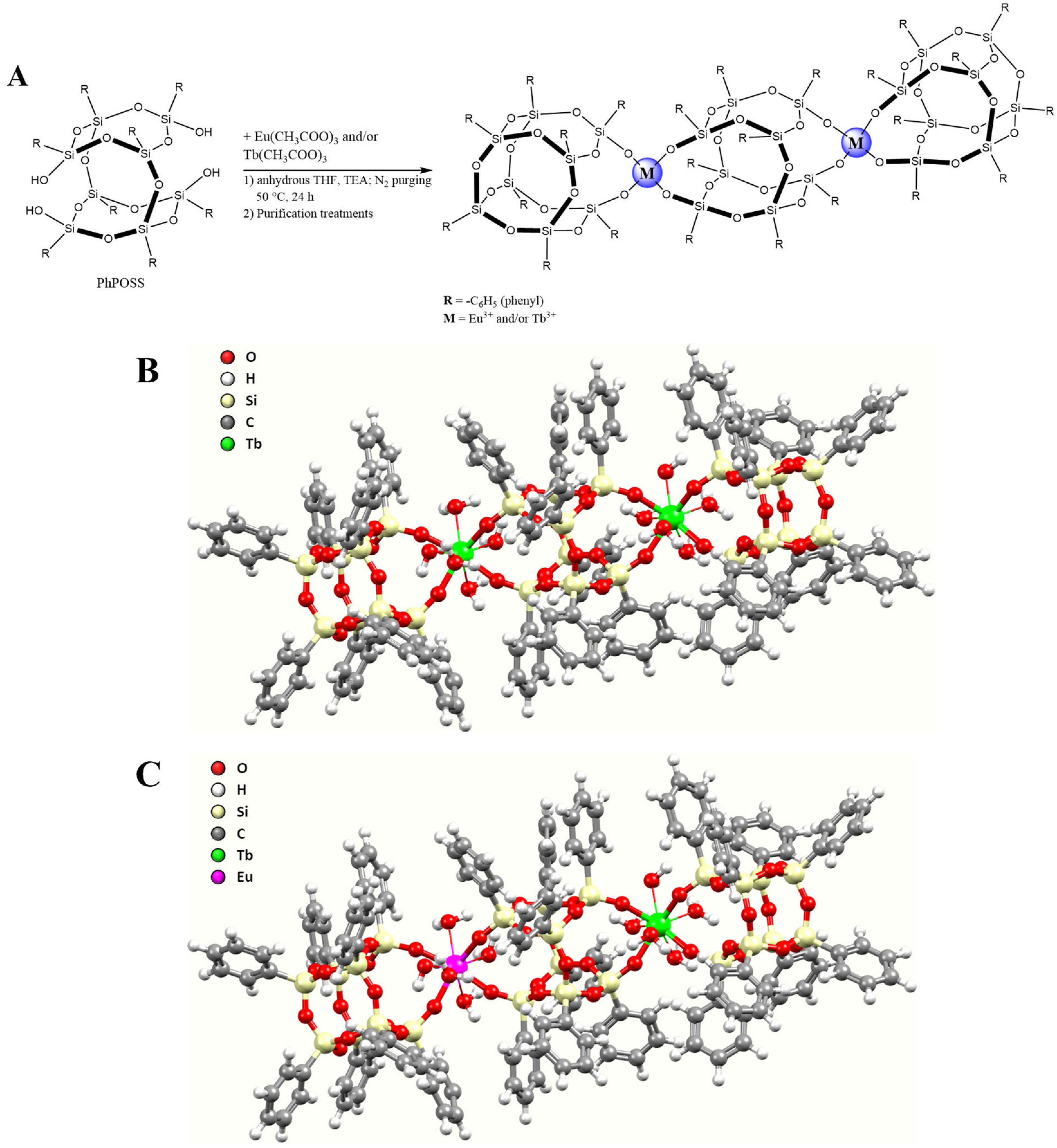

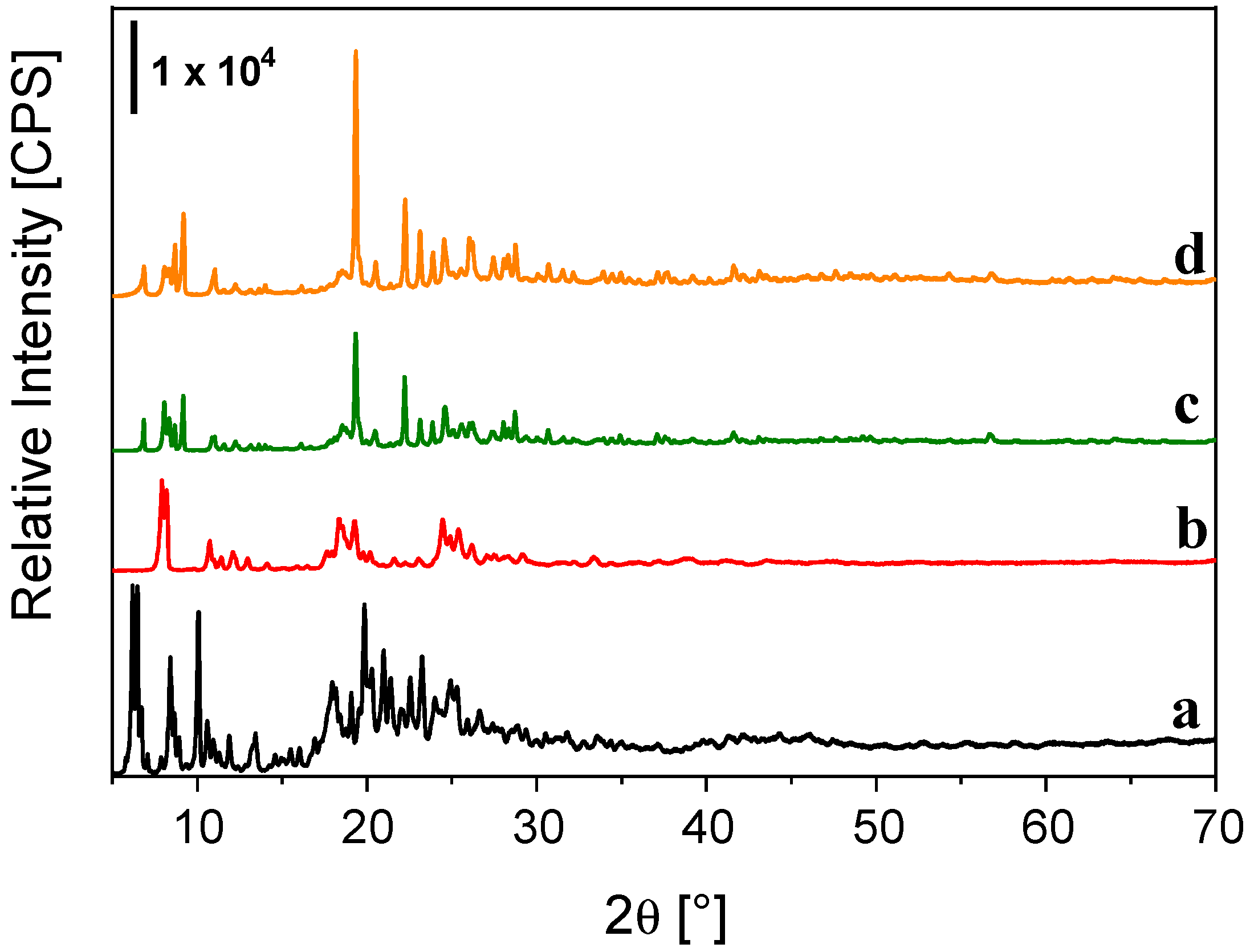

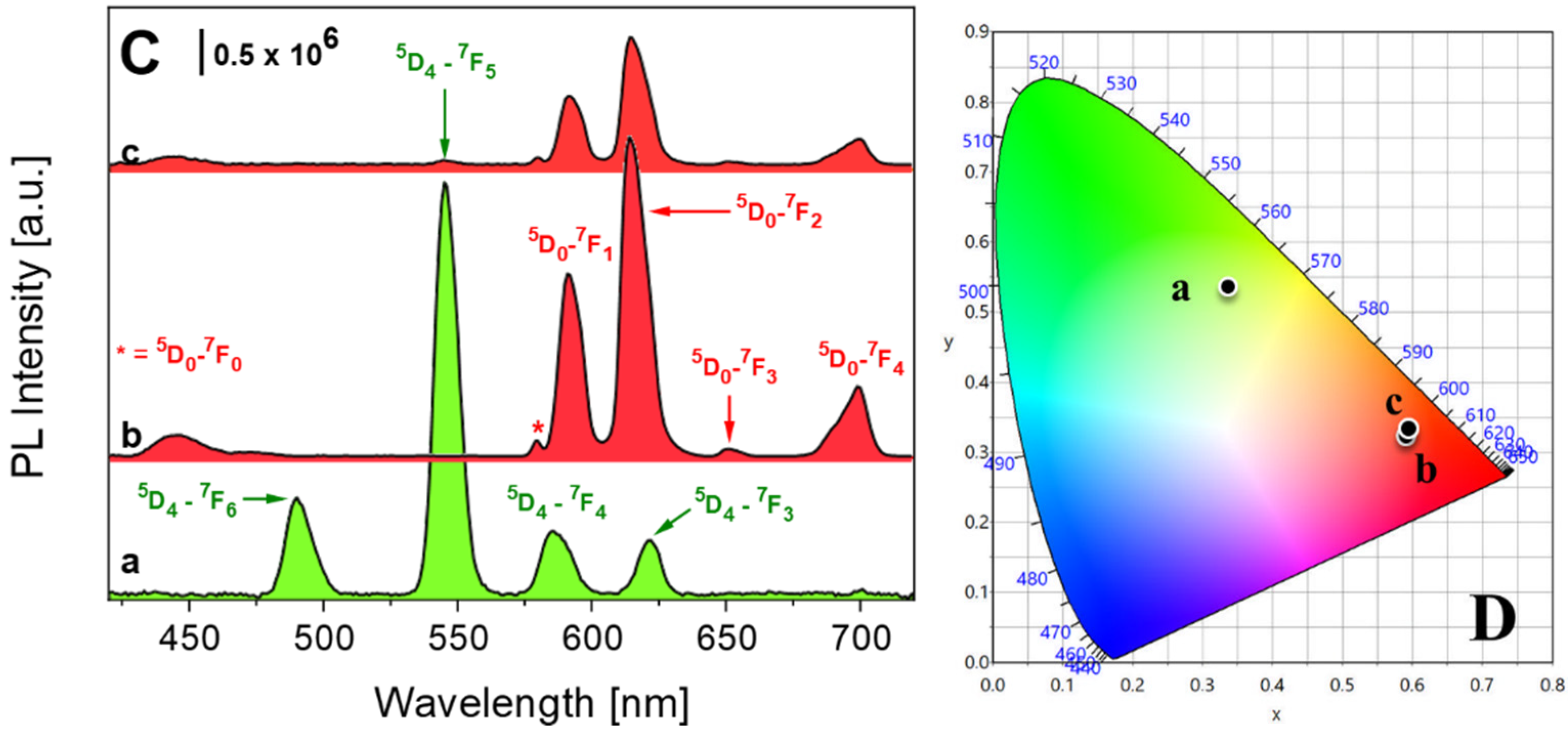
| Sample | Tb3+ Content [mmol/g] | Eu3+ Content [mmol/g] | C Content [mmol/g] |
|---|---|---|---|
| Tb-PhPOSS | 0.63 | - | 42.24 |
| TbEu-PhPOSS | 0.26 | 0.30 | 41.30 |
| τD [s] | τDA [s] | kEnT [s−1] | EEnT [%] |
|---|---|---|---|
| 3.88 × 10−4 | 1.50 × 10−4 | 4.09 × 103 | 61.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesi, S.; Bisio, C.; Carniato, F. Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions. Processes 2022, 10, 758. https://doi.org/10.3390/pr10040758
Marchesi S, Bisio C, Carniato F. Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions. Processes. 2022; 10(4):758. https://doi.org/10.3390/pr10040758
Chicago/Turabian StyleMarchesi, Stefano, Chiara Bisio, and Fabio Carniato. 2022. "Synthesis of Novel Luminescent Double-Decker Silsesquioxanes Based on Partially Condensed TetraSilanolPhenyl POSS and Tb3+/Eu3+ Lanthanide Ions" Processes 10, no. 4: 758. https://doi.org/10.3390/pr10040758






