Abstract
Deep eutectic solvents (DESs) have been used for the pretreatment of lignocellulose and showed selective dissolution for different lignocellulosic components. In this study, six new ternary DESs were synthesized on the basis of anhydrous oxalic acid DES by adding alcohol, acid, and deionized water, respectively, including choline chloride/anhydrous oxalic acid/ethylene glycol (ChCl-OA-EG), choline chloride/anhydrous oxalic acid/glycerol (ChCl-OA-G), choline chloride/anhydrous oxalic acid/lactic acid (ChCl-OA-LA), choline chloride/anhydrous oxalic acid/malonic acid (ChCl-OA-MA), choline chloride/anhydrous oxalic acid/10% H2O (v/v) (ChCl-OA + 10% H2O), and choline chloride/anhydrous oxalic acid/20% H2O (v/v) (ChCl-OA + 20% H2O). The lignin in bagasse was extracted and separated with these ternary DESs, and Fourier Transform Infrared (FTIR), Scanning electron microscope (SEM), X-ray diffraction (XRD), Two-dimensional Heteronuclear Single Quantum Coherence (2D HSQC), and Thermogravimetric analysis (TG) were used to characterize the molecular structures of lignin and cellulose. The results showed that under the mild reaction condition of cooking at 90 °C for 4 h, all six ternary DESs effectively dissolved hemicellulose in bagasse, the DES ChCl-OA-MA prepared with malonic acid significantly increased the removal of lignin (71.64%) by breaking the β-O-4′ ether bond of lignin molecules, and the crystallinity of cellulose was also significantly improved (67.65%).
1. Introduction
The storage capacity of fossil fuels is finite and their unfettered use has caused increasingly major environmental concerns, prompting researchers to look for alternate energy sources [1,2]. At the same time, a considerable quantity of agricultural wastes (straw, corn stover, bagasse, etc.) can only be consumed by incineration, which generates a slew of environmental issues while also making biomass reuse difficult, resulting in resource waste. As a raw material, lignocellulose biomass has inexpensive and renewable properties and has a wide range of research prospects [3,4,5]. Abbott [6] published a study in 2003, in which it was discovered that, combining choline chloride (ChCl) and urea, the mixture’s melting point was lower than the melting point of the separate components, leading to the development of the deep eutectic solvent (DES) idea. DES has been developed for more than ten years and offers the following advantages: environmental protection, low cost, easy synthesis, simple recovery and purification, and strong biocompatibility. DES pretreatment of biomass produces energy chemicals to replace fossil fuels—green, low-cost solvents that are more environmentally friendly than ionic liquids [7].
Abbott [8] and Francisco [9] found that the hydrogen bond formed between ChCl and cellulose can stabilize cellulose structure, making it difficult to dissolve cellulose while allowing lignin to dissolve. Currently, most DES research focuses on screening various hydrogen-bonded acceptors to various hydrogen-bonded donors. Meanwhile, due to the inherent problem of high viscosity in DES systems, it is frequently necessary to use a long pretreatment time, a high-temperature, or a multi-step combined pretreatment to obtain a high solubility of lignin when pretreating lignocellulose [10]. Zhang et al. [11] prepared DES using ChCl, monocarboxylic acids, dicarboxylic acids, and polyols, and they found that through treating corncob at 90 °C for 24 h, ChCl/anhydrous oxalic acid (OA) DES achieved the best delignification rate. Chen et al. [12] treated switchgrass at 120 °C for 1 h with ChCl/glycerol DES acidified with H2SO4 and the results showed that the lignin removal rate increased from 17.72% to 56.62% after a single pretreatment, demonstrating that an acidic environment can effectively improve DES pretreatment performance. Overall, the acidic hydrogen-bond donors of DES can efficiently separate cellulose, hemicellulose, and lignin from lignocellulose [13].
Poplar is now the most common raw material used in the pretreatment of DES systems, and good lignin removal results have been achieved. However, the solubilization of lignin in DESs varies with plant materials. Compared with poplar wood, straw, and other raw materials, bagasse is rich in resources and inexpensive and can be utilized as a biomass feedstock to produce high-value chemicals. Bagasse as a raw material has been used in a series of studies on DES pretreatment. However, high temperatures, prolonged treatment times, and multi-step combined treatments are often required to produce a high lignin yield from bagasse. By comparing the DES formed by ChCl with the hydrogen bond donors, including acids, alcohols, and urea, Li et al. [14] found that ChCl/oxalic acid DES removed 48.20% of lignin from bagasse after pretreatment at 100 °C for 4 h. Still, cellulose carbonation was observed in the treated bagasse. Chourasia et al. [15] pretreated bagasse at 80 °C for 12 h by synthesizing a DES and the results showed that ChCl/lactic acid DES (molar ratio 1:5) removed 81.6% of lignin. Ji et al. [16] removed 83.30% of the lignin by treating bagasse with ChCl, oxalic acid, and AlCl3·6H2O DES (molar ratio 1:1:0.2) in a microwave ultrasound at 110 °C for 20 min.
In this study, to obtain a pretreatment route for bagasse with a low temperature and high pretreatment efficiency, six ternary DESs were composed by adding the new components into the basic ChCl-OA DES and the effects of the pretreatment on the dissolution rate of lignin and its molecular structure, hemicellulose dissolution, and the cellulose crystallinity of bagasse were investigated. This provides a theoretical basis for finding green mild biomass refining routes and for bagasse reuse.
2. Materials and Methods
2.1. Material and Reagents
Bagasse (from Nanning, Guangxi Province, China) was packed into a slurry bag and washed with flowing tap water for 6 h to eliminate pollutants before drying for 48 h at 60 °C. Bagasse was crushed with a disintegrator and sieved with a 40–60 mesh screen after drying, followed by extraction with a benzene/ethanol (2:1, v/v) solution in a water bath at 100 °C for 6 h and four refluxes per hour. The product was washed three times with 100% ethanol after the extractions to ensure no residue of phenyl alcohol solution and placed in a blast drying oven at 60 °C for 12 h and sealed.
Choline chloride, lactic acid, anhydrous oxalic acid, malonic acid, ethylene glycol, glycerol, KBr, and Deuterated Dimethyl sulfoxide (DMSO) were purchased from the Shanghai Aladdin Chemical Co., Ltd. (Shanghai, China). All reagents were of analytical grade and used without further purification.
2.2. Compositional Analysis of Bagasse
The contents of cellulose and hemicellulose in raw and pretreated bagasse were determined using the National Renewable Energy Laboratory (NREL) procedure [17]. GB/T 2677.8-1994, GB/T 10337-2008, GB/T 742-2018, GB/T 2677.2-2011, and GB/T 2677.6-1994 were used to determine the total lignin, ash, moisture, and extract contents of bagasse [18].
2.3. Preparation of DESs
The binary DES was composed of ChCl and anhydrous oxalic acid (OA) (ChCl-OA) in a molar ratio of 1:1. Ternary components of ethylene glycol (EG), glycerol (G), lactic acid (LA), and malonic acid (MA) were added to the binary DES in a molar ratio of 1:1:1 to produce ternary DESs, respectively, and marked as ChCl-OA-EG, ChCl-OA-G, ChCl-OA-LA, and ChCl-OA-MA; ChCl-OA + 10% H2O and ChCl-OA + 20% H2O were created by combining 10% (v/v) and 20% (v/v) of deionized water with the ChCl-OA DES.
All compounds were dried in a vacuum drying oven for 48 h before use. The prepared ternary DESs were magnetically stirred in conical flasks at 80 °C for 6 h until the mixtures became clear without solids and then stored at room temperature (25 ± 2 °C).
2.4. Bagasse Pretreatment by DESs
Bagasse (2 g, oven-dried) was added into the conical flask with different DESs (40 g) and was stirred continuously at 90 °C for 4 h. After dissolution, 50 mL of ethanol was added to the conical flask, and the solid and liquid were separated by vacuum filtration using a G3 filter. The resulting solid residue was washed with anhydrous ethanol several times until the filtrate became colorless and then the G3 filter was dried at 105 °C for 6 h. The filtrate was evaporated at 65 °C with a rotary evaporation device to remove ethanol, then 4–5 volumes of distilled water were added to the filtrate. The supernatant was removed after 48 h precipitation, the lignin solid was obtained by centrifugation, and excess moisture was removed by freeze-drying for backup use. The lignin obtained using this method after DES pretreatment is called DES-Lignin (DES-L).
2.5. Materials Characterizations
2.5.1. Compositional Analysis
The chemical compositions of the original bagasse and DES-pretreated bagasse were determined using the NREL method [17]. In this way, 0.3 g bagasse (absolute dry weight basis) was hydrolyzed by 3 mL of 72% H2SO4 at 30 °C for 1 h and 84 mL of deionized water was added before holding in an autoclave at 121 °C for 45 min. After cooling and standing at the end of the reaction, the mixture was filtered with a G3 filter, and 5 mL of the filtrate was taken and adjusted to pH 2 with 8% NaOH solution and then diluted to 10 mL and transferred to a liquid phase vial using a syringe with 0.22 μm filter tip. The liquid phase vial was placed into a high-performance liquid chromatography (HPLC) Agilent 1260 series (Agilent, Palo Alto, CA, USA), column Sugar SH 1011 300 × 8 mm, (Showa Denko K.K., Tokyo, Japan) to determine the cellulose and hemicellulose content of the samples.
The lignin content was determined by the weight method. For this, 15 mL of 72% H2SO4 and 1 g of dried bagasse were mixed and hydrolyzed at 30 °C for 2 h and transferred to a conical flask. Then, 560 mL of deionized water was added and the mixture was boiled at 100 °C for 4 h in a water bath, during which water was added continuously to keep the volume constant, then the conical flask was cooled and kept for 6 h. Solid–liquid separation was performed using a G3 filter. (The G3 filter is an acid-resistant glass filtration apparatus for solid–liquid separation with an average microporous diameter of 15–40 microns.) After filtration, the G3 filter and the insoluble residue were dried in an oven at 105 °C for 6 h and weighed, then placed in a muffle furnace at 575 °C for 4 h, cooled at the end of the reaction, and weighed.
The equations for lignin, cellulose, and hemicellulose were calculated as follows.
where m1 is the weight of the acid-insoluble residue plus the crucible, mg; m2 is the weight of the ash plus the crucible, mg; m0 is the weight of the raw material, mg; Cglu and Cxyl are, respectively, the concentrations of glucose and xylose measured in the liquid phase, mg/mL; 87 is the total volume of the sample, mL; 0.90 and 0.88 are the correction coefficients of C-6 sugar and C-5 sugar, respectively; and 300 is the weight of the raw material, mg.
The solid recovery was calculated as a percentage of the solid recovered after pretreatment. The component loss was calculated as a percentage of the corresponding component reduced after pretreatment.
The solid recovery and loss of components were calculated using the equations below:
where M0 is the dry weight of the pretreated bagasse; M is the initial dry weight of the untreated bagasse; A is the content (%) of individual components in the pretreated bagasse; A0 is the content (%) of individual components in the untreated bagasse.
2.5.2. The Properties of DESs
The viscosity of seven DESs at different temperatures (20 °C, 40 °C, 60 °C, 80 °C, and 100 °C) were measured using a DV-III viscometer (Brookfield, Middleboro, MA, USA). The DESs were adjusted to 0.5 mol/L by adding deionized water [19] and the pH values were measured using a PHS-3C pH meter (INESA Instrument, Shanghai, China).
2.5.3. Scanning Electron Microscopy (SEM)
The morphology of the samples was analyzed using an SU 5000 field emission SEM (Hitachi, Tokyo, Japan). The samples were gold-coated and then test shots were taken. The accelerating voltage during imaging was 0.5 kV.
2.5.4. X-ray Diffraction (XRD)
An X-ray diffractometer (PANalytical B.V., Almelo, The Netherlands) was used to characterize the cellulose of the post-treatment bagasse at room temperature (25 ± 2 °C) with nickel-filtered Cu Kα1 radiation at 40 kV and 30 mA. At a scan rate of 12°/min, the scattering angle (2θ) ranged from 5 to 40°. The peak height method was used to calculate the crystallinity index (CrI) based on the diffraction intensity of crystalline and amorphous regions using the equation below [20,21]:
where I2 is the diffraction intensity at 2θ ≈ 22°, which corresponds to the (020) lattice diffraction, and I1 is the minimum intensity at 2θ ≈ 18°, which corresponds to the amorphous region.
2.5.5. Fourier Transform Infrared (FTIR) Spectroscopy
On a Nicolet IS50 FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), the chemical structure of cellulose and DES-L from sugarcane bagasse treated under different conditions and milled wood lignin (MWL) were scanned. All samples were mixed with KBr and pressed, dried at 105 °C for 12 h, and scanned in the spectral range of 4000–400 cm−1 with 32 scans per spectrum.
2.5.6. Two-Dimensional Heteronuclear Single Quantum Coherence Analysis of Lignin
Two-dimensional Heteronuclear Single Quantum Coherence nuclear magnetic resonance (2D HSQC NMR) analyses of MWL and DES-L were performed on the Bruker 500 Hz nuclear magnetic resonance spectrometer (Bruker, Karlsruhe, Germany) following the standard procedure for lignin analysis [22]. Lignin was analyzed using deuterated dimethyl sulfoxide (DMSO-d6, deuterated: 0.998) as solvent (50 mg sample per 0.5 mL DMSO-d6). Two-dimensional hydrocarbon correlation spectra of lignin samples were then acquired using the pulse program “hsqcetgp”, in which the spectral widths of 1H and 13C NMR were 2200 and 15,400 Hz, respectively. Of these, 1024 were sampled in the 1H dimension, the relaxation time was 1.5 s, and the number of samples was accumulated 32 times; the 13C dimension was sampled 256 times.
2.5.7. Thermogravimetric Analysis (TGA)
The thermal properties of the samples were analyzed using TA 25 (TA Instruments, New Castle, DE, USA). Approximately 5–10 mg samples were placed in aluminum crucibles with a temperature range from 40 to 800 °C in a nitrogen atmosphere and heated at 10 °C/min.
3. Results and Discussion
3.1. Chemical Compositions of Bagasse
The composition of sugarcane bagasse, which was used as a lignocellulosic raw material, is shown in Table 1. The cellulose, hemicellulose, and lignin contents were 44.68 ± 1.27%, 27.98 ± 0.43%, and 22.84 ± 0.29%, respectively. The contents of acid-soluble lignin and acid-insoluble lignin were 1.57 ± 0. 12% and 21.27 ± 0.17%, respectively. The composition obtained in this study differs slightly from those reported in previous studies, owing to the different origins of bagasse [10].

Table 1.
Content of bagasse biomass (%).
3.2. Solubility Analysis of Bagasse Components in Various DESs
A total of seven DESs were synthesized for bagasse pretreatment; the viscosities of the seven DESs at different temperatures are shown in Figure S1 and Table S1 and their pH values are shown in Table S2. The compositions of bagasse after various pretreatments are shown in Table 2. The results showed that the pretreatment efficiency of DES with the addition of alcohols and acids was improved. The addition of alcohols, acids, and deionized water were all effective in enhancing the flowability of DES and improving the pretreatment effect; more hemicellulose was leached from bagasse at low temperatures compared to ChCl-OA. The 80.01% solid recovery rate treated by ChCl-OA alone was reduced to 60.88% after ChCl-OA-MA pretreatment; this was mainly due to the decrease in the viscosity of DES at 90 °C after the addition of malonic acid (the viscosity decreased from 146.3 mpa·s to 75.3 mpa·s) and the enhancement of the acidity of the system (pH 2.33 to 2.22). The hemicellulose removal rate of all DESs increased from 46.45% to over 63.48%, indicating that they were all effective solvents for the separation of hemicellulose from bagasse.

Table 2.
Effect of various pretreatment solvents on the chemical composition of bagasse.
The lignin removal rate varied depending on the newly added DES components. As shown in Table 2, both alcohol and acid components from ChCl-OA-EG, ChCl-OA-G, ChCl-OA-LA, and ChCl-OA-MA effectively boosted hemicellulose and lignin removal rates, and pretreatment with ChCl-OA-MA resulted in the maximum removal of 71.64% of lignin and 75.82% of hemicellulose. This is because adding acids provides more protons that contribute to breaking chemical bonds in lignocellulose (e.g., ether bonds, ester bonds, and polysaccharide compounds) [23], facilitating the separation of lignin from lignocellulose. Furthermore, carboxyl groups (-COOH) in DESs can easily react with hydroxyl groups (-OH) in cellulose to form monoesters or cross-linked diesters, which prevents cellulose from being dissolved during acid hydrolysis [24]. The addition of alcohols improved the solubility of lignin because the free hydroxyl groups of alcohols can interact with the ether bonds in the lignin structure, thereby facilitating lignin removal. However, in the cases of combination with deionized water, the delignification effects of DES were weakened, although the fluidity of DES solvent was improved.
In conclusion, four DESs, including ChCl-OA-EG, ChCl-OA-G, ChCl-OA-LA, and ChCl-OA-MA, effectively enhanced the pretreatment efficiency of the ChCl-OA system, indicating that they can be used as pretreatment reagents for lignocellulose biomass. Whereas ChCl-OA + 10% H2O and ChCl-OA + 20% H2O improved hemicellulose removal and reduced solids recovery, the lignin removal rate decreased. The different viscosity and pH values of DESs after the addition of different new components resulted in differences in the effects of pretreatment bagasse.
3.3. Analysis of Surface Morphology of Bagasse
SEM was used to view the morphology of bagasse fibers before and after pretreatment with DESs. As shown in Figure 1a, the surface rupture of bagasse fiber was observed after the pretreatment of the other five DESs, except for ChCl-OA-G. Irregular lamellar cells appeared on the surface and some fibers were even broken.
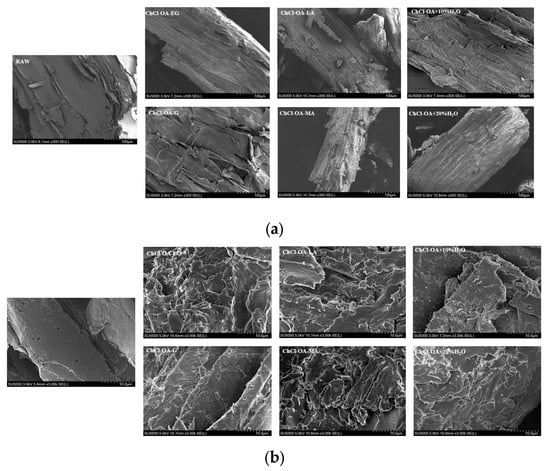
Figure 1.
The Scanning electron microscope (SEM) images of the original and pretreated bagasse at different magnifications: (a) 300×; (b) 3000×.
As shown in Figure 1b, DESs (ChCl-OA-LA and ChCl-OA-MA) formed by the addition of acids showed the most significant effect on the surface morphology of bagasse fiber and a number of cracks and pores appeared on the fiber surface, while after the pretreatment of bagasse with DESs (ChCl-OA-EG, ChCl-OA-G, ChCl-OA + 10% H2O, and ChCl-OA + 20% H2O) formed by the addition of alcohols and deionized water, only some holes existed on the surface and the fiber surface was less fractured.
3.4. Crystallinity Analysis
XRD was used to investigate bagasse’s crystalline structure and relative crystallinity (Figure 2). The samples before and after pretreatment showed absorption at 15.0°, 16.4°, 22.5°, and 34.5°, indicating the presence of cellulose I. The crystal planes of cellulose II are typically found at 2θ = 13°, 20.0° and around 22.0° [20,21]. The absorption spectrum had the main peak at 22.5°, indicating that cellulose I was the predominant crystal form.
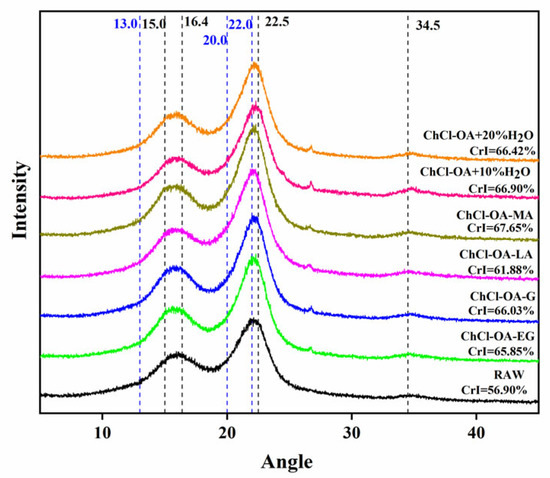
Figure 2.
The X-ray diffraction (XRD) patterns of bagasse cellulose treated with different deep eutectic solvents (DESs).
Compared with the feedstock crystallinity of 56.9%, pretreatment with ChCl-OA-EG and ChCl-OA-G increased the crystallinity of cellulose to 65.85% and 66.03%. After pretreatment with ChCl-OA-LA and ChCl-OA-MA, it increased to 61.88% and 67.65%. After pretreatment with ChCl-OA + 10% H2O and ChCl-OA + 20% H2O, it increased to 66.90% and 66.42%.
3.5. Chemical Composition Analysis of Bagasse
FTIR spectra of bagasse treated by various DESs are shown in Figure 3a. The signal attribution in the FTIR spectrum is shown in Table S3. The peak of spectra at 3300–3500 cm−1 represented the stretching vibrations of hydroxyl groups of lignin or carbohydrates (cellulose or hemicellulose). Lignin has absorption peaks of 1600, 1510, 1456, and 826 cm−1, where the skeletal vibrational absorption peaks of the benzene rings are positioned at 1600, 1510, and 1456 cm−1, and the vibrational absorption peaks of C-H linked with benzene rings are placed at 826 cm−1 [25]. The peak at 1600 cm−1 was weakened after ChCl-OA-MA pretreatment, indicating that it removed lignin from bagasse more effectively than other DESs. The absorption peak at 1245 cm−1 was weakened or disappeared after being pretreated with all the six DESs, indicating the breakage of ester bonds between lignin and hemicellulose. These weaker absorption peaks proved that DESs effectively removed lignin from bagasse. Figure 3b depicts photographs of the samples before and after pretreatment, these six DES-pretreated samples showed minor macroscopic changes and none of them showed carbonization of fibers.
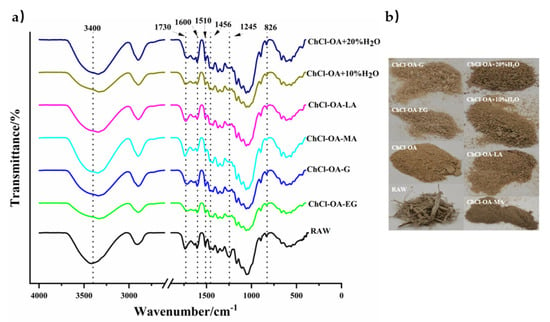
Figure 3.
The Fourier Transform Infrared (FTIR) spectra (a) and images (b) of bagasse treated with different DESs.
Figure 4 shows the FTIR spectra of the DES-L and MWL. The results showed that different DES treatments had no significant effect on DES-L structure. The characteristic bands of lignin aromatic ring vibrations were observed at 1595, 1512, 1462, and 1423 cm−1. The absorption vibration of the clove nucleus, guaiacyl group, the C-C/C-O/C=O vibration of the guaiacyl group, and the C-H vibration are attributed to the peaks at 1271 cm−1, 1224 cm−1, 1125 cm−1, and 1036/831 cm−1 near 1325 and 1220 cm−1 [26]. MWL had a peak at 1657 cm−1, which was derived from non-conjugated carbonyl and ester groups and conjugated carbonyl vibrations, while DES-L had no peak there. This finding suggested that DES treatment may have disrupted the conjugated structure of lignin by breaking the double bond at the α-position of the lignin molecule. It is worth noting that the peak at 918 cm−1 indicated the presence of a small amount of cellulose and the six DES-Ls had a weak peak here.
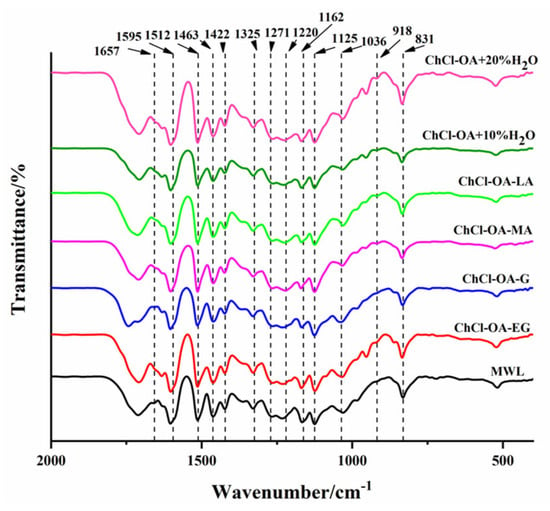
Figure 4.
FTIR spectra of the Lignin was extracted after DES treatment (DES−L) prepared with different DESs.
3.6. Two-Dimensional HSQC Analysis of Lignin
Two-dimensional HSQC analysis aimed to compare the structural changes in the MWL and DES-L. The side chain (δC/δH 50–90/2.5–6.0) and aromatic (δC/δH 90–150/5.0–8.0) regions of the 2D HSQC spectra of MWL and DES-L are shown in Figure 5. The main substructures observed in MWL and DES-L by 2D HSQC are shown in Figure 6. The 2D HSQC spectra of other DES-Ls are shown in Figure S2 and Table S4 shows the main cross-linking signal peak in lignin samples. The abundances of the principal lignin linkages and the S/G ratios were estimated based on the previous literature [27].
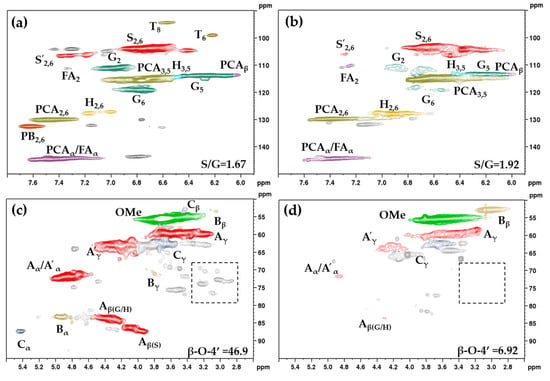
Figure 5.
Two-dimensional Heteronuclear Single Quantum Coherence nuclear magnetic resonance (2D HSQC NMR) spectra of milled wood lignin (MWL) (a,c) and DES-L from ChCl-OA-MA (b,d).
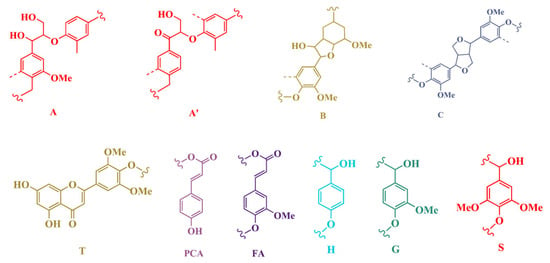
Figure 6.
Main substructures observed in MWL and DES-Ls by 2D HSQC: (A) β-O-4′ ether linkages, (A’) β-O-4′ ether linkages with, acetylated at γ-carbon, (B) resinol, (C) phenyl-coumaran, (PCA) p-coumarate acid, (FA) ferulic acid, (T) flavone tricin, (H) p-hydroxyphenyl units, (G) guaiacyl units, (S) syringyl units.
The aromatic regions of lignin (δC/δH 90–150/5.8–7.8) are shown in Figure 5a,b. The signal peaks in the spectra corresponded to different lignin units, including p-hydroxyphenyl units (H), guaiacyl units (G), syringyl units (S), coumaric acid (PCA), and ferulic acid (FA) [28]. According to the signal intensity, the MWL of bagasse mainly consisted of S-type units, G-type units, and a few H-type units. The increased content of S-type units was observed only in the lignins from pretreatments with ChCl-OA-G, ChCl-OA-LA, and ChCl-OA-MA, and the relative content of G-type units in all six DES-Ls decreased, indicating that the DESs mainly destroyed G-type units. The ratios of S/G in all DES-Ls (1.70–1.92) were higher than that in MWL (1.67).
Two special and typical flavone tricin (T) signal peaks were found in the 2D HSQC of the bagasse lignin sample [29]. However, the flavone tricin structure was not detected in DES-Ls pretreated with ChCl-OA-MA and ChCl-OA-LA, indicating that the above pretreatments disrupted the flavone tricin structure of the lignin molecules.
The side chain regions of lignin (δC/δH 50–95/2.7–5.5) are shown in Figure 5c,d which include β-aromatic ether linkage (β-O-4′, A), resinol structure (β-β′, B), and phenyl-coumaran structure (β-5′, C). It was calculated that the content of the β-O-4′ ether bond in MWL was 46.9/100 Ar, which was higher than that of all DES-Ls (6.92–28.20/100 Ar), and this suggested that DES mainly disrupted the β-O-4′ ether bond of lignin molecules. In comparison to the content of β-β′ bonds (3.30/100 Ar) and β-5′ bonds (1.50/100 Ar) in MWL, among the six DES-Ls, only ChCl-OA-Gs were detected with β-β′ bonds (2.47/100 Ar) and β-5′ bonds (0.07/100 Ar), owing to their trace amounts after pretreatments.
In addition, except for ChCl-OA-G-treated DES-L, the other five DES-Ls showed no signal of carbohydrates in the side chain region (boxed areas in Figure 5c,d), indicating the high purity of DES-Ls.
3.7. Thermal Stability
The pyrolysis of lignocellulose is divided into four stages, with water degraded at 40–100 °C, hemicellulose at 215–310 °C, cellulose at 310–390 °C, and lignin at 390–515 °C [30]. The thermogravimetric (TG) analysis of bagasse treated with different DESs is shown in Table S5. Figure 7 shows the TG and differential thermogravimetric (DTG) curves of bagasse under different treatment conditions. As shown in Figure 7, the degradation process of bagasse raw materials includes three stages: hemicellulose, cellulose, and lignin. However, the samples treated with DESs have no weight loss stage with lignin, indicating that lignin was removed through pretreatments. Moreover, within the second decomposition temperature range, the weight losses of all six DES samples were less than that of the raw material, indicating that substantial hemicellulose had been successfully separated by pretreatment. According to the above results, the crystallinities of the samples treated with DES were significantly higher than that of the raw material, which is manifested in the larger decomposition amount of cellulose at a higher temperature, especially ChCl-OA-MA.
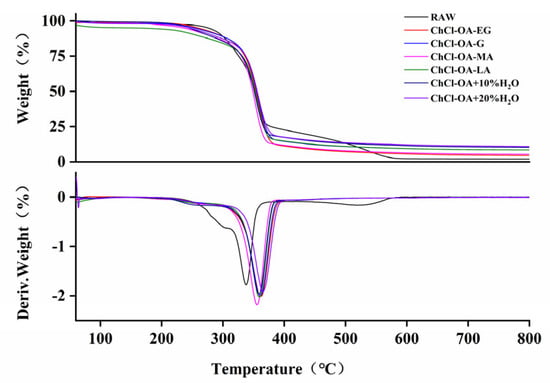
Figure 7.
Thermogravimetric (TG)/Differential Thermogravimetric (DTG) curves of bagasse treated with different DESs.
The pyrolysis stage of lignin has three segments. The first stage occurs at 80–160 °C, which is mainly ascribed to the dehydration and degradation of low-molecular weight lignin; the second stage is the pyrolysis of lignin phenylpropane polymer at 160–470 °C; the third stage is the lignin carbonization stage at 470–600 °C [31]. The TG analysis of different DES-Ls is shown in Table S6. Figure 8 shows the TG and DTG curves of different DES-Ls. As shown in Figure 8, the thermal degradation behaviors of the six DES-Ls are consistent. In addition, the thermal stability of MWL is superior to DES-Ls due to its undamaged structure. In the second stage, all the six DES-Ls have two inflection point temperatures (T1 and T2), while T2’ of MWL occurs in the third stage (470–600 °C) at higher temperature of lignin degradation. The T1 and T2 of DES-Ls correspond to a large number of cleavages of lignin side chains and depolymerization of phenylpropane units, respectively. The residue reacts into coke with the increase in temperature, and the mass of MWL and DES-L remain constant until 730 °C and 600 °C, respectively.
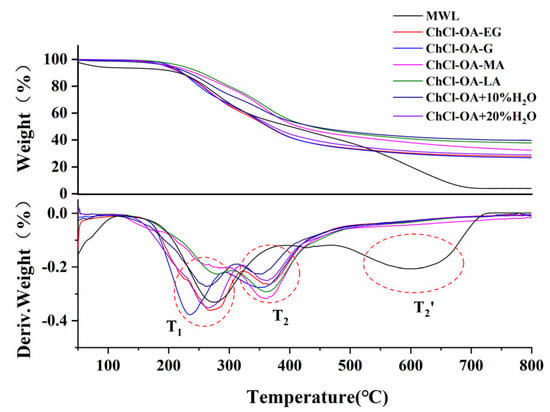
Figure 8.
TG/DTG curves of different DES−Ls.
According to the results of 2D HSQC, a large number of β-O-4′ ether bonds in lignin were broken in the process of ChCl-OA-MA treatment, which resulted in less weight loss at 200–300 °C and the maximum weight loss at 300–400 °C for its corresponding DES-L, while lignin from ChCl-OA-G treatment, with only a small amount of β-O-4′ ether bond fracture, still had a maximum weight loss at 200–300 °C.
4. Conclusions
In this study, six ternary DESs were synthesized on the base of ChCl-OA by adding various third components. Except for the water-added ternary DESs, the other four effectively dissolved lignin from bagasse at low temperatures. The ChCl-OA-MA formed by the addition of malonic acid achieved the maximum lignin removal rate of up to 71.64% through pretreatment at 90 °C for 4 h and the β-O-4′ ether bond of G-type units in the bagasse lignin molecule was effectively destroyed. On the other hand, the ternary DES system with deionized water did not promote lignin removal and instead interfered with it. All six DESs effectively separated hemicellulose from bagasse with extraction rates over 63.48%, and the crystallinity of cellulose from pretreated bagasse was elevated to over 61.88%.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10040778/s1: Figure S1: Effect of temperature on the viscosity of different ternary DES, Figure S2: 2D-HSQC NMR spectra of the other DES-L from bagasse, Table S1: The viscosity of different ternary DES at 90 °C, Table S2: The pH value of different ternary DES, Table S3: Band assignments for FTIR spectra, Table S4: Assignments of 13C-1H cross-peaks in 2D HSQC spectra of the lignin fractions, Table S5: The TG analysis of bagasse treated with different DESs, Table S6: The TG analysis of different DES-Ls.
Author Contributions
Conceptualization, Y.Y. and L.Z.; methodology, Y.Y.; software, Y.Y.; validation, Y.Y. and L.Z.; formal analysis, Y.Y.; investigation, Y.Y.; resources, L.Z., J.R. and B.H.; data curation, Y.Y.; writing—original draft preparation, Y.Y.; writing—review and editing, L.Z.; visualization, Y.Y.; supervision, L.Z.; project administration, L.Z., J.R. and B.H.; funding acquisition, L.Z., J.R. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Area R&D Program of Guangdong Province (2020B0101070001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Raghavi, S.; Sindhu, R.; Binod, P.; Gnansounou, E.; Pandey, A. Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour. Technol. 2016, 199, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Hamilton, S.K.; Barham, B.L.; Dale, B.E.; Izaurralde, R.C.; Jackson, R.D.; Landis, D.A.; Swinton, S.M.; Thelen, K.D.; Tiedje, J.M. Cellulosic biofuel contributions to a sustainable energy future: Choices and outcomes. Science 2017, 356, eaal2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.-J.; Wen, J.-L.; Mei, Q.-Q.; Chen, X.; Sun, D.; Yuan, T.-Q.; Sun, R.-C. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Dominguez de Maria, P.; Maugeri, Z. Ionic liquids in biotransformations: From proof-of-concept to emerging deep-eutectic-solvents. Curr. Opin. Chem. Biol. 2011, 15, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. Cationic functiona lisation of cellulose using a choline based ionic liquid analogue. Green Chem. 2006, 8, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. Engl. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Arni, S.A. Extraction and isolation methods for lignin separation from sugarcane bagasse: A review. Ind. Crops Prod. 2018, 115, 330–339. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Reznicek, W.D.; Wan, C. Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Chourasia, V.R.; Pandey, A.; Pant, K.K.; Henry, R.J. Improving enzymatic digestibility of sugarcane bagasse from different varieties of sugarcane using deep eutectic solvent pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient cleavage of strong hydrogen bonds in sugarcane bagasse by ternary acidic deep eutectic solvent and ultrasonication to facile fabrication of cellulose nanofibers. Cellulose 2021, 28, 6159–6182. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- New, E.K.; Wu, T.Y.; Tien Loong Lee, C.B.; Poon, Z.Y.; Loow, Y.-L.; Wei Foo, L.Y.; Procentese, A.; Siow, L.F.; Teoh, W.H.; Nik Daud, N.N.; et al. Potential use of pure and diluted choline chloride-based deep eutectic solvent in delignification of oil palm fronds. Process Saf. Environ. Prot. 2019, 123, 190–198. [Google Scholar] [CrossRef]
- Skulcova, A.; Russ, A.; Jablonsky, M.; Sima, J. The pH behavior of seventeen deep eutectic solvents. BioResources 2018, 13, 5042–5051. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A., Jr.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Sanchez, P.B.; Tsubaki, S.; Padua, A.A.H.; Wada, Y. Kinetic analysis of microwave-enhanced cellulose dissolution in ionic solvents. Phys. Chem. Chem. Phys. 2020, 22, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ralph, J.; Akiyama, T. Solution-state 2D NMR of Ball-milled Plant Cell Wall Gels in DMSO-d6. BioEnergy Res. 2008, 1, 56–66. [Google Scholar] [CrossRef]
- Weng, L.; Toner, M. Janus-faced role of water in defining nanostructure of choline chloride/glycerol deep eutectic solvent. Phys. Chem. Chem. Phys. 2018, 20, 22455–22462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Chandra, R.P.; Zhang, X.; Saddler, J.N. Non-productive celluase binding onto deep eutectic solvent (DES) extracted lignin from willow and corn stover with inhibitory effects on enzymatic hydrolysis of cellulose. Carbohydr. Polym. 2020, 250, 116956. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, L.; Wang, Z.; Laskar, D.D.; Swita, M.S.; Cort, J.R.; Yang, B. Characterization of lignin derived from water-only and dilute acid flowthrough pretreatment of poplar wood at elevated temperatures. Biotechnol. Biofuels 2015, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, L.; Yu, J.; Lu, Y.; Jiang, B.; Fan, Y.; Wang, Z. High-purity lignin isolated from poplar wood meal through dissolving treatment with deep eutectic solvents. R. Soc Open Sci. 2019, 6, 181757. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.Y.; Gao, X.; Peng, X.P.; Gao, Y.F.; Liu, J.; Wen, J.L.; Yuan, T.Q. Microwave-assisted deep eutectic solvents (DES) pretreatment of control and transgenic poplars for boosting the lignin valorization and cellulose bioconversion. Ind. Crops Prod. 2021, 164, 113415. [Google Scholar] [CrossRef]
- Rencoret, J.; Prinsen, P.; Gutierrez, A.; Martinez, A.T.; Del Rio, J.C. Isolation and structural characterization of the milled wood lignin, dioxane lignin, and cellulolytic lignin preparations from brewer’s spent grain. J. Agric. Food Chem. 2015, 63, 603–613. [Google Scholar] [CrossRef]
- del Rio, J.C.; Rencoret, J.; Prinsen, P.; Martinez, A.T.; Ralph, J.; Gutierrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Yang, H.; Rong, Y.; Chen, H.; Dong, H.L.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).