Abstract
Saline water necessarily contained in crude oil forms complex and stable water-in-oil (w/o) emulsions with oil. Due to the negative impact of this emulsion on the oil’s transportation and refining, special materials are added to help break the emulsion and separate water. Herein, a comparative study of the demulsifying ability concerning w/o emulsion of the original and freshly milled quartz (FMQ) particles isolated from river sand was carried out. The effect of quartz with a mesh size of 75 μm on reducing emulsion stability was investigated using rheological measurements, interfacial tension measurements, demulsification tests, as well as routine methods for characterizing solid and liquid materials. With the addition of 3 wt% FMQ, 97% demulsification efficiency was achieved after 100 min of settling, against 140 min for the original quartz. The role of milling quartz is to increase the ability of water to adhere and thus locally increase the pH value; this results in a reduction in the stability of the emulsion and its destruction. The prolonging effect of quartz milling lasted about 2.5–3.0 h, after which the demulsifying ability of milled quartz became comparable to that of the starting material.
1. Introduction
Crude oil contains saline water. Being dispersed in a continuous phase of the crude oil, droplets of water form complex and extremely stable water-in-oil (w/o) emulsions [1,2,3]. In addition to becoming bound in emulsions, saline water becomes fixed in waxes, solids, asphaltenes, resins, and other components natively existing in crude oil and stabilizing the w/o emulsions [4,5,6]. Emulsions in crude oil are very undesired in the petroleum sector, as they cause significant problems during transportation and processing, such as pipeline corrosion, restricted flow line pressure, and poisoning of downstream refinery catalysts, among other things [7,8]. For these reasons, before the transportation of crude oil to the refining plant, the content of emulsified water must be removed beyond a certain extent, generally to less than 0.5 wt% [9].
Breaking down w/o emulsions is challenging due to their high stability. Various physical and chemical methods can be applied to achieve the demulsification of crude oils. Physical methods, such as ultrasound-assisted demulsifying [10,11,12], microwave irradiation [13,14], microfiltration through membranes [15], thermal heating [16], solar demulsification [17,18], and electrodemulsification [19,20], despite their benefits, are high energy-consuming; this limits the application of physical methods for crude oil demulsifying. Chemical techniques involve introducing small amounts of additives into crude oil; these additives, called demulsifiers, destroy the interfacial films surrounding water droplets, thereby promoting the association of water droplets into globules [21,22,23,24]. The breakdown of the emulsion continues with the phase separation; the water and oil phases are spatially positioned at the top or bottom of the container, depending on the density of the oil relative to the water. Polymeric surfactants [25,26,27,28], ionic liquids [29,30,31,32], and magnetic [33,34,35,36] and non-magnetic [37,38,39,40] nanoparticles have been suggested as effective chemical demulsifiers. At the same time, the use of chemical demulsifiers often has disadvantages, including a high cost. Further, many demulsifiers cannot be reused.
For developing low-cost demulsifiers, naturally occurring materials could be a good choice. The literature describes several examples of the use of natural materials for crude oil demulsifying. Adewumni and co-workers investigated the efficiency of palm oil fuel ash (POFA), a byproduct of the palm oil industry, for this purpose [41]. Adding 3 wt% of the POFA to the w/o emulsion, followed by agitation, led to the recovery of more than 90% water, which is comparable to the efficiency of commercial demulsifiers. Eva Knapic proposed applying natural extracts derived from Saponaria officianalis for the recovery of crude oil from produced water [42]. This «green» natural demulsifier at a concentration of 0.5 g/L allowed the recovery of up to 95% of oil from the water. The natural rock alginite was tested as a crude oil demulsifier by Hippman et al. [43]. The addition of 0.5 wt% of alginite to the emulsion led to its splitting and, after two hours, the water content of the oil was below 1%.
One of the most abundant and relatively inexpensive natural materials is quartz (SiO2). Recently, Adewumni and colleagues for the first time investigated the possibility of applying quartz particles from sand as a demulsifier of crude oil [44]. It was demonstrated that a moderate concentration of quartz (≤5 wt%) with a minimum particle size of 75 μm provides a demulsification efficiency of 98–99%. Adewumni et al. went on to investigate the effect of several monovalent and divalent salts in the emulsion, as well as pH, on the efficiency of crude oil demulsifying using quartz particles [45].
It is generally accepted that milling quartz noticeably changes its surface structure and energy [46,47]. In particular, the adsorption properties of the surface of freshly ground quartz change in comparison with the original mineral [48]. Since the surface properties of the solid demulsifier have a significant effect on the breaking of the emulsion, we hypothesized differences in the demulsifying ability of the original and freshly milled quartz. In addition, the understanding of the mechanism of the quartz particles’ demulsifying ability toward w/o emulsions was expanded. To the best of the authors’ knowledge, this is the first study on the demulsification of crude oil using freshly milled quartz.
2. Materials and Methods
2.1. Materials
A crude oil sample originating from the Kumkol oilfield was used for the experiments. The crude oil characterization is presented in Table 1.

Table 1.
Crude oil characterization.
The sand sample was collected on the banks of the Badam River (the South region of Kazakhstan).
2.2. Sand Sample Milling
The sand sample was milled for 10 min using a laboratory ball mill (IBMT-30, HT Machinery, Taipei, Taiwan) equipped with a 0.5 L ceramic drum and ceramic balls of 10 mm in diameter, at a ball-to-powder ratio of 8. Freshly milled sand was sieved and the 75 µm fraction was used in demulsification tests. In comparison, a fraction of raw (unmilled) sand of the same size was used to demulsify the crude oil.
2.3. Preparation of the Water-in-Oil Emulsion
The crude oil sample was centrifuged to remove solid impurities and pre-heated at 75 °C. Then, 13 mL of NaCl aqueous solution (105 g/L) was mixed with 150 mL of the crude oil using a mechanical stirrer (Mslos 18-23, Guangzhou Medsinglong Medical Equipment Co., Guangzhou, China) at 2500 rpm for 5 min. The resulting w/o emulsion contained 8.9 vol% water, according to the Dean–Stark technique [49]. The pH of the w/o emulsions was adjusted by using NaOH.
2.4. Demulsification Tests
A bottle test [50] was applied for the demulsification tests. A volume of 100 mL of the emulsion was transferred to graduated glass bottles with a volume of 200 mL. The pre-determined amounts of the sand (0.84 g (1 wt%), 2.53 g (3 wt%), or 4.22 g (5 wt%)) were added to the emulsion as the demulsifier. The bottle was shaken for 7–9 min to thoroughly mix the sand and the w/o emulsion, followed by transferring to the incubator, which was maintained at a temperature of 85 °C. The position of the w/o interface was used for monitoring the process of phases separation. The blank (without sand) sample of the emulsion was shaken for the same period before it was placed in the incubator. Demulsifier efficiency was measured by the percentage of water separated from the oil, which is calculated as
where (mL) is the volume of separated water, and 8.9 mL is the volume of the water in the emulsion before settling.
Three repetitions of each experiment were performed until reproducibility ±1% was achieved.
2.5. Rheological Measurements
The viscosity of the w/o emulsion (blank and with sand addition) was measured by using the viscometer (Rheotec RUV 2, PSL-Rheotek, Essex, UK) at a temperature of 30 ± 1 °C and a share rate of 1 s−1.
2.6. Interfacial Tension Measurements
Interfacial tension values for the interface of the oil and aqueous solution, as well as the oil and sand–aqueous solution, as a function of the concentrations of demulsifier or pH, were measured using a tensiometer (K20, Kruss, Hamburg, Germany) according to the Wilhelmy plate method. All the experiments were performed at 25 ± 1 °C and repeated thrice to assess the reproducibility of 0.1 Pa·s.
2.7. Characterization of Sand and Emulsion
The composition of the crude oil sample was determined by using high-performance liquid chromatography (Varian Pro). The droplet size distribution (DSD) in an as-prepared w/o emulsion was measured by the light-scattering technique (Mastersizer 2000, Malvern Panalytical, Malvern, UK). The emulsion’s structure before and after demulsification was analyzed using an optical microscope Levenhuk D 70L. The pH of the w/o emulsion was measured using a pH meter/ionomer (ITAN, Tomanalyt, Tomsk, Russia) equipped with a glass redox combination electrode (Ag/AgCl was a reference electrode and a platinum rod tip was a working electrode). The XRD pattern of the sand sample was recorded by a diffractometer (D8 Advance, Bruker, Ettlingen, Germany) with CuKa (40 kV, 40 mA) radiation in a 2 theta range of 0–70. X-ray fluorescence analysis was performed using a portable analyzer EDX POCKET. The specific surface area of the sand samples was determined using the nitrogen adsorption technique (NOVA 1200e, Quantachrome Instruments, Boynton Beach, FL, USA).
3. Results and Discussion
3.1. Characterization of the Sand Sample and w/o Emulsion
The original sand sample was fine grains with particle sizes of about 70–840 μm (Figure 1).
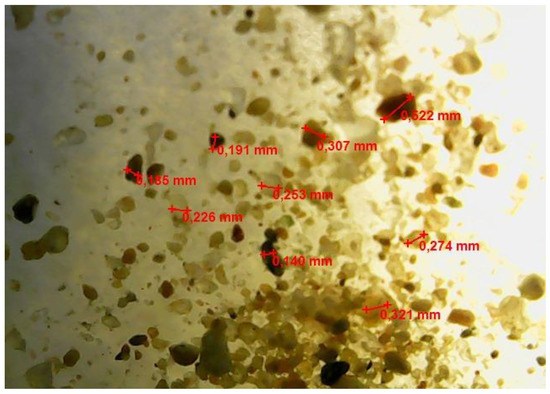
Figure 1.
Original sand sample.
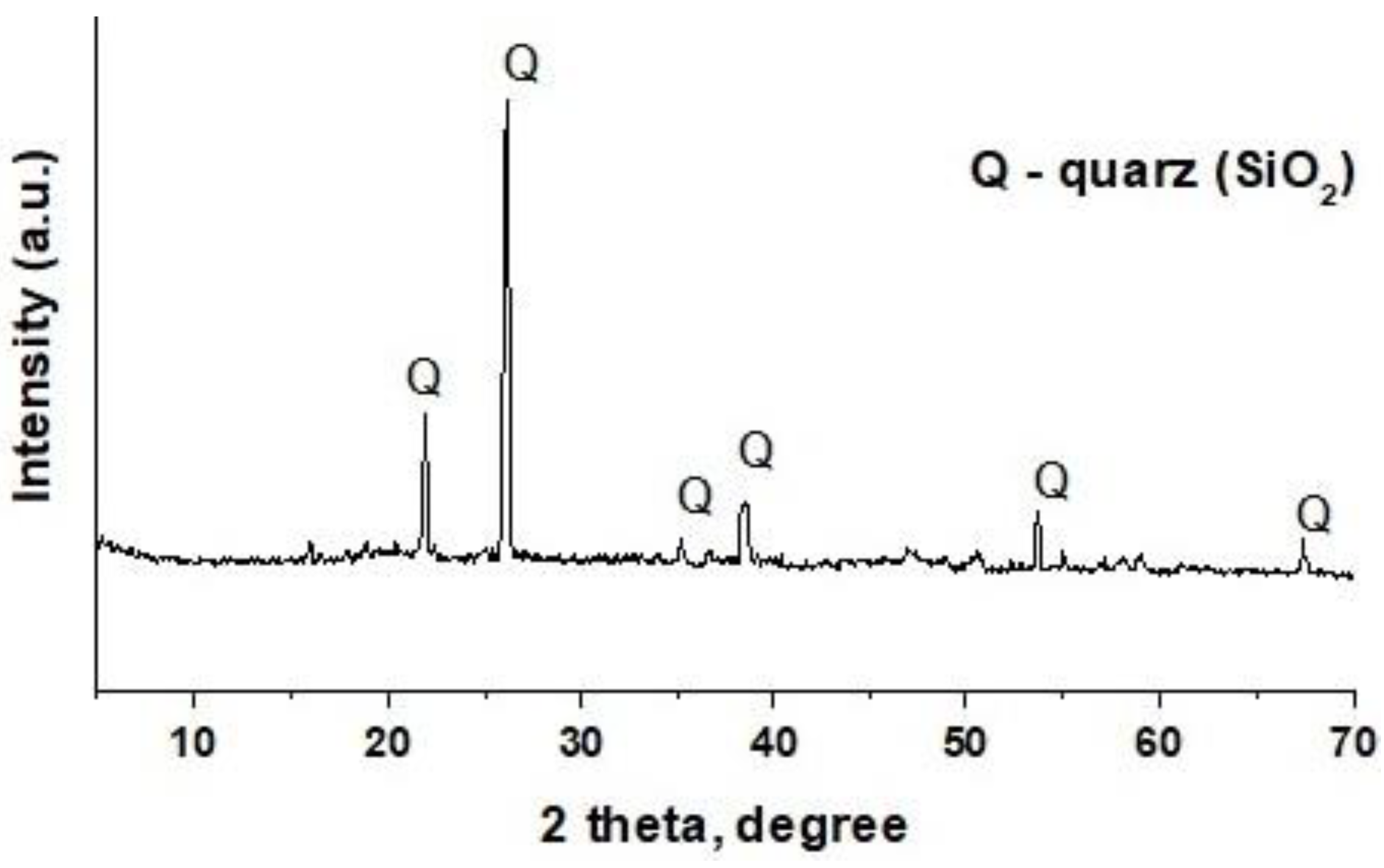
The sand is represented by particles of both almost transparent and dark colours. Only mostly transparent sand particles were manually selected for further research. X-ray fluorescence analysis showed the following elemental composition of sand, wt%: Si 45.8; Ca 0.4; and Fe 0.1. XRD analysis revealed the presence of only a quartz (SiO2) phase in the sand (Figure 2).
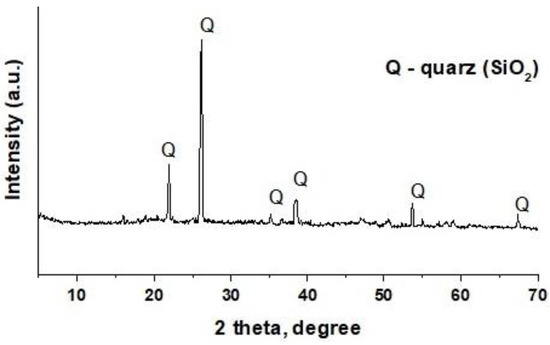
Figure 2.
XRD pattern of the sand sample.
Thus, the sand sample used was almost entirely quartz particles. As a result, the utilised demulsifiers are subsequently referred to as quartz particles in this article.
After milling the selected portion of sand, particles of a more or less uniform shape were obtained (Figure 3).

Figure 3.
Freshly milled sand sample.
The specific surface areas of the 75 μm fractions for both the original and milled sand were 1.2 m2/g.
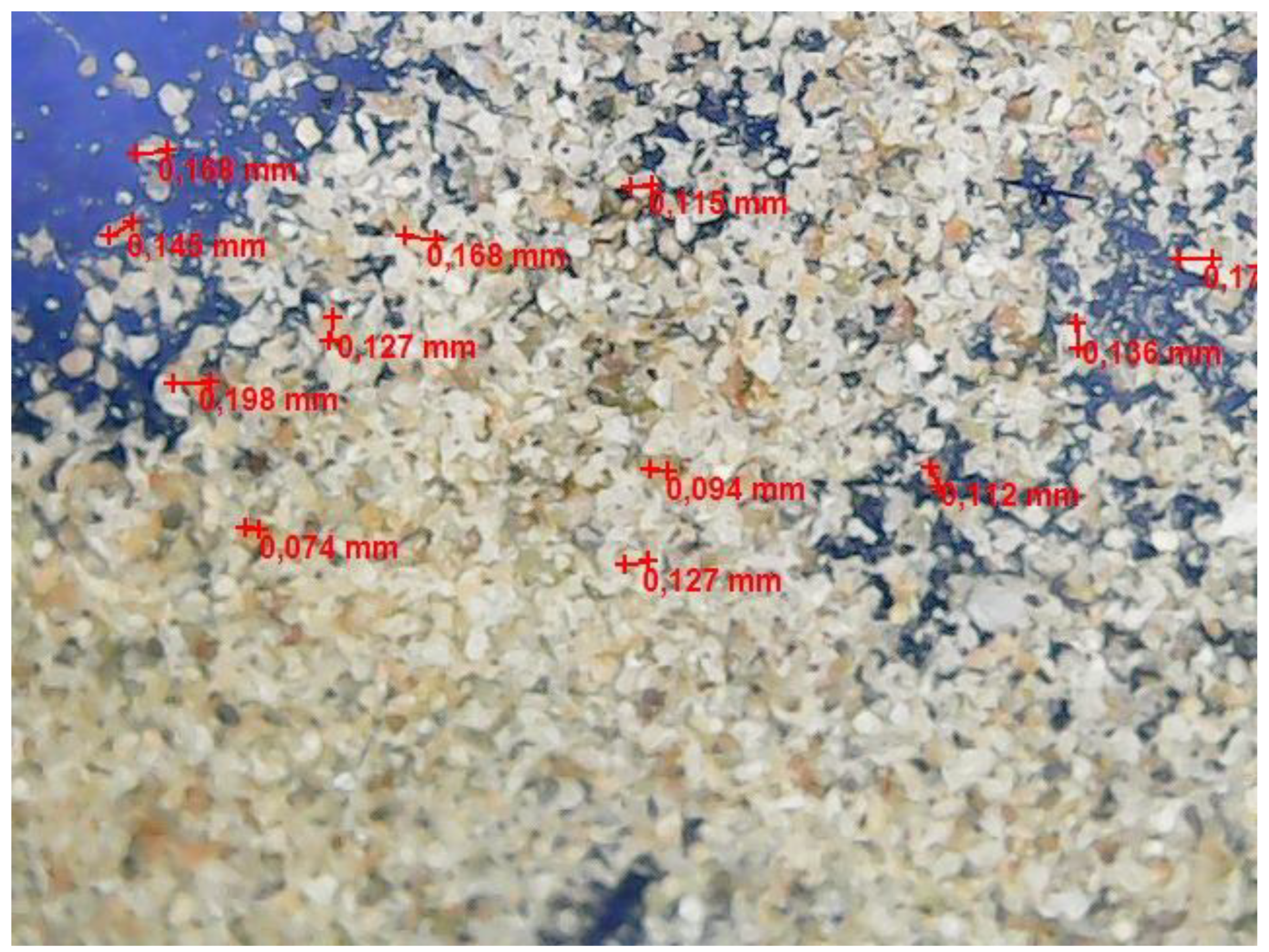
A micrograph of a freshly prepared w/o emulsion is shown in Figure 4.
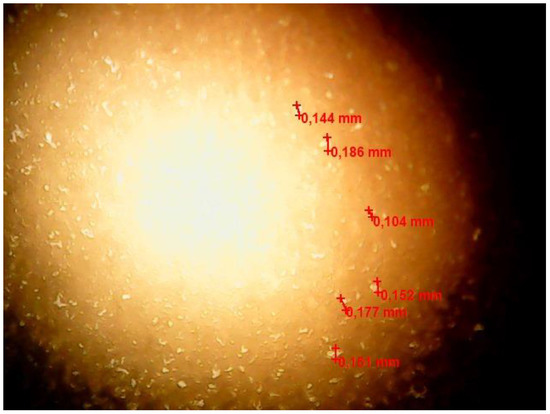
Figure 4.
Optical microscopic image of a freshly prepared w/o emulsion sample.
Figure 4 demonstrates water droplets with a size of about 0.100–0.190 mm, more or less evenly distributed in the volume of the emulsion.
3.2. Demulsification Performance of the Untreated and Freshly Milled Quartz Particles
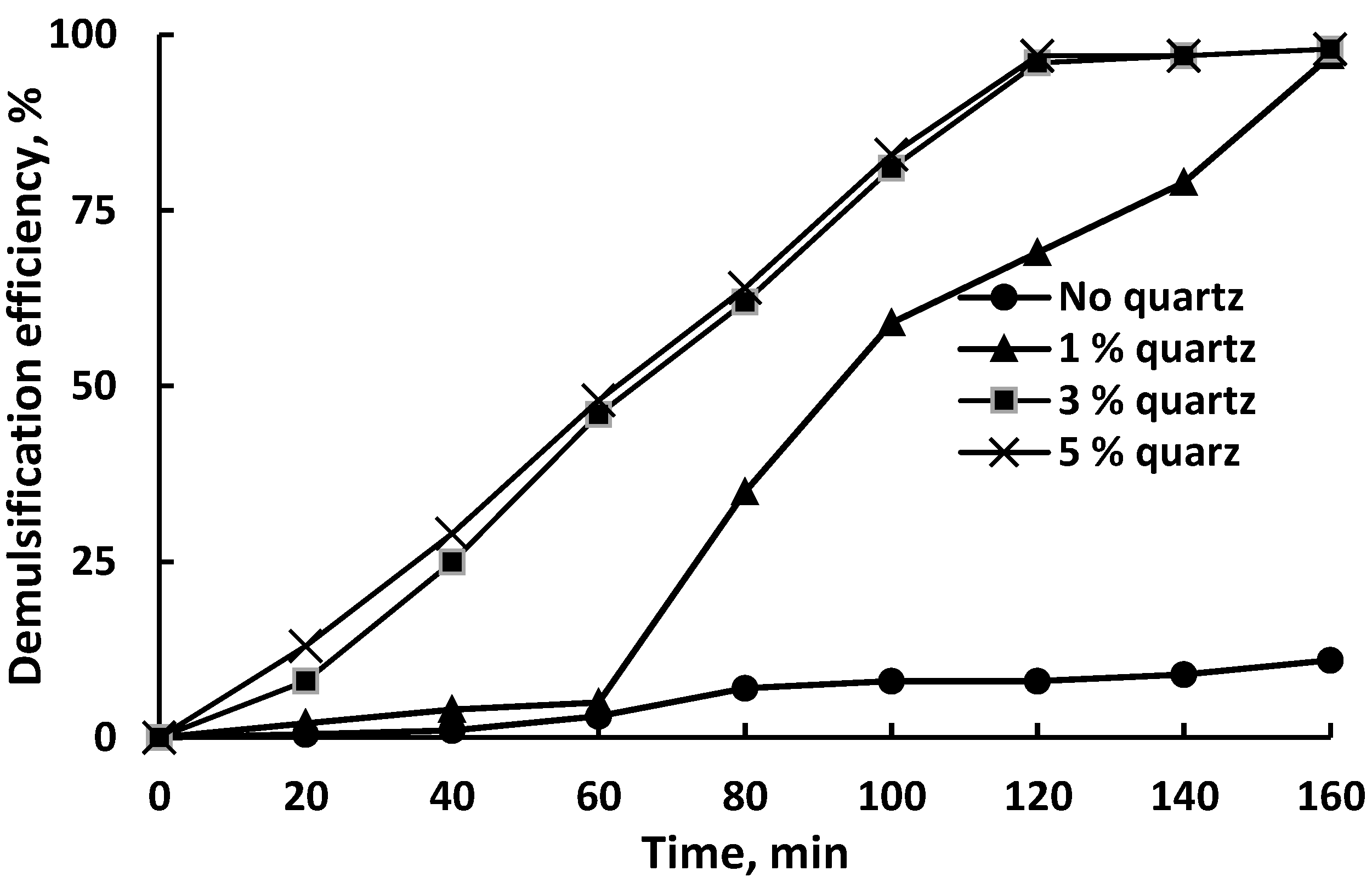
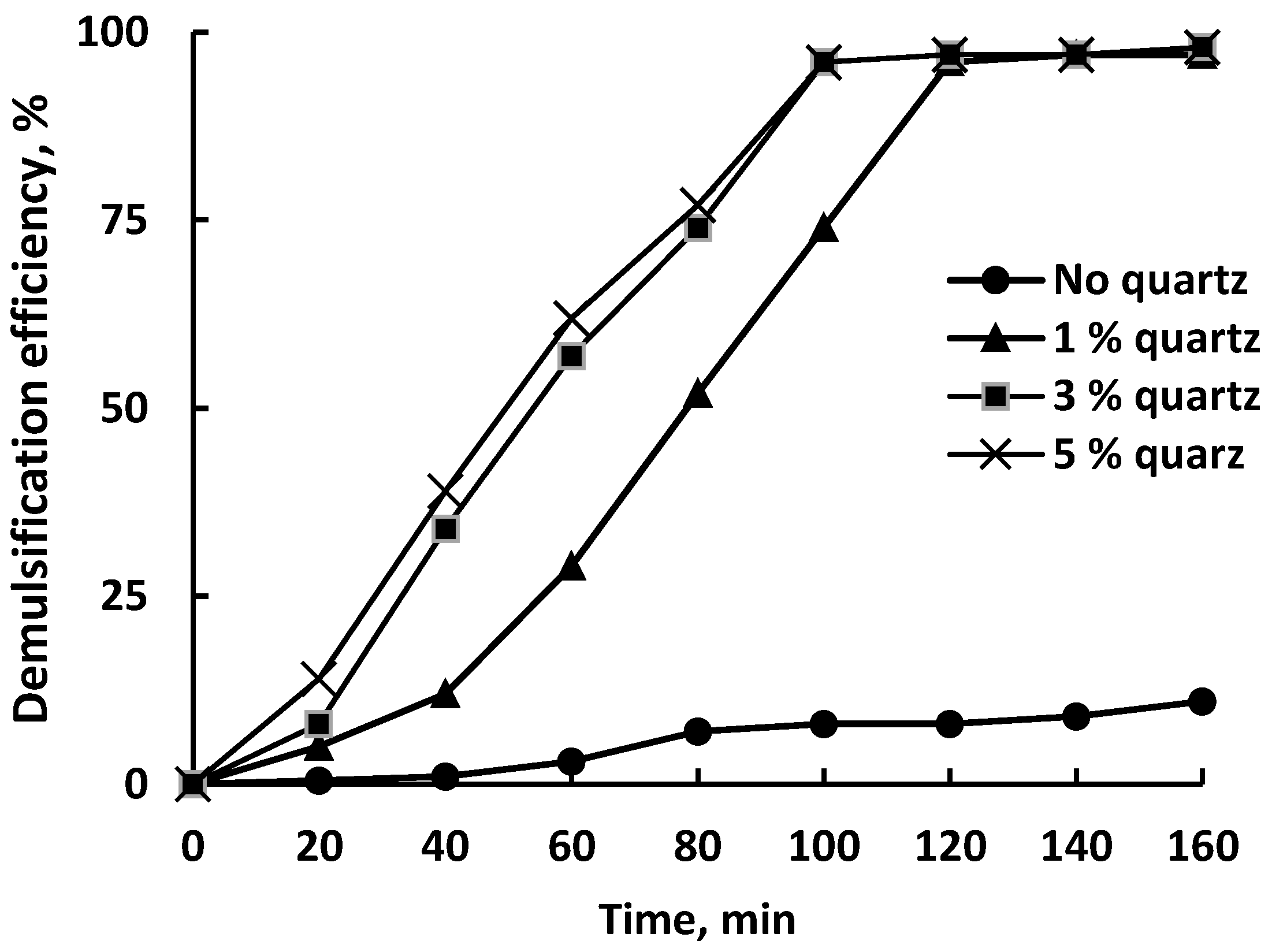
Figure 5 demonstrates the demulsification efficiency (DE) of untreated quartz particles obtained from a sieve with a mesh size of 75 μm, depending on the contact duration and the amount of quartz added.
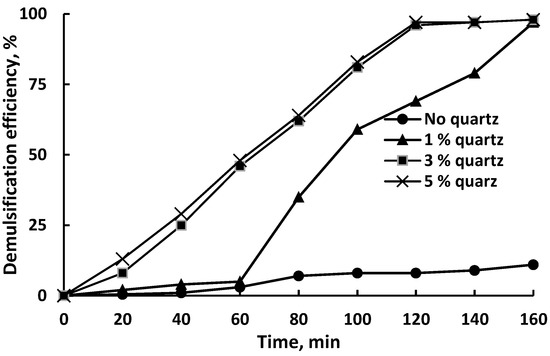
Figure 5.
Demulsification efficiency of the untreated quartz particles with a mesh size of 75 μm.
The separation of water from the emulsion occurred even in the absence of a demulsifier; however, this process was very slow as no more than 10% of the water was separated after 160 min of settling. The addition of 1 wt% quartz to the emulsion had very little effect on demulsification up to 60 min of settling; after the specified time, the degree of demulsification increased sharply and reached almost 60% in 100 min. The maximum degree of demulsification reached 97% after 160 min of settling. Increasing the dosage of quartz up to 3 wt% dramatically increased the efficiency of demulsification; up to 120 min of settling and near-linear growth was observed. After 120 min, the DE value was 97% and reached a plateau. Increasing the dosage of quartz to 5 wt% had no effect on the behavior of the DE vs. time curve compared to the dosage of 3 wt%.
The results obtained are generally consistent with the data on crude oil demulsification using quartz from sand obtained by Adewumni et al. [44].
Figure 6 shows the DE of freshly milled quartz (FMQ) particles obtained from a sieve with a mesh size of 75 μm; the conditions of the experiment were the same as those used in unmilled quartz.
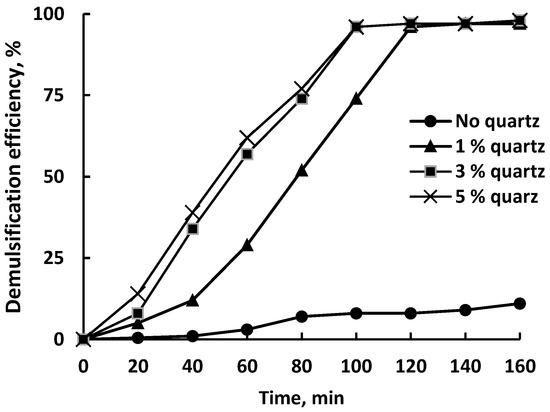
Figure 6.
Demulsification efficiency of the FMQ particles with a mesh size of 75 μm.
In general, DE vs. time curves in Figure 6 showed the same trends as in Figure 5. The maximum degree of demulsification was 97–98%, as in the presence of untreated quartz. However, the use of milled quartz significantly increased the efficiency of demulsification; the addition of 1 wt% demulsifier led to the separation of 97% of water after 120 min of settling, while untreated quartz required 160 min. Further, with the addition of 3 wt% FMQ, 97% of DE was achieved after 100 min of settling, against 140 min for the original quartz.
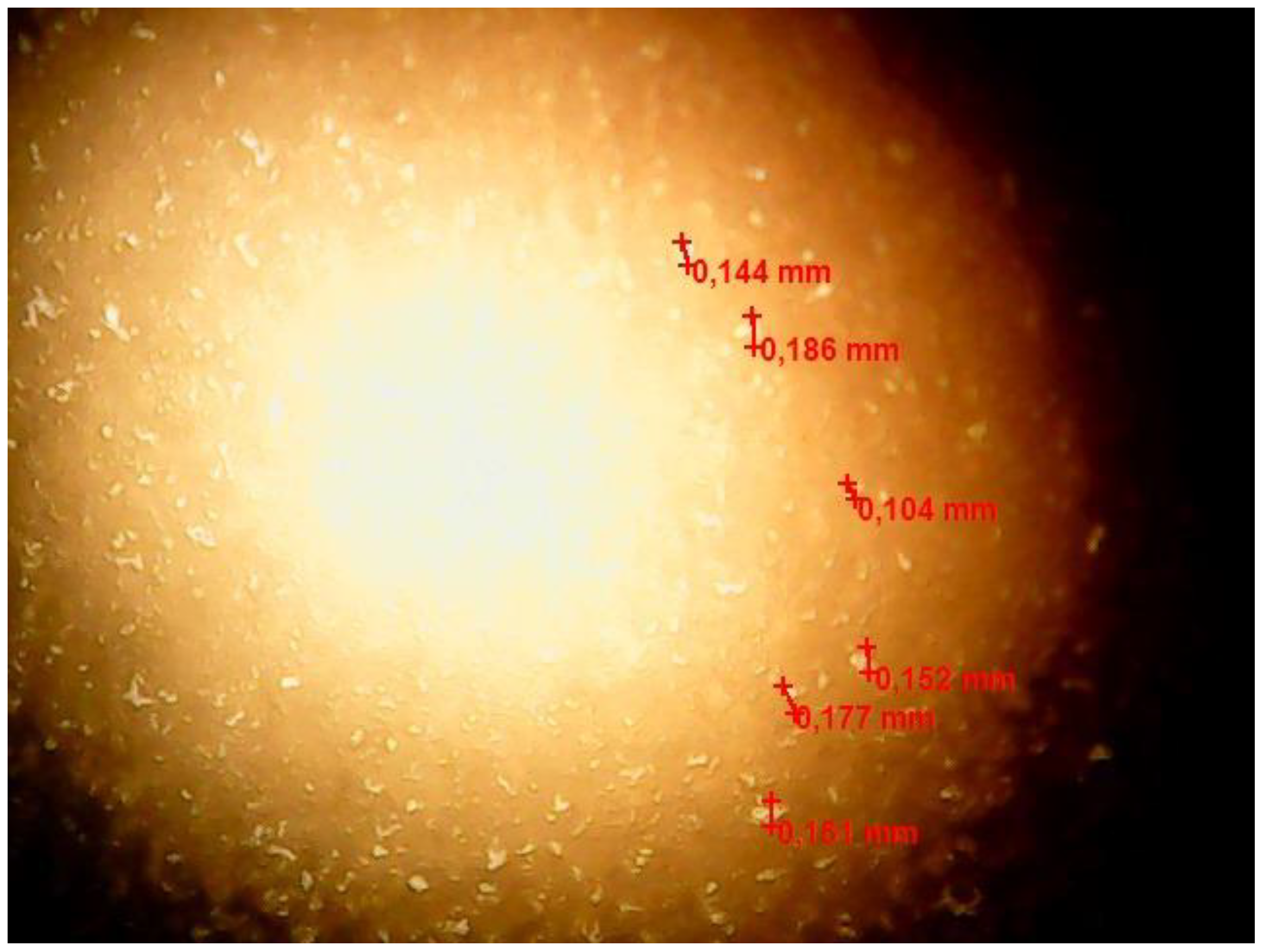
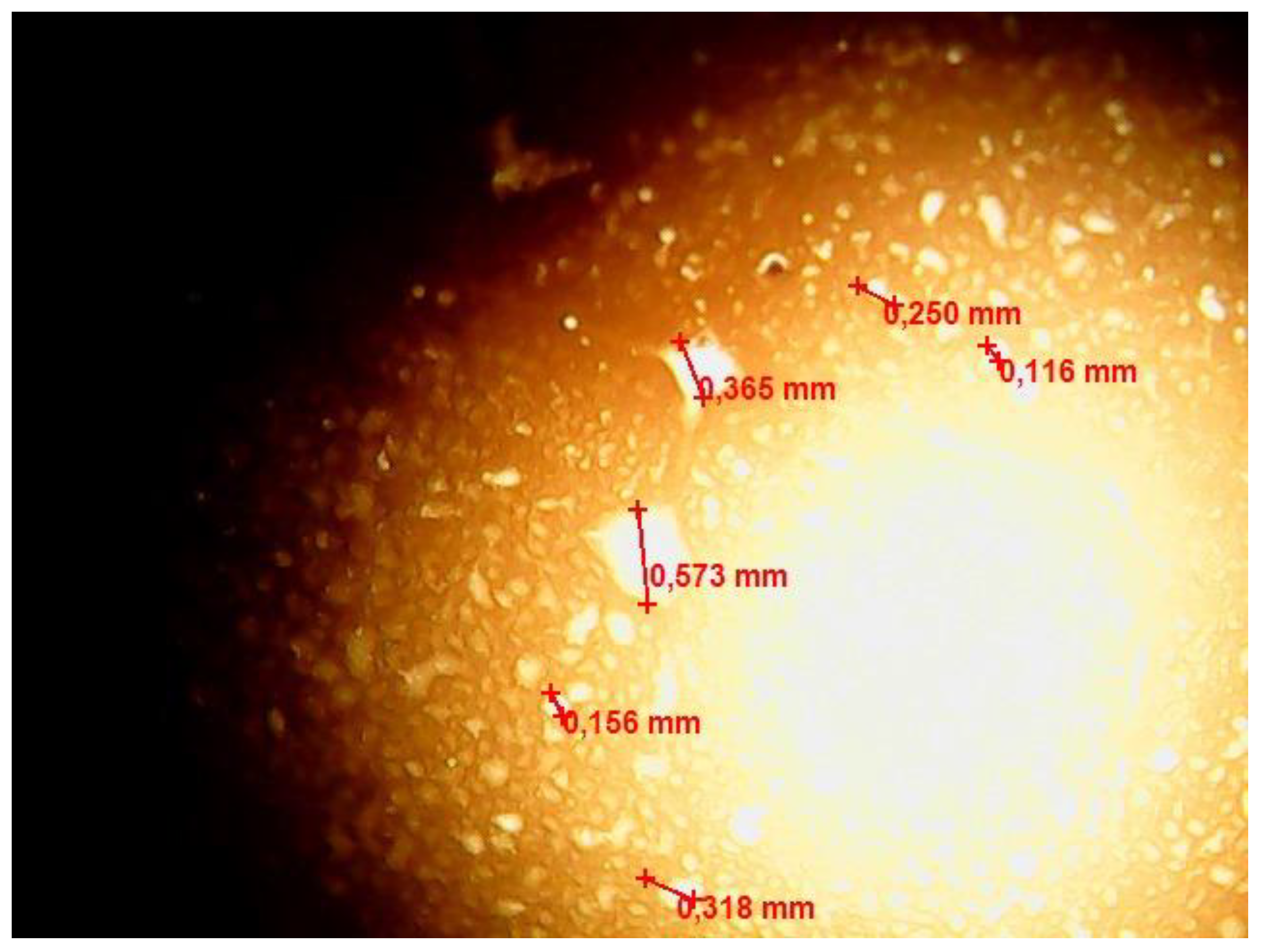
Figure 7 shows the results of optical microscopy of the w/o emulsion after 60 min of contact with FMQ (1 wt%).
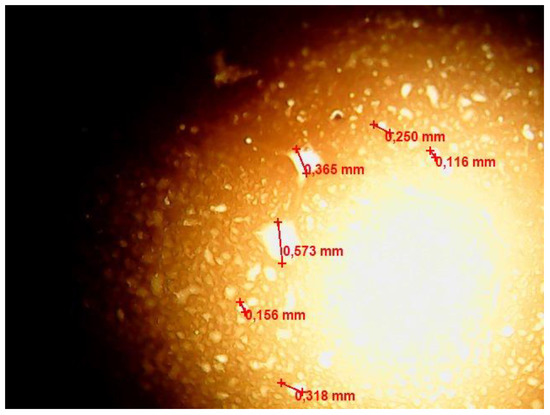
Figure 7.
Optical microscopic image of the w/o emulsion sample after contact with the FMQ particles for 60 min.
Figure 7 shows formed water globules with a size of about 350–550 μm, which were absent in the freshly prepared emulsion.
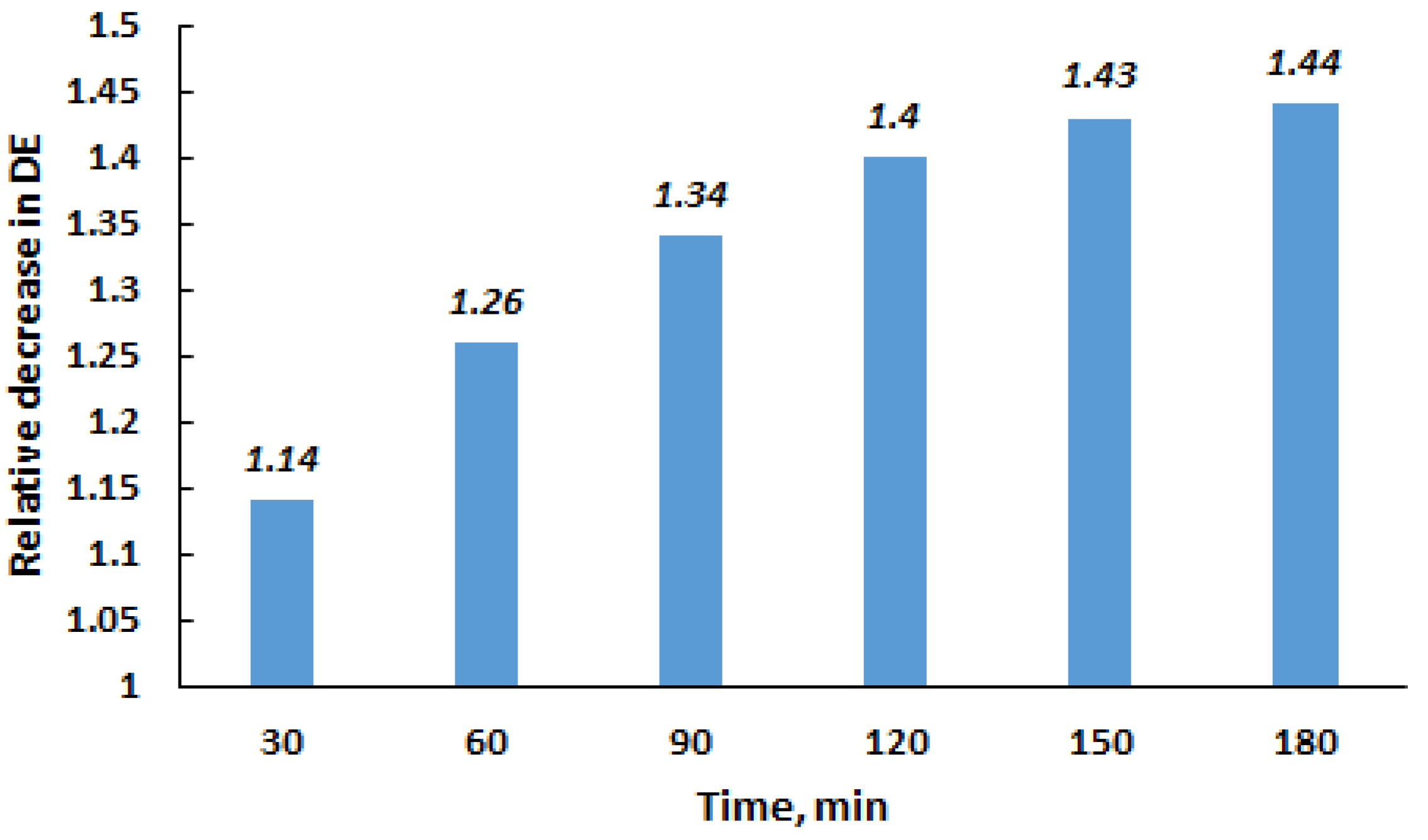
To compare the DE for the original and milled quartz, the following conditions were selected: 1 wt% of quartz and a settling time of 80 min. A comparison of Figure 5 and Figure 6 indicates that under the specified conditions, the DE for the original and FMQ particles were 35 and 52%, respectively. This result demonstrates that the efficiency of milled quartz was 1.49 times higher than that of the untreated demulsifier. It was interesting to observe how long FMQ retained the increased ability to demulsify the crude oil. To clarify this issue, experiments were carried out with quartz aged for a certain time after milling. The relative decrease in the efficiency of FMQ, depending on the time interval between milling and its use as a demulsifier, is presented in Figure 8.
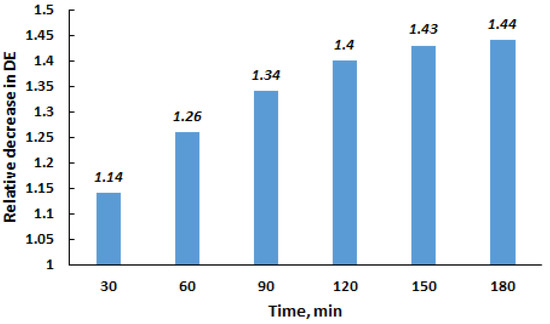
Figure 8.
Influence of the time interval between quartz milling and the use as a demulsifier on a relative decrease in the demulsification efficiency.
Over time after milling, the increased demulsifying ability of quartz particles gradually decreases and approaches that of the original quartz. After 150–180 min, the demulsifying performance of the milled quartz becomes almost comparable to that of the original material.
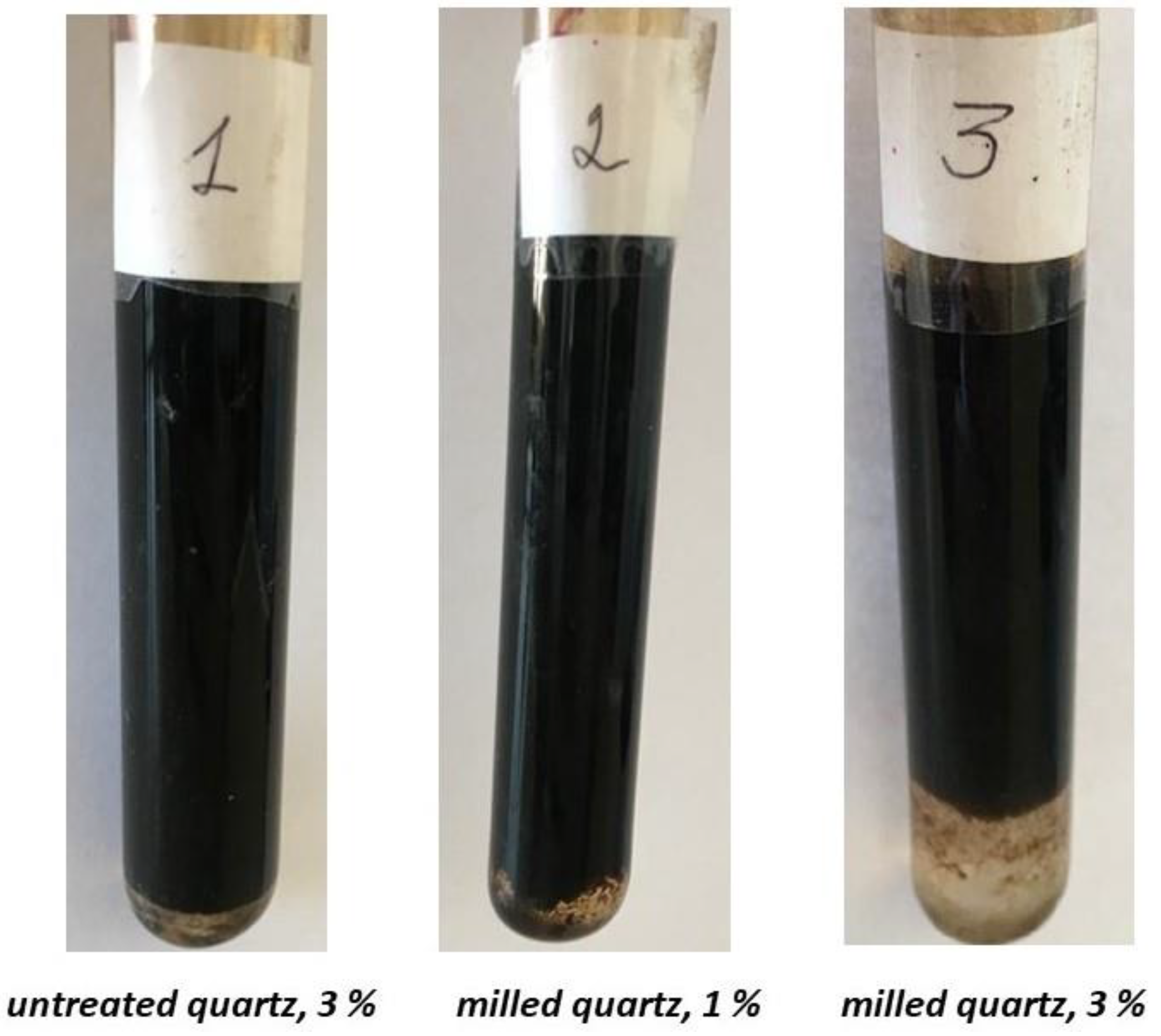
The comparative effectiveness of the original and FMQ as a demulsifier can be estimated based on Figure 9. At 140 min of settling, the addition of 1 wt% FMQ showed approximately the same demulsifying ability as the addition of 3 wt% of the original quartz. At the same time, an increase in the content of FMQ to 3 wt% sharply increased the volume of separated water.
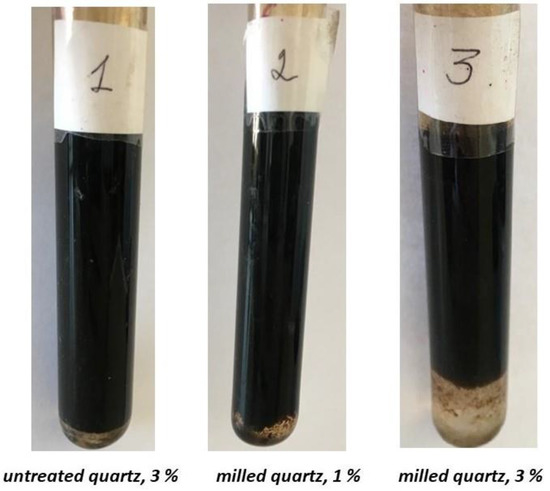
Figure 9.
Samples of the w/o emulsions after settling for 140 min in the presence of the original and freshly milled quartz particles.
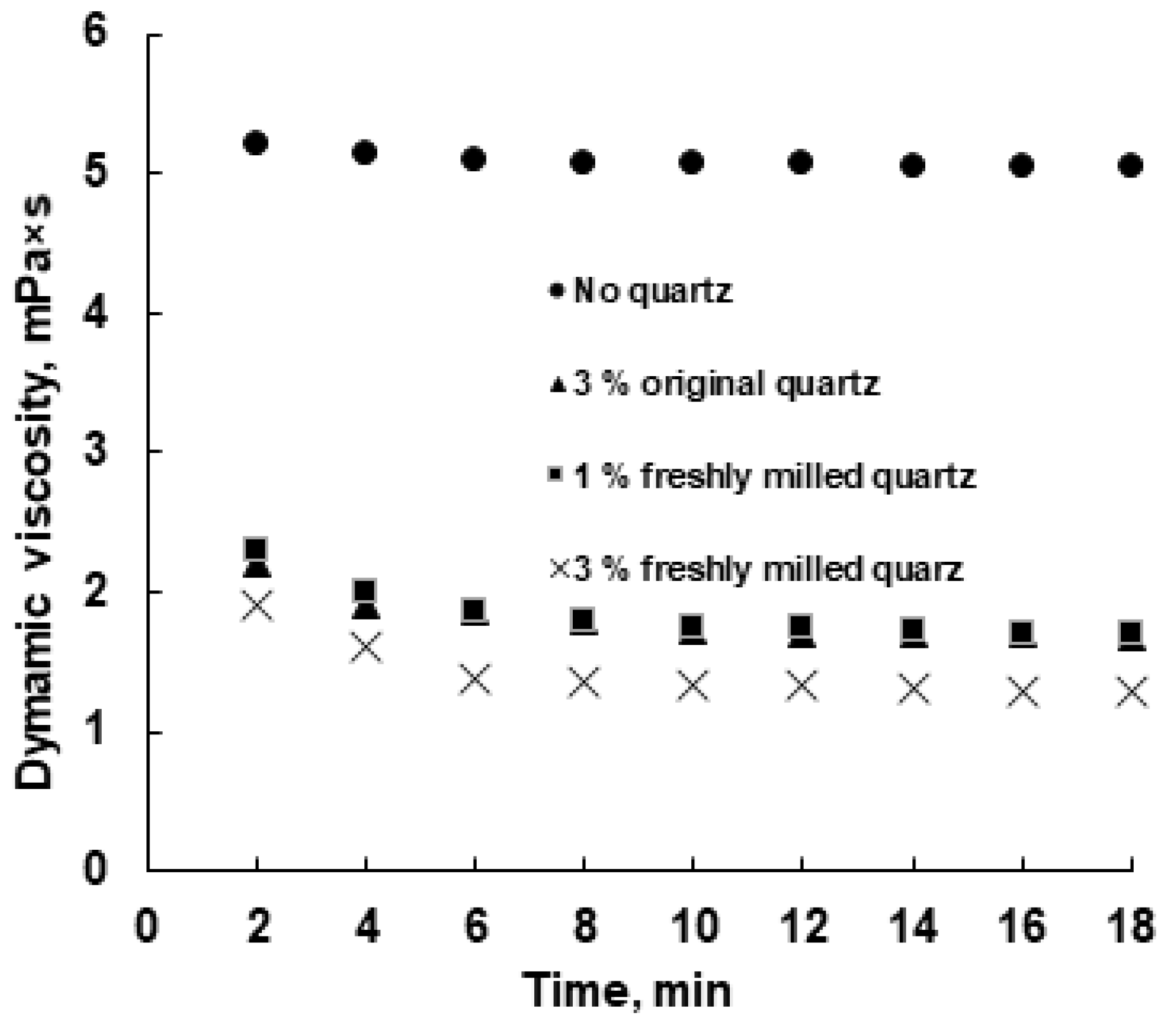
3.3. Viscosity Behavior
Figure 10 illustrates the viscosity behavior of the emulsion with and without the addition of quartz particles. It is clearly shown that the addition of quartz particles, both original and freshly milled, sharply reduced the viscosity of the emulsion. At the same time, the efficiency of the initial quartz at a concentration of 3 wt% was similar to that of FMQ at a concentration of 1 wt%; the value of the dynamic viscosity of the emulsion was 1.68–1.70 mPa·s after 16 min of contact with the named demulsifiers. An increase in the FMQ content to 3 wt% made it possible to reduce the viscosity of the emulsion to 1.29 mPa·s. A decrease in the viscosity of the emulsion indicates its partial destruction [17,51]; thus, this reveals that there is an approximately equal demulsifying ability of 3 wt% of the original quartz and 1 wt% FMQ, while 3 wt% FMQ showed greater efficiency.
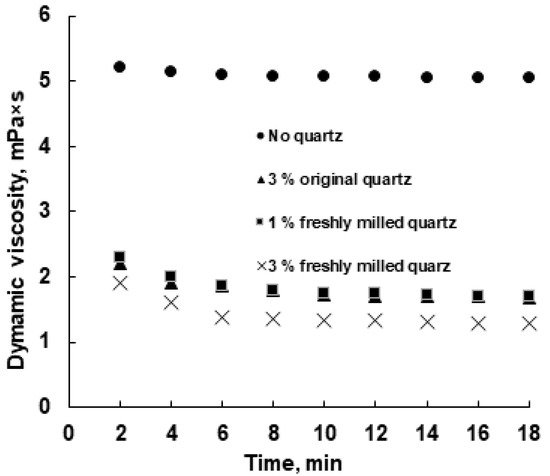
Figure 10.
Influence of the original and freshly milled quartz particles on the w/o emulsion viscosity.
3.4. Interfacial Tension Measurements
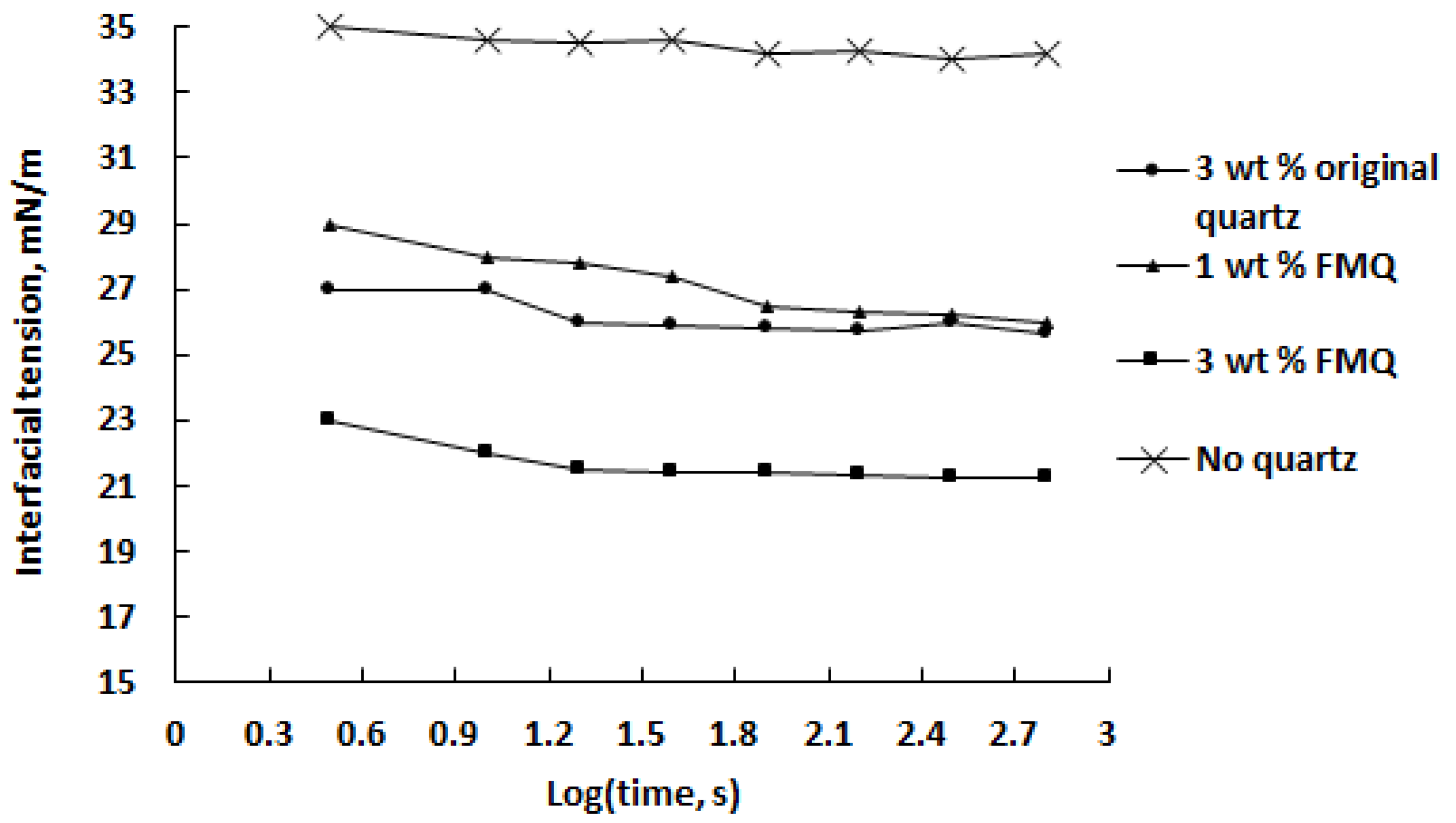
Interfacial tension (IFT) at the w/o interface is one of the most important factors affecting the stability of emulsions. The presence of a third phase that adsorbs at the w/o interface increases IFT and inhibits coalescence of the dispersed aqueous phase. Emulsion stability can be reduced by conditions reducing the film-forming capacity of the crude oil, in particular by adding a demulsifier. The degree of IFT reduction in the presence of a demulsifier can serve as one of the indicators of its effectiveness [52]. Figure 11 shows the change in the IFT of the saline water–crude oil interface as a function of contact time with quartz samples and quartz dosage; for comparison, the values of the IFT over time in the absence of the demulsifier are also given.
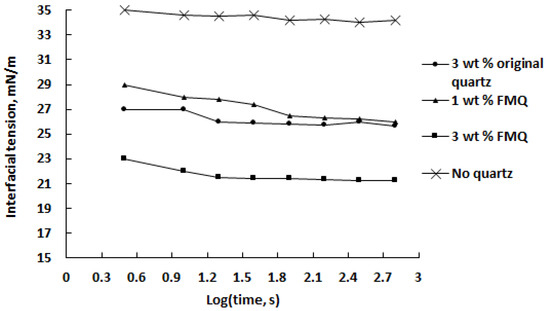
Figure 11.
Interfacial tension of the saline water–crude oil interface as a function of contact time with quartz at different demulsifier dosages.
It can be noted that the presence of quartz significantly reduces the IFT, and in the first 2 min, the efficiency of demulsifiers decreases in the series 3 wt% FMQ > 3 wt% original quartz > 1 wt% FMQ. However, after 250 s from the start of the emulsion’s contact with the quartz samples, 3 wt% original quartz showed the same ability to reduce the IFT as 1 wt% FMQ. A strong correlation exists between the data presented in Figure 11 with those in Figure 10; namely, all of the studied quartz samples had a destructive effect on the emulsion. At the same time, FMQ showed greater efficiency in this regard than the original quartz.
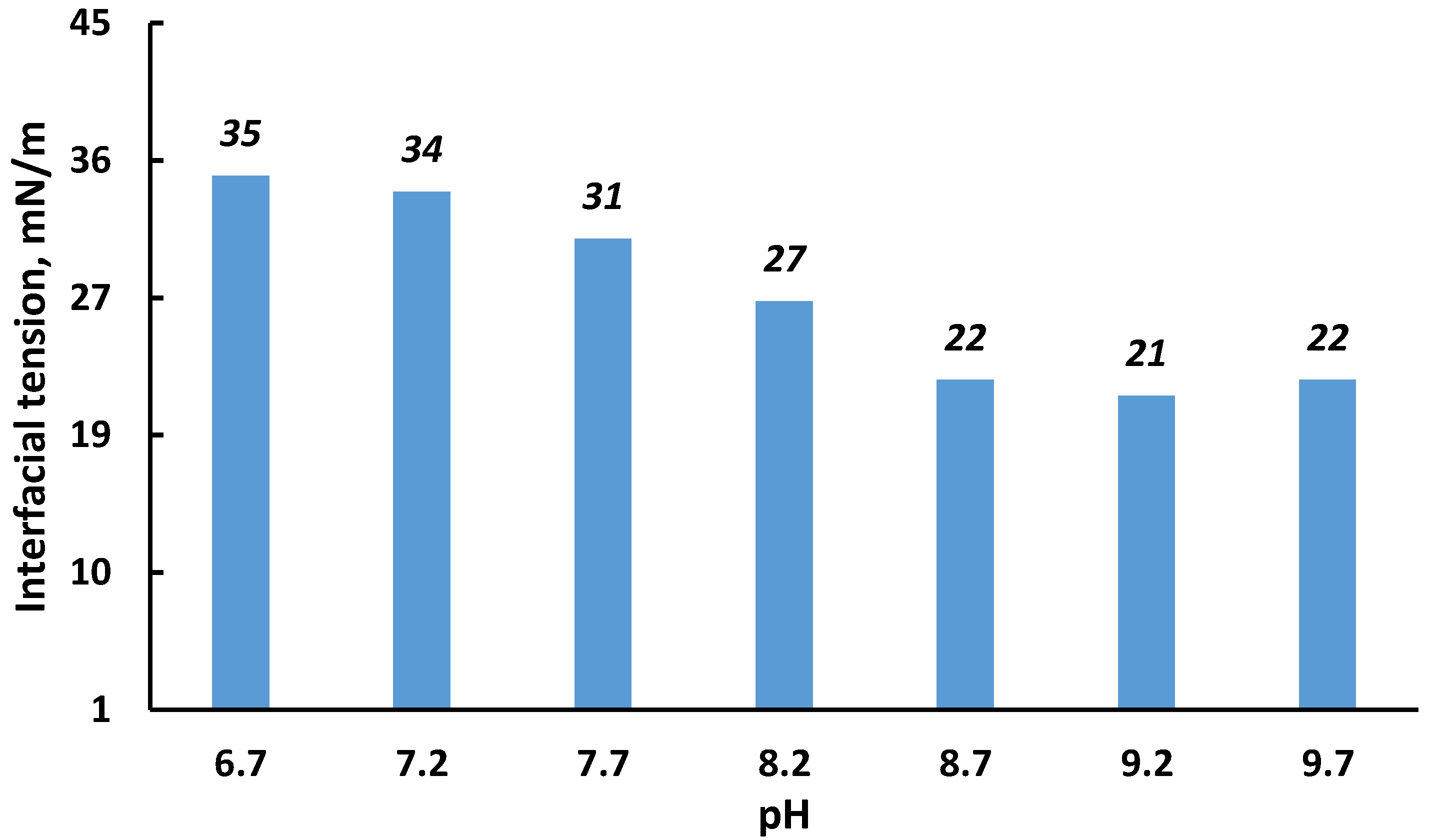
It is generally accepted that pH changes largely determine the stability of w/o emulsions. For a particular oil, it is essentially impossible to theoretically predict the effect of pH on the stability of the interfacial film surrounding the water droplets in the emulsion. However, it is known that an optimum pH range, at which value maximum emulsion-breaking occurs, exists for most crude oils [53,54,55]. As a rule, an IFT close to the minimum value is an indicator that crude oil emulsions demonstrate minimum stability. Figure 12 shows the dependence of initial tension on the pH of the emulsion; the initial emulsion had a pH = 6.7. With an increase in pH, the interfacial tension decreased; this effect was especially pronounced in the range of pH = 7.2–8.7, after which this trend disappeared. Thus, one should expect the least stability of the studied emulsion at a pH of about 8.7 and higher.
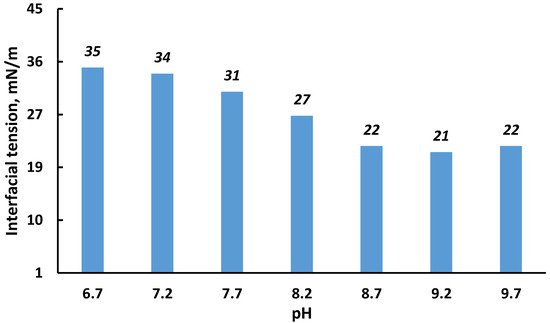
Figure 12.
Influence of pH on the initial interfacial tension of the saline water–crude oil interface.
3.5. Proposed Mechanism of w/o Demulsification by Freshly Milled Quartz
When milling quartz, fractures in crystals occur. In this case, a new surface is formed, which can later be subjected to deformations. The breaking of silicon–oxygen bonds in quartz, which occurs during its milling, leads to the formation of unsatisfied valence forces, contributing to the formation of metastable surface groups represented as >Si=O [56]. Upon contact with water, these groups form silanol groups [56]. Concurrently, in acid solution, the water adhering to the quartz surface increases the pH value in the medium [56]. It follows from the article by Ahmed and van Cleave [56] that the cause for the increase in pH may be the adsorption of H+ ions on the quartz surface in acid solution. Based on the indicated prerequisites in the literature, the following mechanism of the demulsification action of freshly milled quartz can be assumed. Quartz particles penetrate the water–oil interface; this effect of quartz particles during oil demulsification was also indicated in [44]. Because of the strong adhesion of water to the surface of the quartz due to the presence of metastable surface groups, there is an increase in pH in the microenvironment surrounding the quartz particles. As shown in Figure 12, an increase in pH leads to a decrease in surface tension and, hence, a decrease in the stability of the emulsion. This results in a significant breakdown of the emulsion. In this case, significant destruction of the emulsion occurs. The released water droplets surround the quartz particles due to the partial hydration of its surface, and coalesce has a higher density compared to oil; thus, water settles to the bottom of the vessel, reducing the water content in oil.
The above effect is prolonged in the first minutes after the quartz is crushed. After 2.5–3.0 h, the number of metastable surface groups is significantly reduced due to the high reactivity. This entails a strong decrease in the adhesion of water to the surface of the quartz, and the increased demulsifying ability of the quartz particles disappears.
4. Conclusions
A comparative study of the demulsifying ability concerning the w/o emulsion of the original and freshly milled quartz (FMQ) particles isolated from river sand was carried out and presented herein. The demulsifying efficiency of FMQ at a dosage of 1 wt% was found to be comparable to that of 1 wt% of the original quartz particles, at the same mesh size of 75 μm. The increase in the efficiency of FMQ was caused by the formation of metastable groups on its surface, which have an increased ability to form bonds with water molecules. At the same time, in an acidic environment, there is a local increase in pH in the microenvironment surrounding quartz particles. Studies have shown that the pH of a raw emulsion is 6.7, and increasing the pH decreased the surface tension of the saline water–crude oil interface; this indicates that the stability of the emulsion was reduced. Thus, the role of milling quartz is to increase the ability to adhere to water and, thus, locally increase the pH value. After 2.5–3.0 h following milling, the quartz particles lost their increased efficiency, and their demulsifying ability became equal to that of the original quartz.
The best available demulsifiers usually require several tens of ppm to achieve 97% efficiency, that is, the same efficiency as 3% of FMQ [2,4,22]. However, due to the high environmental friendliness and low cost, the use of FMQ from river sand for crude oil demulsification seems to be very promising.
The proposed method for the demulsification of crude oil is convenient to scale up. It is necessary to grind quartz sand in a continuous ball mill located near the demulsification plant. The solid demulsifier settles to the bottom of the vessel together with the water and can be easily separated from it, preferably by decantation.
Author Contributions
Conceptualization, K.N. and M.Z.; methodology, Z.N.; validation, G.B. and N.O.; investigation, G.B. and N.O.; resources, K.N.; data curation, M.Z. and N.O.; writing—original draft preparation, R.N.; writing—review and editing, R.N.; visualization, Z.N.; project administration, K.N.; funding acquisition, K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant no. AP08857586).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the results can be made available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-crude oil emulsion stabilization: Review and unanswered questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Greaves, D.; Boxall, J.; Mulligan, J.; Sloan, E.D.; Koh, C.A. Hydrate formation from high water content-crude oil emulsions. Chem. Eng. Sci. 2008, 63, 4570–4579. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; de Lourdes Mosqueira, M.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Cruz Clavel-López, J.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Kolotova, D.S.; Kuchina, Y.A.; Petrova, L.A.; Voron’ko, N.G.; Derkach, S.R. Rheology of water-in-crude oil emulsions: Influence of concentration and temperature. Colloids Interfaces 2018, 2, 64. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Rousseau, D. Fat crystals and water-in-oil emulsion stability. Curr. Opin. Colloid Interface Sci. 2011, 16, 421–431. [Google Scholar] [CrossRef]
- Kang, W.; Xu, B.; Wang, Y.; Li, Y.; Shan, X.; An, F.; Liu, J. Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 555–560. [Google Scholar] [CrossRef]
- Ghannam, M.T. Water-in-crude oil emulsion stability investigation. Pet. Sci. Technol. 2005, 23, 649–667. [Google Scholar] [CrossRef]
- Spiecker, P.M.; Gawrys, K.L.; Trail, C.B.; Kilpatrick, P.K. Effects of petroleum resins on asphaltene aggregation and water-in-oil emulsion formation. Colloids Surf. A Physicochem. Eng. Asp. 2003, 220, 9–27. [Google Scholar] [CrossRef]
- Xu, X.; Cao, D.; Liu, J.; Gao, J.; Wang, X. Research on ultrasound-assisted demulsification/dehydration for crude oil. Ultrason. Sonochem. 2019, 57, 185–192. [Google Scholar] [CrossRef]
- Adeyemi, I.; Meribout, M.; Khezzar, L. Recent developments, challenges, and prospects of ultrasound-assisted oil technologies. Ultrason. Sonochem. 2021, 82, 105902. [Google Scholar] [CrossRef] [PubMed]
- Nadirova, Z.K.; Ivakhnenko, O.P.; Zhantasov, M.K.; Bimbetova, G.Z.; Nadirov, K.S. Ultrasound-assisted dewatering of crude oil from Kumkol oilfield. Chem. Bull. Kazakh Natl. Univ. 2021, 101, 4–10. [Google Scholar] [CrossRef]
- Santos, D.; da Rocha, E.C.; Santos, R.L.; Cancelas, A.J.; Franceschi, E.; Santos, A.F.; Dariva, C. Demulsification of water-in-crude oil emulsions using single mode and multimode microwave irradiation. Sep. Purif. Technol. 2017, 189, 347–356. [Google Scholar] [CrossRef]
- Lv, X.; Song, Z.; Yu, J.; Su, Y.; Zhao, X.; Sun, J.; Wang, W. Study on the demulsification of refinery oily sludge enhanced by microwave irradiation. Fuel 2020, 279, 118417. [Google Scholar] [CrossRef]
- Ezzati, A.; Gorouhi, E.; Mohammadi, T. Separation of water in oil emulsions using microfiltration. Desalination 2005, 185, 371–382. [Google Scholar] [CrossRef]
- Akbari, S.; Nour, A.; Jamari, S.; Rajabi, A. Demulsification of water-in-crude oil emulsion via conventional heating and microwave heating technology in their optimum conditions. Aust. J. Basic Appl. Sci. 2016, 10, 66–74. [Google Scholar]
- Fan, M.; Nie, C.; Du, H.; Ni, J.; Wang, B.; Wang, X. An insight into the solar demulsification of highly emulsified water produced from oilfields by monitoring the viscosity, zeta potential, particle size and rheology. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 144–154. [Google Scholar] [CrossRef]
- Nie, C.; Du, H.; Zhang, Y.; Han, G.; Wang, H.; Yuan, D.; Wang, X. Towards efficient solar demulsification (I): A solar electrical role on interfacial film of emulsions. Sustain. Mater. Technol. 2021, 30, e00344. [Google Scholar] [CrossRef]
- Xu, B. Fast and Energy-efficient Demulsification for Crude Oil Emulsions Using Pulsed Electric Field. Int. J. Electrochem. Sci. 2017, 12, 9242–9249. [Google Scholar] [CrossRef]
- Taleghani, S.T.; Jahromi, A.F.; Elektorowicz, M. Electro-demulsification of water-in-oil suspensions enhanced with implementing various additives. Chemosphere 2019, 233, 157–163. [Google Scholar] [CrossRef]
- Alao, K.T.; Alara, O.R.; Abdurahman, N.H. Trending approaches on demulsification of crude oil in the petroleum industry. Appl. Petrochem. Res. 2021, 11, 281–293. [Google Scholar] [CrossRef]
- Saad, M.A.; Kamil, M.; Abdurahman, N.H.; Yunus, R.M.; Awad, O.I. An overview of recent advances in state-of-the-art techniques in the demulsification of crude oil emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Abdulredha, M.M.; Aslina, H.S.; Luqman, C.A. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arab. J. Chem. 2020, 13, 3403–3428. [Google Scholar] [CrossRef]
- Otarbaev, N.S.; Kapustin, V.M.; Nadirov, K.S.; Bimbetova, G.Z.; Zhantasov, M.K.; Nadirov, R.K. New potential demulsifiers obtained by processing gossypol resin. Indones. J. Chem. 2019, 19, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Roostaie, T.; Farsi, M.; Rahimpour, M.R.; Biniaz, P. Performance of biodegradable cellulose based agents for demulsification of crude oil: Dehydration capacity and rate. Sep. Purif. Technol. 2017, 179, 291–296. [Google Scholar] [CrossRef]
- Shehzad, F.; Hussein, I.A.; Kamal, M.S.; Ahmad, W.; Sultan, A.S.; Nasser, M.S. Polymeric surfactants and emerging alternatives used in the demulsification of produced water: A review. Polym. Rev. 2018, 58, 63–101. [Google Scholar] [CrossRef]
- Du, Y.; Si, P.; Wei, L.; Wang, Y.; Tu, Y.; Zuo, G.; Ye, S. Demulsification of acidic oil-in-water emulsions driven by chitosan loaded Ti3C2Tx. Appl. Surf. Sci. 2019, 476, 878–885. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Hou, J.; Wang, L.; Cepuch, C.; Masliyah, J.; Xu, Z. Effect of hydroxyl content and molecular weight of biodegradable ethylcellulose on demulsification of water-in-diluted bitumen emulsions. Ind. Eng. Chem. Res. 2011, 50, 6347–6354. [Google Scholar] [CrossRef]
- Abullah, M.M.; Al-Lohedan, H.A.; Attah, A.M. Synthesis and application of amphiphilic ionic liquid based on acrylate copolymers as demulsifier and oil spill dispersant. J. Mol. Liq. 2016, 219, 54–62. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A.; Abdullah, M.M. Dipoles poly (ionic liquids) based on 2-acrylamido-2-methylpropane sulfonic acid-co-hydroxyethyl methacrylate for demulsification of crude oil water emulsions. J. Mol. Liq. 2016, 222, 680–690. [Google Scholar] [CrossRef]
- Ezzat, A.O.; Atta, A.M.; Al-Lohedan, H.A.; Abdullah, M.M.; Hashem, A.I. Synthesis and application of poly (ionic liquid) based on cardanol as demulsifier for heavy crude oil water emulsions. Energy Fuels 2018, 32, 214–225. [Google Scholar] [CrossRef]
- Ismail, A.I.; Atta, A.M.; El-Newehy, M.; El-Hefnawy, M.E. Synthesis and Application of New Amphiphilic Asphaltene Ionic Liquid Polymers to Demulsify Arabic Heavy Petroleum Crude Oil Emulsions. Polymers 2020, 12, 1273. [Google Scholar] [CrossRef] [PubMed]
- Adewunmi, A.A.; Kamal, M.S.; Solling, T.I. Application of magnetic nanoparticles in demulsification: A review on synthesis, performance, recyclability, and challenges. J. Pet. Sci. Eng. 2020, 196, 107680. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, X.; Liu, N.; Cao, Y.; Lu, F.; Xu, L.; Feng, L. Magnetically recoverable efficient demulsifier for water-in-oil emulsions. ChemPhysChem 2015, 16, 595–600. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Ma, J.; Li, X.; Al-Shiaani, N.H.; He, L. Fast demulsification of oil-water emulsions at room temperature by functionalized magnetic nanoparticles. Sep. Purif. Technol. 2021, 274, 118967. [Google Scholar] [CrossRef]
- Farrokhi, F.; Jafari Nasr, M.R.; Rahimpour, M.R.; Arjmand, M.; Vaziri, S.A. Application of a novel magnetic nanoparticle as demulsifier for dewatering in crude oil emulsion. Sep. Sci. Technol. 2018, 53, 551–558. [Google Scholar] [CrossRef]
- Nikkhah, M.; Tohidian, T.; Rahimpour, M.R.; Jahanmiri, A. Efficient demulsification of water-in-oil emulsion by a novel nano-titania modified chemical demulsifier. Chem. Eng. Res. Des. 2015, 94, 164–172. [Google Scholar] [CrossRef]
- Huang, Z.; Li, P.; Luo, X.; Jiang, X.; Liu, L.; Ye, F.; Mi, Y. Synthesis of a novel environmentally friendly and interfacially active CNTs/SiO2 demulsifier for W/O crude oil emulsion separation. Energy Fuels 2019, 33, 7166–7175. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Wu, J.Y. Development and characterization of novel and stable silicon nanoparticles-embedded PCM-in-water emulsions for thermal energy storage. Appl. Energy 2019, 238, 1407–1416. [Google Scholar] [CrossRef]
- Javadian, S.; Sadrpoor, S.M. Demulsification of water in oil emulsion by surface modified SiO2 nanoparticle. J. Pet. Sci. Eng. 2020, 184, 106547. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S.; Solling, T.I.; Salami, B.A. Palm oil fuel ash (POFA) as a demulsifier for crude oil emulsions: Performance and mechanism. J. Pet. Sci. Eng. 2019, 183, 106430. [Google Scholar] [CrossRef]
- Knapik, E. Biodemulsification combined with fixed-bed biosorption for the recovery of crude oil from produced water. J. Water Process. Eng. 2020, 38, 101614. [Google Scholar] [CrossRef]
- Hippmann, S.; Ahmed, S.S.; Fröhlich, P.; Bertau, M. Demulsification of water/crude oil emulsion using natural rock Alginite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 71–79. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Amao, A.O.; Kamal, M.S.; Solling, T.I. Demulsification and breaking mechanism of variable quartz concentrates obtained from sand. J. Pet. Sci. Eng. 2020, 192, 107263. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S.; Amao, A.O.; Solling, T.I. Extracted quartz as efficient natural demulsifier for crude oil-water emulsions: Effect of monovalent/divalent salts, pH and modeling study. J. Pet. Sci. Eng. 2021, 206, 109069. [Google Scholar] [CrossRef]
- Parks, G.A. Surface and interfacial free energies of quartz. J. Geophys. Res. Solid Earth 1984, 89, 3997–4008. [Google Scholar] [CrossRef]
- Murashov, V.V.; Demchuk, E. A comparative study of unrelaxed surfaces on quartz and kaolinite, using the periodic density functional theory. J. Phys. Chem. B 2005, 109, 10835–10841. [Google Scholar] [CrossRef]
- Parks, G.A. Surface energy and adsorption at mineral/water interfaces: An introduction. Miner.-Water Interface Geochem. 2018, 23, 133–176. [Google Scholar]
- Dean, E.W.; Stark, D.D. A Convenient Method for the Determination of Water in Petroleum and Other Organic Emulsions. Ind. Eng. Chem. 1920, 12, 486–490. [Google Scholar] [CrossRef] [Green Version]
- Márquez, N.; Antón, R.E.; Graciaa, A.; Lachaise, J.; Salager, J.L. Partitioning of ethoxylated alkylphenol surfactants in microemulsion-oil-water systems. Part II: Influence of hydrophobe branching. Colloids Surface A 1998, 131, 45–49. [Google Scholar] [CrossRef]
- Tao, J.; Shi, P.; Fang, S.; Li, K.; Zhang, H.; Duan, M. Effect of rheology properties of oil/water interface on demulsification of crude oil emulsions. Ind. Eng. Chem. Res. 2015, 54, 4851–4860. [Google Scholar] [CrossRef]
- Kang, W.; Yin, X.; Yang, H.; Zhao, Y.; Huang, Z.; Hou, X.; Aidarova, S. Demulsification performance, behavior and mechanism of different demulsifiers on the light crude oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 197–204. [Google Scholar] [CrossRef]
- Strassner, J.E. Effect of pH on interfacial films and stability of crude oil-water emulsions. J. Pet. Technol. 1968, 20, 303–312. [Google Scholar] [CrossRef]
- Arla, D.; Sinquin, A.; Palermo, T.; Hurtevent, C.; Graciaa, A.; Dicharry, C. Influence of pH and water content on the type and stability of acidic crude oil emulsions. Energy Fuels 2007, 21, 1337–1342. [Google Scholar] [CrossRef]
- Behrang, M.; Hosseini, S.; Akhlaghi, N. Effect of pH on interfacial tension reduction of oil (Heavy acidic crude oil, resinous and asphaltenic synthetic oil)/low salinity solution prepared by chloride-based salts. J. Pet. Sci. Eng. 2021, 205, 108840. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Van Cleave, A.B. Adsorption and flotation studies with quartz: Part I. Adsorption of calcium hydrogen and hydroxyl ions on quartz. Can. J. Chem. Eng. 1965, 43, 23–26. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).