Study on the Heat Transfer Characteristics of an Ambient Air Vaporizer with Multi-Component Fluids
Abstract
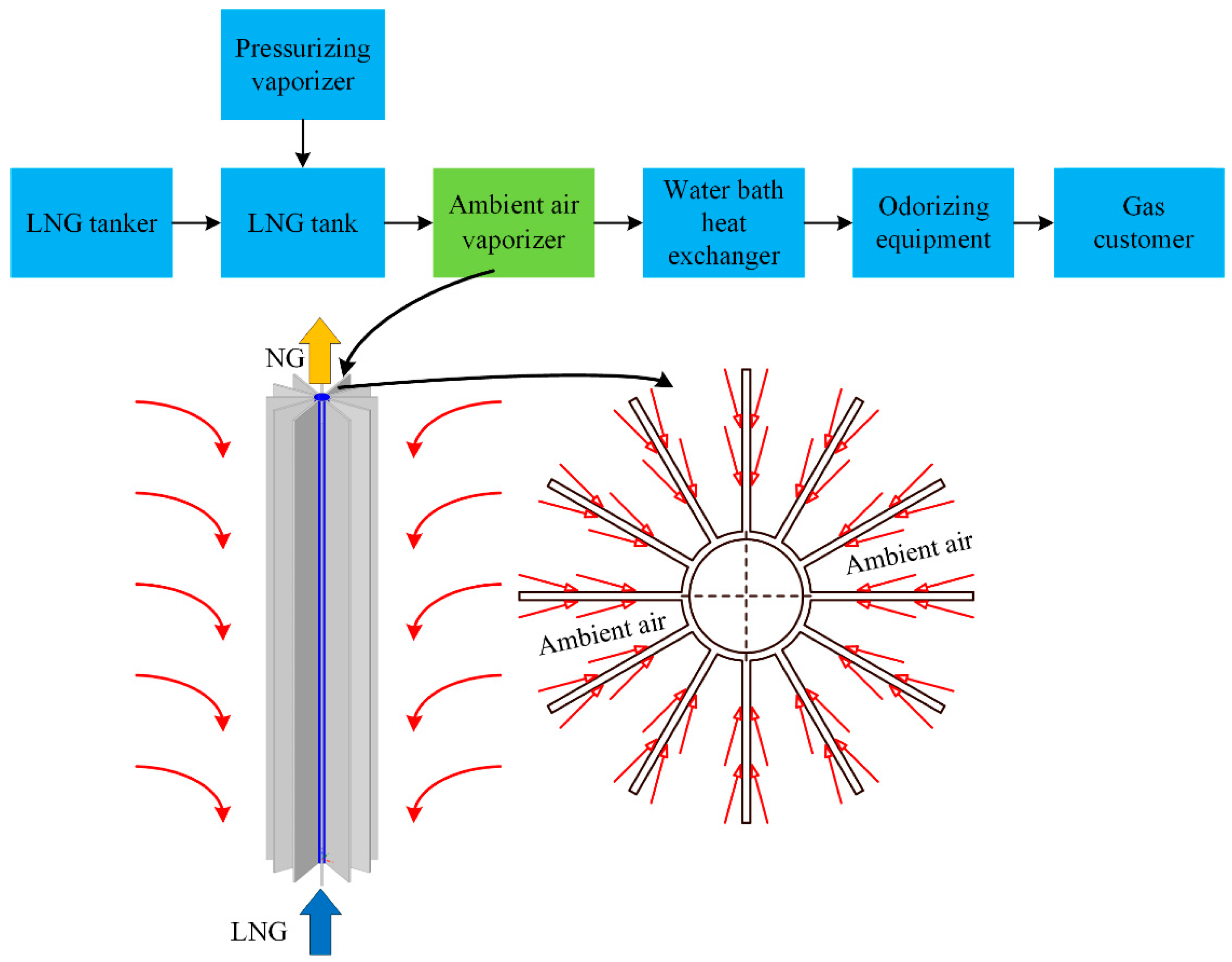
:1. Introduction
2. Mathematical Model of AAV
2.1. External Heat and Mass Transfer Process of AAV
2.2. Internal Heat Transfer Process of AAV
2.3. Framework
3. Results and Discussions
3.1. Model Validation
3.2. Analysis of Single-Component and Multi-Component Heat Transfer Characteristics
3.2.1. Analysis of Heat Transfer Characteristics in Two Components
3.2.2. Analysis of Heat Transfer Characteristics in Multiple Components
4. Conclusions
- (1)
- The whole length of the three phases caused by multi-component LNG was 42.4% of the pure due to the difference in fluid composition in AAV when it was in operation, which has an obvious difference in the mixed phase. The distinction can be reflected in the fluid and surface temperature, the thickness of the frost layer, etc. It can also be proved from the change curve of the convective heat transfer coefficient of the internal fluid.
- (2)
- From the study of methane content in multicomponent fluids, it can be admitted that the difference in heat transfer mechanism is mainly because the thermodynamic dryness degree x is different when it is in a phase change. It was found that the slope of the pure component in the pure liquid phase and the mixed phase was half that of the multi-component in the same zone.
- (3)
- It was further found that the influence of operating pressure on the , and is greater than that of the design flow rate. When the acceleration of the design pressure increase was 0.1 MPa and the average deviation rate of the was 4.87%, the average rate of drop of the AAV was only 0.125 K, the average peak heat transfer coefficient increased by 0.71 , and the average relative position lagged 1.2 m. However, for every 0.01 increase in the design the flow rate, the average deviation of the was 14.5%, the average drop in was 2.99 K, the peak heat transfer coefficient increased by 4.17 on average, and the average relative position lagged 4.2 m.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Characteristic length, | |
| Specific heat of air at constant pressure, | |
| Radiation emission rate | |
| Heat transfer coefficient, | |
| Interfacial mass transfer coefficient, () | |
| Length, | |
| Lewis number | |
| Latent heat of sublimation of water vapor, | |
| Mass of vapor through the frost surface per unit area, | |
| Mass flow rate per unit area, | |
| Mass flux of the water vapor through the frost surface, | |
| Nusselt number | |
| Prandtl number | |
| Saturation pressure of water vapor at far-field, | |
| Saturation pressure of water vapor at frost surface, | |
| q | Heat flux, |
| Rayleigh number | |
| Universal gas constant of the water vapor, | |
| s | Supersaturation degree |
| T | Temperature, |
| x | Thermodynamic dryness degree |
| Greek symbol | |
| Density, | |
| The thickness of the frost, | |
| Relative humidity of the air | |
| Thermal conductivity, | |
| Strengthening factor | |
| The frost porosity | |
| The minimum thermal conductivity of the frost layer, | |
| The maximum thermal conductivity of the frost layer, | |
| Boltzmann factor, | |
| Surface tension | |
| Time, | |
| Subscripts | |
| a | Air |
| c | Convective heat transfer |
| f | Frost |
| ice | Ice |
| L | Liquid phase |
| N | Vapor phase |
| r | Radiation heat transfer |
| re | Requirement temperature of working fluids |
| v,a | Water vapor of the far-field |
| v,f | Water vapor of the frost surface |
| w | Tube wall |
| out | Output temperature |
References
- Cheng, H.; Ju, Y.; Fu, Y. Experimental and simulation investigation on heat transfer characteristics of supercritical nitrogen in a new rib tube of open rack vaporizer. Int. J. Refrig. 2019, 111, 103–112. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, W.; Ren, L.; Tian, X. Thermal resistance analysis of cryogenic frosting and its effect on performance of LNG ambient air vaporizer. Renew. Energy 2019, 149, 917–927. [Google Scholar] [CrossRef]
- Lisowski, F.; Lisowski, E. Influence of longitudinal fin tubes arrangement in lng ambient air vaporizers on the wind load. Energies 2022, 15, 405. [Google Scholar] [CrossRef]
- Ruan, B.; Lin, W. Experimental study on heat transfer in a model of submerged combustion vaporizer. Appl. Therm. Eng. 2021, 201, 117744. [Google Scholar] [CrossRef]
- Wang, B.; Wang, W.; Qi, C.; Kuang, Y.; Xu, J. Simulation of performance of intermediate fluid vaporizer under wide operation conditions. Front. Energy 2020, 14, 452–462. [Google Scholar] [CrossRef]
- Li, S.; Ju, Y. Review of the LNG intermediate fluid vaporizer and its heat transfer characteristics. Front. Energy 2021, 1–16. [Google Scholar] [CrossRef]
- Hayashi, Y.; Aoki, A.; Adachi, S.; Hori, K. Study of frost properties correlating with frost formation types. J. Heat Transf. 1977, 99, 239–245. [Google Scholar] [CrossRef]
- Na, B.; Webb, R.L. A fundamental understanding of factors affecting frost nucleation. Int. J. Heat Mass Transf. 2003, 46, 3797–3808. [Google Scholar] [CrossRef]
- Na, B.; Webb, R.L. New model for frost growth rate. Int. J. Heat Mass Transf. 2004, 47, 925–936. [Google Scholar] [CrossRef]
- Na, B.; Webb, R.L.; Na, B.; Webb, R.L. Mass transfer on and within a frost layer. Int. J. Heat Mass Transf. 2004, 47, 899–911. [Google Scholar] [CrossRef]
- Iragorry, J.; Tao, Y.-X.; Jia, S. Review article: A critical review of properties and models for frost formation analysis. HVAC&R Res. 2004, 10, 393–420. [Google Scholar] [CrossRef]
- Song, M.; Dang, C. Review on the measurement and calculation of frost characteristics. Int. J. Heat Mass Transf. 2018, 124, 586–614. [Google Scholar] [CrossRef]
- Cui, J.; Li, W.; Liu, Y.; Jiang, Z. A new time- and space-dependent model for predicting frost formation. Appl. Therm. Eng. 2011, 31, 447–457. [Google Scholar] [CrossRef]
- Kandula, M. Frost growth and densification in laminar flow over flat surfaces. Int. J. Heat Mass Transf. 2011, 54, 3719–3731. [Google Scholar] [CrossRef]
- Kandula, M. Frost growth and densification on a flat surface in laminar flow with variable humidity. Int. Commun. Heat Mass Transf. 2012, 39, 1030–1034. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Chu, F.; Ma, Q. Frosting model based on phase change driving force. Int. J. Heat Mass Transf. 2017, 110, 760–767. [Google Scholar] [CrossRef]
- Schneider, H. Equation of the growth rate of frost forming on cooled surfaces. Int. J. Heat Mass Transf. 1978, 21, 1019–1024. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, W.-S.; Lee, T.-H. A one-dimensional model for frost formation on a cold flat surface. Int. J. Heat Mass Transf. 1997, 40, 4359–4365. [Google Scholar] [CrossRef]
- Lee, Y.; Ro, S. Analysis of the frost growth on a flat plate by simple models of saturation and supersaturation. Exp. Therm. Fluid Sci. 2005, 29, 685–696. [Google Scholar] [CrossRef]
- Hermes, C.J.; Piucco, R.O.; Barbosa, J.R.; Melo, C. A study of frost growth and densification on flat surfaces. Exp. Therm. Fluid Sci. 2009, 33, 371–379. [Google Scholar] [CrossRef]
- Hermes, C.J. An analytical solution to the problem of frost growth and densification on flat surfaces. Int. J. Heat Mass Transf. 2012, 55, 7346–7351. [Google Scholar] [CrossRef]
- Jones, B.W.; Parker, J.D. Frost formation with varying environmental parameters. J. Heat Transf. 1975, 97, 255–259. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.Y. Quick estimation of frost growth on cold fins through thermal network analysis. Int. J. Refrig. 2014, 47, 153–163. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, H.; Wang, H. Study on transient distributed model of frost on heat pump evaporator. J. Asian Arch. Build. Eng. 2005, 4, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, J.; Kim, D.R.; Lee, K.-S. Modeling of frost layer growth considering frost porosity. Int. J. Heat Mass Transf. 2018, 126, 980–988. [Google Scholar] [CrossRef]
- El Cheikh, A.; Jacobi, A. A mathematical model for frost growth and densification on flat surfaces. Int. J. Heat Mass Transf. 2014, 77, 604–611. [Google Scholar] [CrossRef]
- Leoni, A.; Mondot, M.; Durier, F.; Revellin, R.; Haberschill, P. Frost formation and development on flat plate: Experimental investigation and comparison to predictive methods. Exp. Therm. Fluid Sci. 2017, 88, 220–233. [Google Scholar] [CrossRef]
- Kuang, Y.; Yi, C.; Wang, W. Numerical simulation of frosting behavior and its effect on a direct-contact ambient air vaporizer. J. Nat. Gas Sci. Eng. 2015, 27, 55–63. [Google Scholar] [CrossRef]
- Li, X.; Wei, W.; Wang, R.; Shi, Y. Numerical and experimental investigation of heat transfer on heating surface during subcooled boiling flow of liquid nitrogen. Int. J. Heat Mass Transf. 2009, 52, 1510–1516. [Google Scholar] [CrossRef]
- Guyun, Z. Heat and Mass Transfer in Engineering; Aviation Industry Press: Beijing, China, 1989; p. 603. [Google Scholar]
- Castillo, J.E.; Huang, Y.; Pan, Z.; Weibel, J.A. Quantifying the pathways of latent heat dissipation during droplet freezing on cooled substrates. Int. J. Heat Mass Transf. 2020, 164, 120608. [Google Scholar] [CrossRef]
- Bergman, T.L.; Bergman, T.L.; Incropera, F.P.; Dewitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Chilton, T.H.; Colburn, A.P. Mass transfer (absorption) coefficients prediction from data on heat transfer and fluid friction. Ind. Eng. Chem. 1934, 26, 1183–1187. [Google Scholar] [CrossRef]
- Auracher, H. Heat transfer in frost and snow. In Der Waermetransport bei Frost und Schnee; Toronto, ON, Canada, 1978; pp. 25–30. Available online: https://www.ihtcdigitallibrary.com/conferences/2d9b67ad1b92ab15,7082010052da3d73,1b7afe206152d81d.html (accessed on 29 March 2022).
- Zhang, X. Heat Transfer; China Construction Industry Press: Beijing, China, 2001; p. 322. [Google Scholar]
- Klimenko, V. A generalized correlation for two-phase forced flow heat transfer. Int. J. Heat Mass Transf. 1988, 31, 541–552. [Google Scholar] [CrossRef]
- Klimenko, V. A generalized correlation for two-phase forced flow heat transfer—Second assessment. Int. J. Heat Mass Transf. 1990, 33, 2073–2088. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Yao, S.; Jin, S.; Yang, J.; Liu, D.; Li, J.; Mao, H. Experimental study on heat transfer characteristics of air-heating cryogenics finned-tube vaporizer under frosting conditions. Cryogenics 2017, 3, 55–69. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, W.; Ren, L.; Wang, H.; Zhang, P. Dynamic heat transfer analysis of liquefied natural gas ambient air vaporizer under frost conditions. Appl. Therm. Eng. 2017, 110, 999–1006. [Google Scholar] [CrossRef]
- Dauger, J.-M. The LNG Industry GIIGNL Annual Report for 2018; International Group of Liquefied Natural Gas Importers: Neuilly-sur-Seine, France, 2018. [Google Scholar]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, C.; Jia, W.; Wang, K. Study on the Heat Transfer Characteristics of an Ambient Air Vaporizer with Multi-Component Fluids. Processes 2022, 10, 851. https://doi.org/10.3390/pr10050851
Wang J, Li C, Jia W, Wang K. Study on the Heat Transfer Characteristics of an Ambient Air Vaporizer with Multi-Component Fluids. Processes. 2022; 10(5):851. https://doi.org/10.3390/pr10050851
Chicago/Turabian StyleWang, Jie, Changjun Li, Wenlong Jia, and Ke Wang. 2022. "Study on the Heat Transfer Characteristics of an Ambient Air Vaporizer with Multi-Component Fluids" Processes 10, no. 5: 851. https://doi.org/10.3390/pr10050851
APA StyleWang, J., Li, C., Jia, W., & Wang, K. (2022). Study on the Heat Transfer Characteristics of an Ambient Air Vaporizer with Multi-Component Fluids. Processes, 10(5), 851. https://doi.org/10.3390/pr10050851





