Improving Epoxy Resin Performance Using PPG and MDI by One-Step Modification
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Polyurethane Prepolymer
2.3. Preparation of Modified Epoxy Resin
2.4. Testing Methods
2.5. Kinetic Analysis of Thermal Decomposition
3. Results and Discussion
3.1. FT-IR of P-M-Modified Epoxy Resin

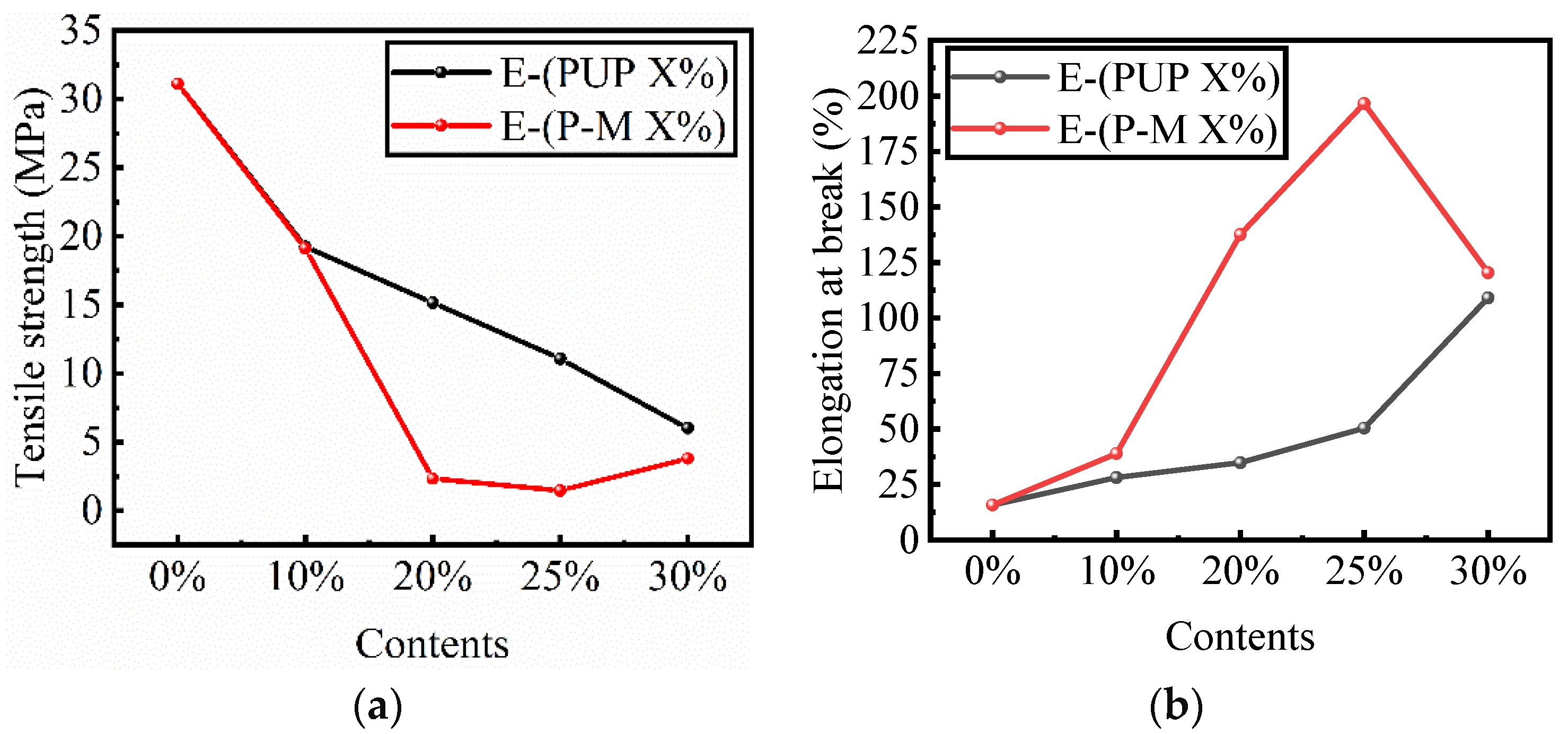
3.2. Properties of Modified Epoxy Resin
3.3. Thermal Properties
3.4. Thermal Decomposition Kinetic
Kissinger Method
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilčáková, J.; Lenka, K.; Marek, J.; Moučka, R.; Vicha, R.; Sedlacik, M.; Kovalcik, A.; Machovsky, M.; Zazantseva, N. Enhanced Charpy impact strength of epoxy resin modified with vinyl-terminated polydimethylsiloxane. J. Appl. Polym. Sci. 2018, 135, 45720. [Google Scholar] [CrossRef]
- Ma, H.; Xin, Z.; Feifei, J.; Tsai, S.-B. A Study on Curing Kinetics of Nano-Phase Modified Epoxy Resin. Sci. Rep. 2018, 8, 3045. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Papa, I.; Langella, A.; Langella, T.; Lopresto, V.; Antonucci, V. Mechanical properties of glass fibre composites based on nitrile rubber toughened modified epoxy resin. Compos. Part B Eng. 2018, 139, 259–267. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Yu, L.-X.; Long, J.-W.; Qi, M.; Chen, L.; Wang, Y.-Z. Piperazine-modified ammonium polyphosphate as monocomponent flame-retardant hardener for epoxy resin: Flame retardance, curing behavior and mechanical property. Polym. Chem. 2016, 7, 3003–3012. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, C.; Qian, X.; Jing, J.; Jin, L. DOPO/Silicon/CNT Nanohybrid Flame Retardants: Toward Improving the Fire Safety of Epoxy Resins. Polymers 2022, 14, 565. [Google Scholar] [CrossRef]
- Yahyaie, H.; Morteza, E.; Hamed-Vakili, T.; Mafi, E.R. Toughening mechanisms of rubber modified thin film epoxy resins. Prog. Org. Coat. 2013, 76, 286–292. [Google Scholar] [CrossRef]
- Wang, H.; Qunying, H.; Yutao, Z. Influences of Modified Sm2O3 on Thermal Stability, Mechanical and Neutron Shielding Properties of Aminophenol Trifunctional Epoxy Resin. Polymers 2022, 14, 638. [Google Scholar] [CrossRef]
- Yang, J.; Hengxu, W.; Xiaohuan, L.; Fu, S.; Song, P. A nano-TiO2/regenerated cellulose biohybrid enables simultaneously improved strength and toughness of solid epoxy resins. Compos. Sci. Technol. 2021, 212, 108884. [Google Scholar] [CrossRef]
- Mao, D.; Chen, J.; Ren, L.; Zhang, K.; Yuen, M.M.F.; Zeng, X.; Sun, R.; Xu, J.-B.; Wong, C.-P. Spherical core-shell Al@Al2O3 filled epoxy resin composites as high-performance thermal interface materials. Compos. Part A Appl. Sci. Manuf. 2019, 123, 260–269. [Google Scholar] [CrossRef]
- Misumi, J.; Oyama, T. Low viscosity and high toughness epoxy resin modified by in situ radical polymerization method for improving mechanical properties of carbon fiber reinforced plastics. Polymer 2018, 156, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, G.; Yu, B.; Peng, M. New reactive rigid-rod aminated aromatic polyamide for the simultaneous strengthening and toughening of epoxy resin and carbon fiber/epoxy composites. Compos. Part B Eng. 2020, 197, 108044. [Google Scholar] [CrossRef]
- Zhang, J.; Mi, X.; Chen, S.; Xu, Z.; Zhang, D.; Miao, M.; Wang, J. A bio-based hyperbranched flame retardant for epoxy resins. Chem. Eng. J. 2020, 381, 122719. [Google Scholar] [CrossRef]
- Liu, X.-F.; Liu, B.-W.; Luo, X.; Guo, D.-M.; Zhong, H.-Y.; Chen, L.; Wang, Y.-Z. A novel phosphorus-containing semi-aromatic polyester toward flame retardancy and enhanced mechanical properties of epoxy resin. Chem. Eng. J. 2020, 380, 122471. [Google Scholar] [CrossRef]
- Kou, Y.; Zhou, W.; Li, B.; Dong, L.; Duan, Y.-E.; Hou, Q.; Liu, X.; Cai, H.; Chen, Q.; Dang, Z.-M. Enhanced mechanical and dielectric properties of an epoxy resin modified with hydroxyl-terminated polybutadiene. Compos. Part A Appl. Sci. Manuf. 2018, 114, 97–106. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, J.; Liu, X.; Liu, R. Polyurethane modified epoxy acrylate resins containing ε-caprolactone unit. Prog. Org. Coat. 2020, 141, 105543. [Google Scholar] [CrossRef]
- Frisch, H.-L.; Frisch, K.-C.; Klempner, D. Glass Transitions of Topologically Interpenetrating Polymer Networks. Polym. Eng. Sci. 1974, 14, 646–650. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Wang, Q.; Wang, T. Physical Properties of Micro Hollow Glass Bead Filled Castor Oil-Based Polyurethane/Epoxy Resin IPN Composites. J. Macromol. Sci. Part B 2017, 56, 161–169. [Google Scholar] [CrossRef]
- Huo, L.; Wang, D.; Liu, H.; Jia, P.; Gao, J. Cytoxicity, dynamic and thermal properties of bio-based rosin-epoxy resin/ castor oil polyurethane/carbon nanotubes bio-nanocomposites. J. Biomater. Sci. Polym. Ed. 2016, 27, 1100–1114. [Google Scholar] [CrossRef]
- Bakar, M.; Duk, R.; Przybyłek, M.; Kostrzewa, M. Mechanical and Thermal Properties of Epoxy Resin Modified with Polyurethane. J. Reinf. Plast. Compos. 2008, 28, 2107–2118. [Google Scholar] [CrossRef]
- Bakar, M.; Hausnerova, B.; Kostrzewa, M. Effect of diisocyanates on the properties and morphology of epoxy/polyurethane interpenetrating polymer networks. J. Thermoplast. Compos. Mater. 2013, 26, 1364–1376. [Google Scholar] [CrossRef] [Green Version]
- Bakar, M.; Kostrzewa, M.; Pawelec, Z. Preparation and properties of epoxy resin modified with polyurethane based on hexamethylene diisocyanate and different polyols. J. Thermoplast. Compos. Mater. 2014, 27, 620–631. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Hausnerova, B.; Bakar, M.; Dalka, M. Property evaluation and structure analysis of polyurethane/epoxy graft interpenetrating polymer networks. J. Appl. Polym. Sci. 2011, 122, 1722–1730. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Hausnerova, B.; Bakar, M.; Siwek, E. Effects of various polyurethanes on the mechanical and structural properties of an epoxy resin. J. Appl. Polym. Sci. 2011, 119, 2925–2932. [Google Scholar] [CrossRef]
- Li, Z.-H.; Huang, Y.-P.; Ren, D.-Y.; Zheng, Z.-Q. Structural characteristics and properties of polyurethane modified TDE-85/MeTHPA epoxy resin with interpenetrating polymer networks. J. Cent. South Univ. Technol. 2008, 15, 305–308. [Google Scholar] [CrossRef]
- Li, Z.-H.; Huang, Y.-P.; Ren, D.-Y.; Zheng, Z.-Q. Structural characteristics and properties of PU-modified TDE-85/MeTHPA epoxy resin. J. Cent. South Univ. Technol. 2007, 14, 753–758. [Google Scholar] [CrossRef]
- Ren, D.-Y.; Li, X.-H.; Li, Z.-H. Influence Factors Study on the Properties of Epoxy Resin Modified by Polyurethane. Adv. Mater. Res. 2012, 472–475, 1937–1940. [Google Scholar] [CrossRef]
- Sivanesan, D.; Kim, S.; Jang, T.W.; Kim, H.J.; Song, J.; Seo, B.; Lim, C.-S.; Kim, H.-G. Effects of flexible and rigid parts of ε-caprolactone and tricyclodecanediol derived polyurethane on the polymer properties of epoxy resin. Polymer 2021, 237, 124374. [Google Scholar] [CrossRef]
- Othman, M.B.H.; Khan, A.; Ahmad, K.; Zakaria, M.R.; Ullah, F.; Akil, H.M. Kinetic investigation and lifetime prediction of Cs–NIPAM–MBA-based thermo-responsive hydrogels. Carbohydr. Polym. 2016, 136, 1182–1193. [Google Scholar] [CrossRef]
- Díaz, E.G.; González, E.; García, M.F.; Asensio, I.A. Kinetic Study of the Pyrolysis of Canary Pine: The Relationship between the Elemental Composition and the Kinetic Parameters. Ind. Eng. Chem. Res. 2018, 57, 9094–9101. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Fan, L.; Jiang, Y.; Cao, L.; Tang, Z.; Zhu, J. Synthesis and Properties of a Bio-Based Epoxy Resin with High Epoxy Value and Low Viscosity. ChemSusChem 2014, 7, 555–562. [Google Scholar] [CrossRef]
- Jia, M.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Polyurethanes from Direct Organocatalytic Copolymerization of p-Tosyl Isocyanate with Epoxides. Angew. Chem. Int. Ed. Engl. 2021, 60, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; He, X.; Wu, Q.; Sun, P.-C.; Wang, C.-J.; Liu, X.-Z. A new recyclable crosslinked polymer combined polyurethane and epoxy resin. Polymer 2018, 149, 154–163. [Google Scholar] [CrossRef]
- Koradiya, S.B.; Adroja, P.-P.; Patel, J.P.; Ghumara, R.Y.; Parsania, P.H. Curing, Spectral and Thermal Study of Epoxy Resin of Bisphenol-C and Its Polyester Polyols Based Polyurethanes. Polym. Plast. Technol. Eng. 2012, 51, 1545–1549. [Google Scholar] [CrossRef]
- Mothé, C.G.; de Freitas, J.S. Lifetime prediction and kinetic parameters of thermal decomposition of cashew gum by thermal analysis. J. Therm. Anal. Calorim. 2018, 131, 397–404. [Google Scholar] [CrossRef]







| P-M Contents | R2 | Slope | Intercept | Ea (KJ·mol−1) |
|---|---|---|---|---|
| EP-(P-M 0%) | 0.9765 | −7093.42 | 10.49 | 58.97 |
| EP-(P-M 10%) | 0.9822 | −5933.12 | 7.07 | 49.33 |
| EP-(P-M 20%) | 0.9960 | −8454.20 | 13.86 | 70.29 |
| EP-(P-M 25%) | 0.9920 | −7870.63 | 12.21 | 65.44 |
| EP-(P-M 30%) | 0.9392 | −5211.02 | 4.94 | 43.32 |
| α | Ea (KJ·mol−1) | ||||
|---|---|---|---|---|---|
| EP-(P-M 0%) | EP-(P-M 10%) | EP-(P-M 20%) | EP-(P-M 25%) | EP-(P-M 30%) | |
| 10% | 42.92 | 49.09 | 50.81 | 41.90 | 34.44 |
| 20% | 51.34 | 51.43 | 57.83 | 56.73 | 52.05 |
| 30% | 56.03 | 52.45 | 57.85 | 59.37 | 55.58 |
| 40% | 57.58 | 53.84 | 58.06 | 61.46 | 57.11 |
| 50% | 58.48 | 55.79 | 58.64 | 58.58 | 57.38 |
| 60% | 59.97 | 56.61 | 58.89 | 58.01 | 57.81 |
| 70% | 62.54 | 59.51 | 62.06 | 60.08 | 59.93 |
| 80% | 67.34 | 63.51 | 66.21 | 63.24 | 63.32 |
| Average | 57.03 | 55.28 | 58.79 | 57.42 | 54.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Liu, X.; Liu, L. Improving Epoxy Resin Performance Using PPG and MDI by One-Step Modification. Processes 2022, 10, 929. https://doi.org/10.3390/pr10050929
Wen Y, Liu X, Liu L. Improving Epoxy Resin Performance Using PPG and MDI by One-Step Modification. Processes. 2022; 10(5):929. https://doi.org/10.3390/pr10050929
Chicago/Turabian StyleWen, Yong, Xudong Liu, and Lang Liu. 2022. "Improving Epoxy Resin Performance Using PPG and MDI by One-Step Modification" Processes 10, no. 5: 929. https://doi.org/10.3390/pr10050929
APA StyleWen, Y., Liu, X., & Liu, L. (2022). Improving Epoxy Resin Performance Using PPG and MDI by One-Step Modification. Processes, 10(5), 929. https://doi.org/10.3390/pr10050929





