Development of a Novel Green Bio-Nanofluid from Sapindus Saponaria for Enhanced Oil Recovery Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. AGC Extraction
2.2.2. Characterization of AGC and Silica-Based Nanoparticles
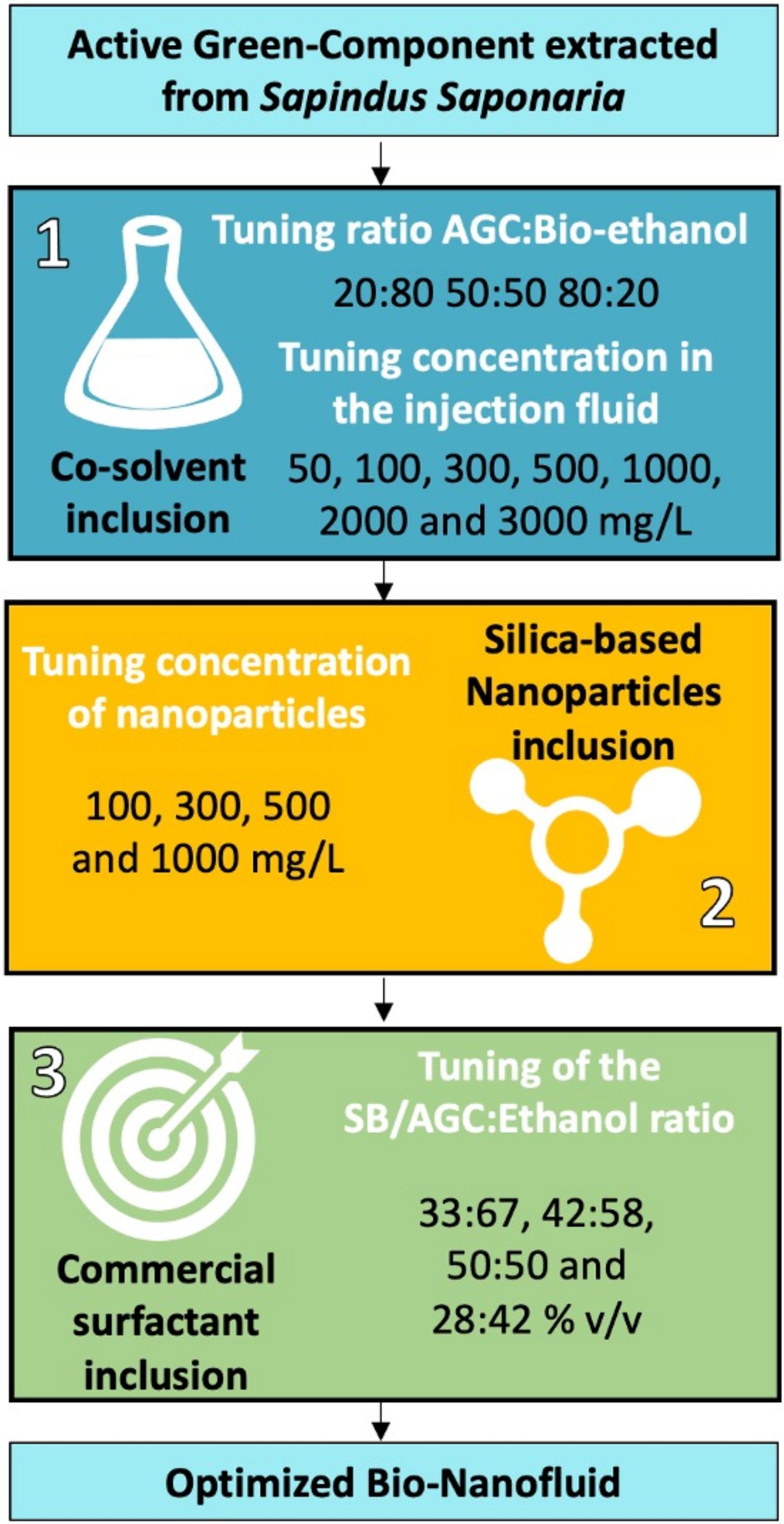
2.2.3. Bio-Nanofluid Design
2.2.4. Adsorption Test
2.2.5. Capillary Number
2.2.6. Coreflooding Test
3. Results
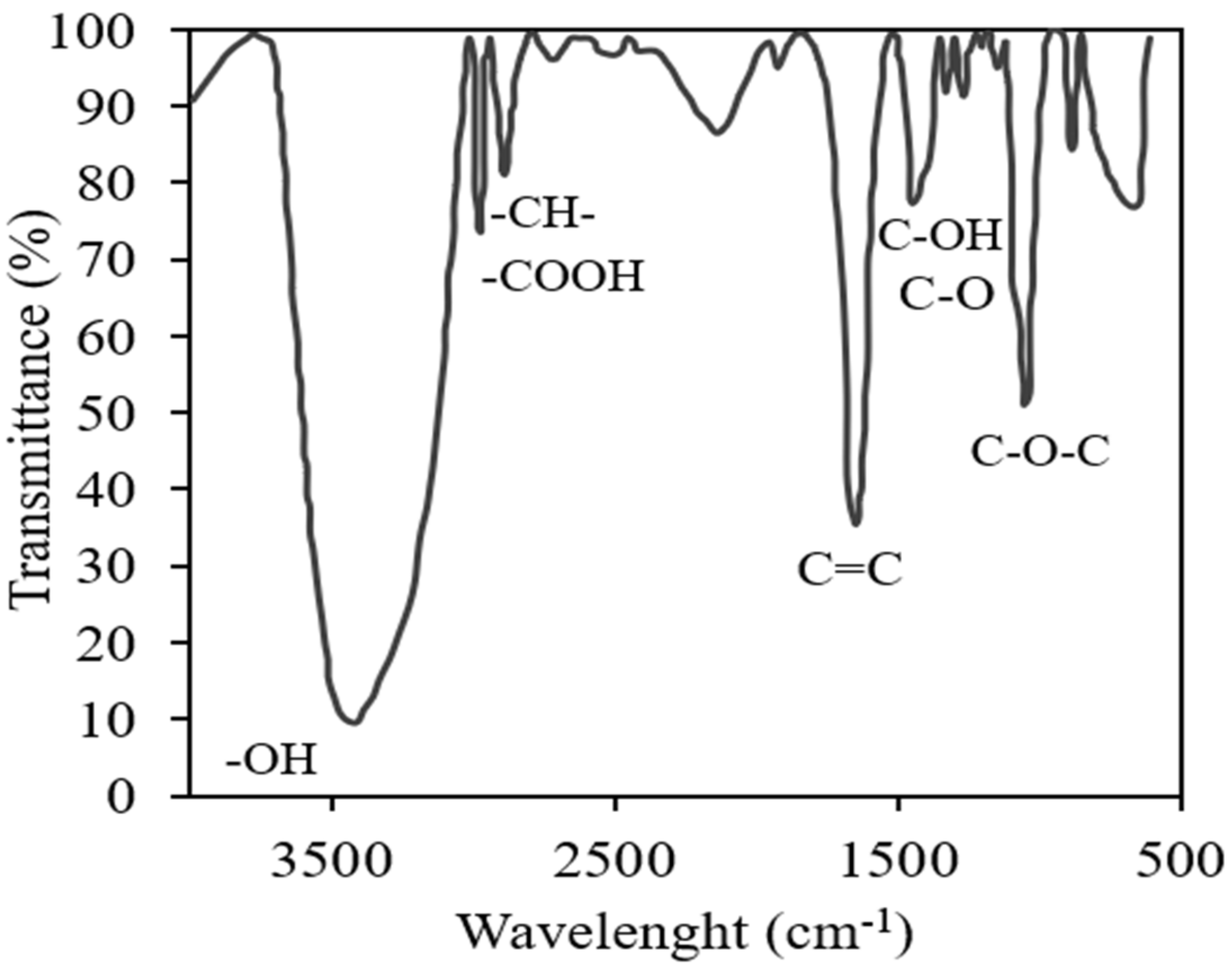
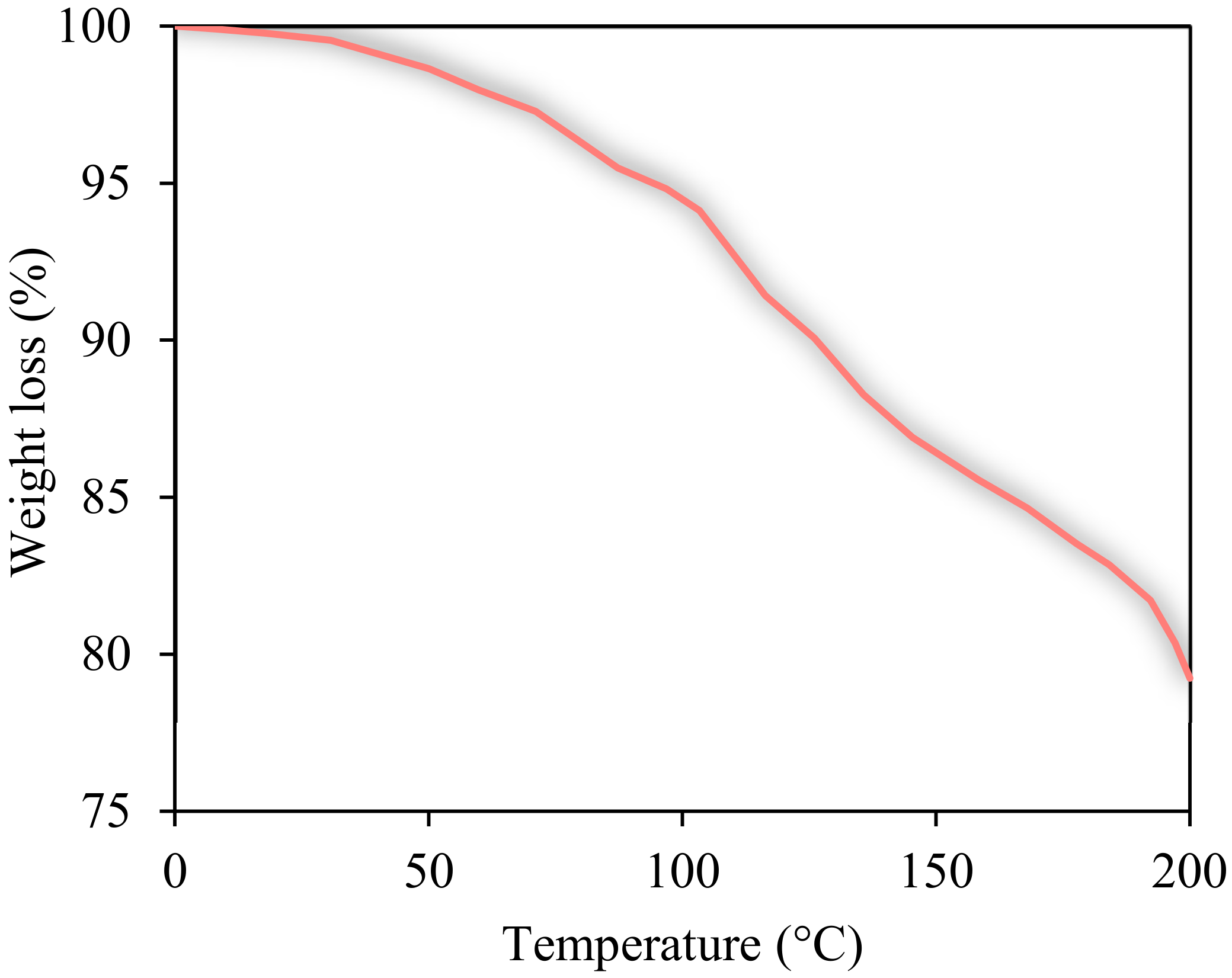
3.1. Characterization of AGC and Nanomaterials
3.2. Nanoparticles Characterization
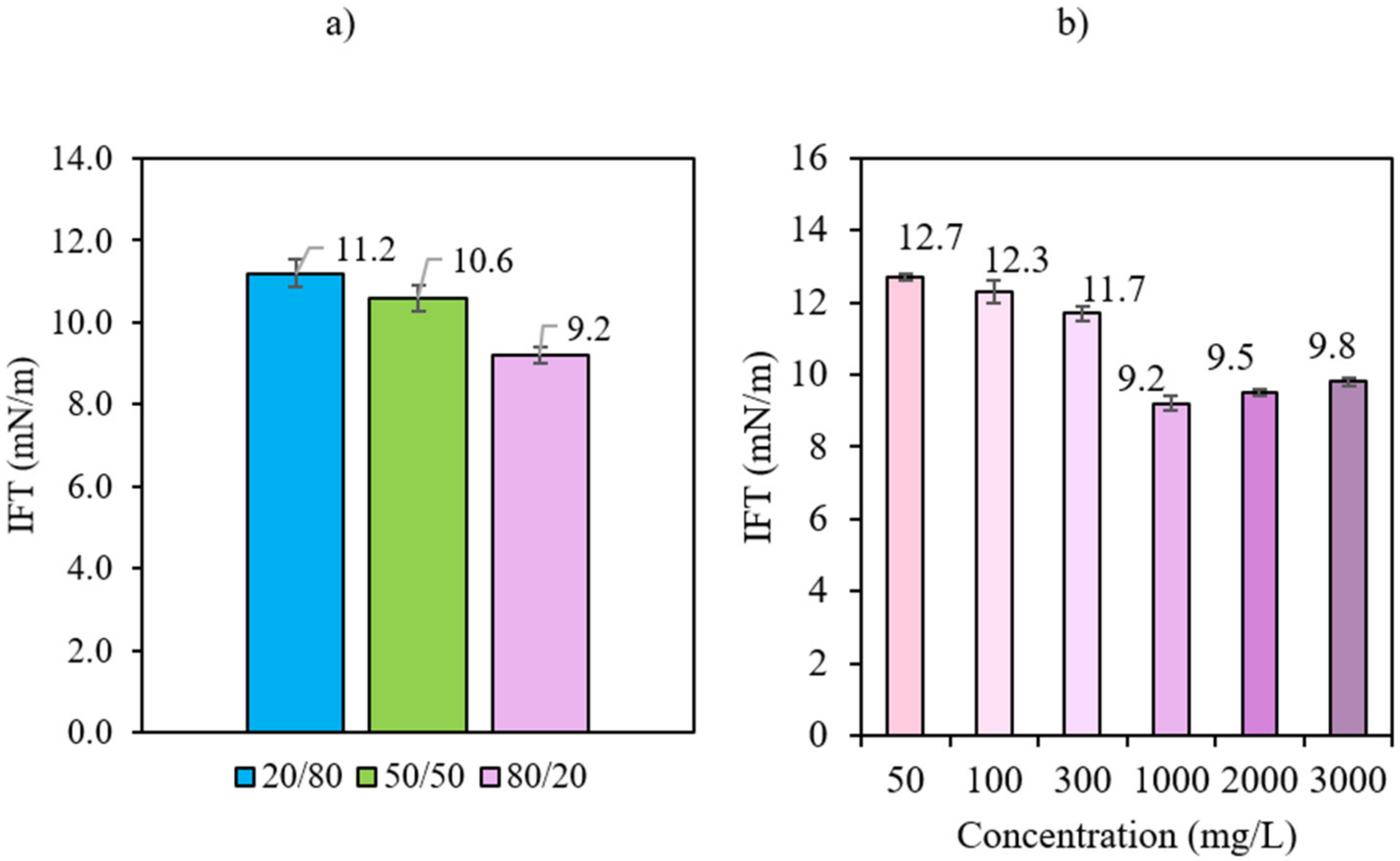
3.3. Effect of Bio-Ethanol Inclusion
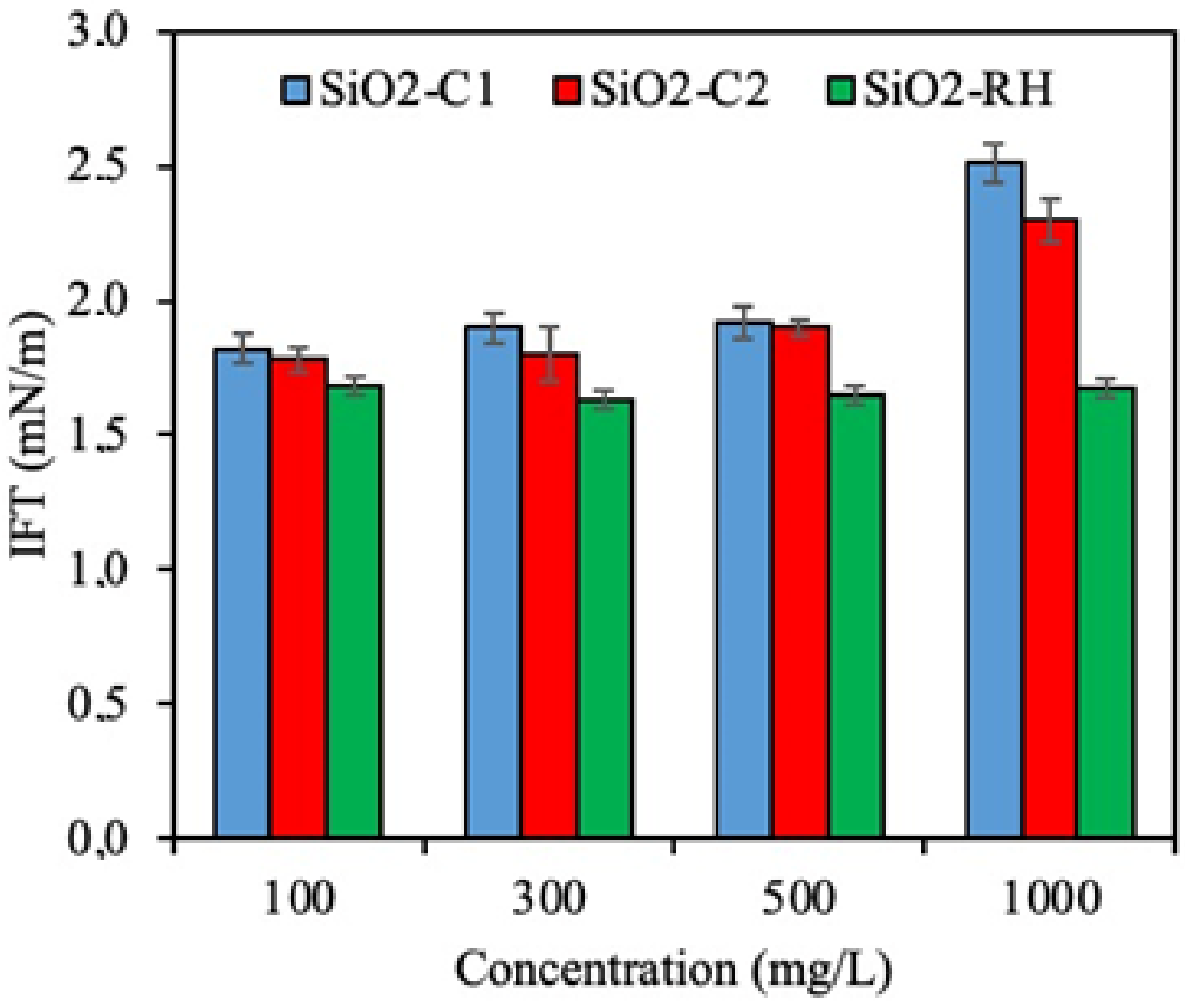
3.4. Effect of Silica-Based Nanoparticles Inclusion
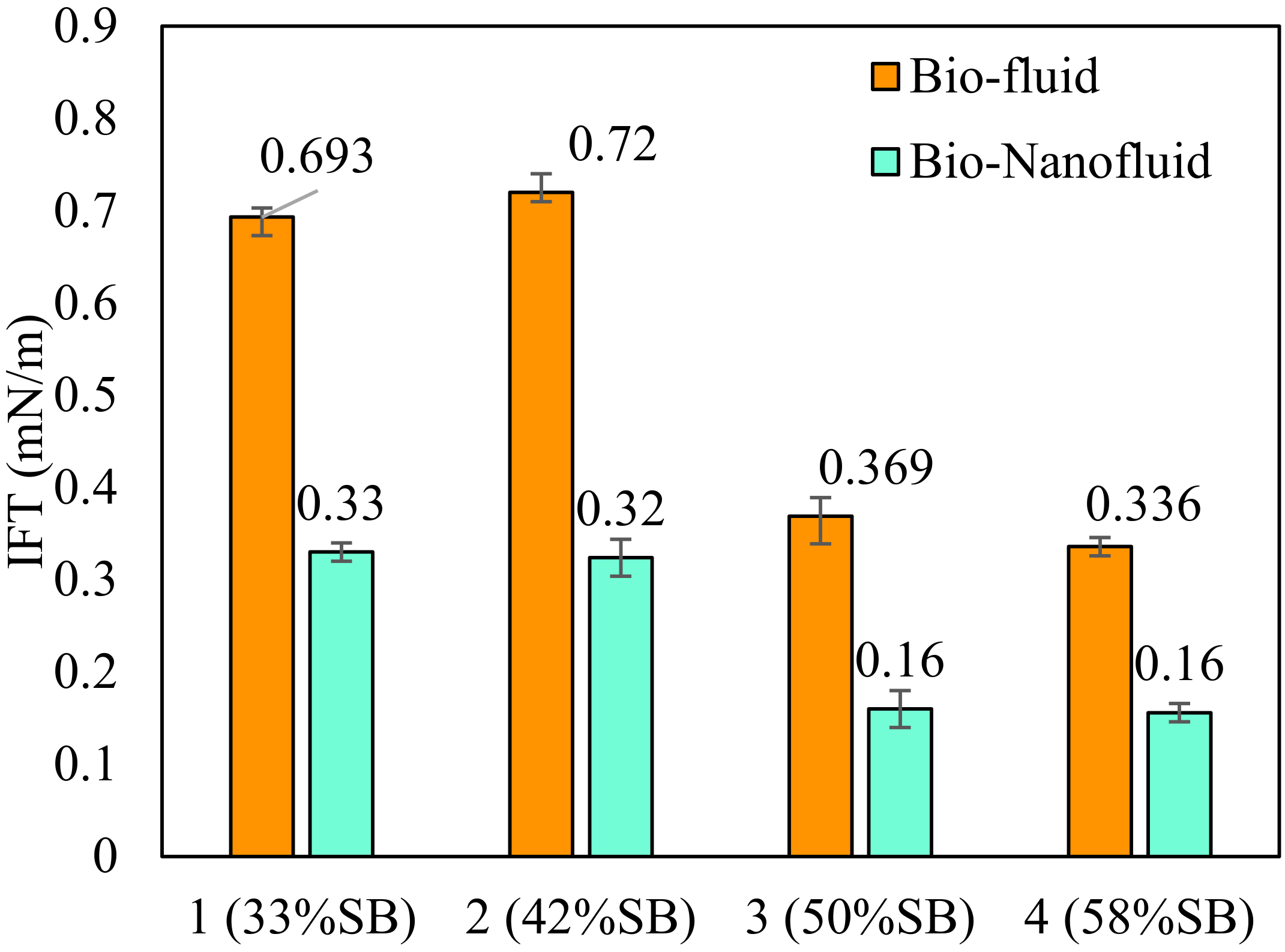
3.5. Effect of SB Inclusion
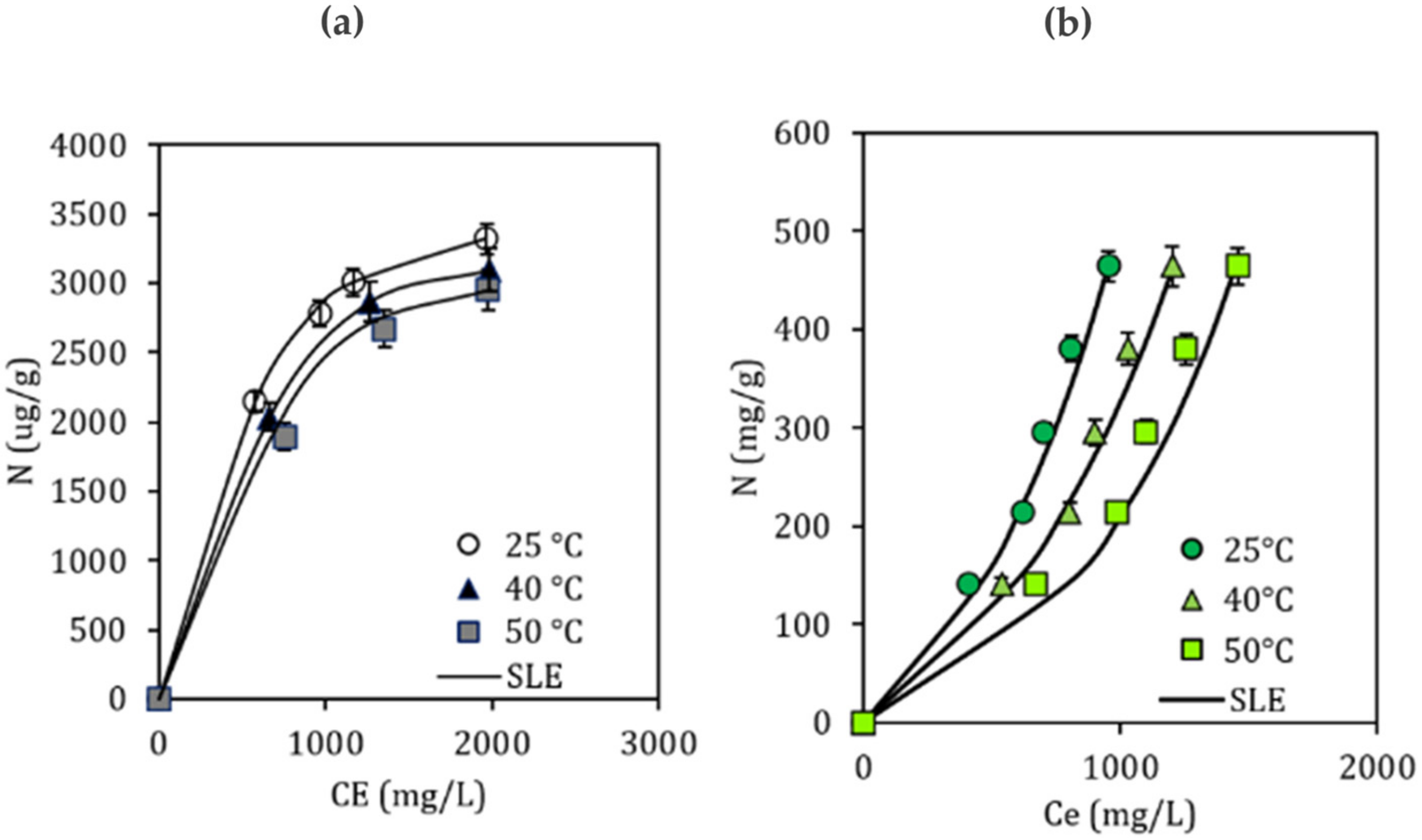
3.6. Adsorption Test
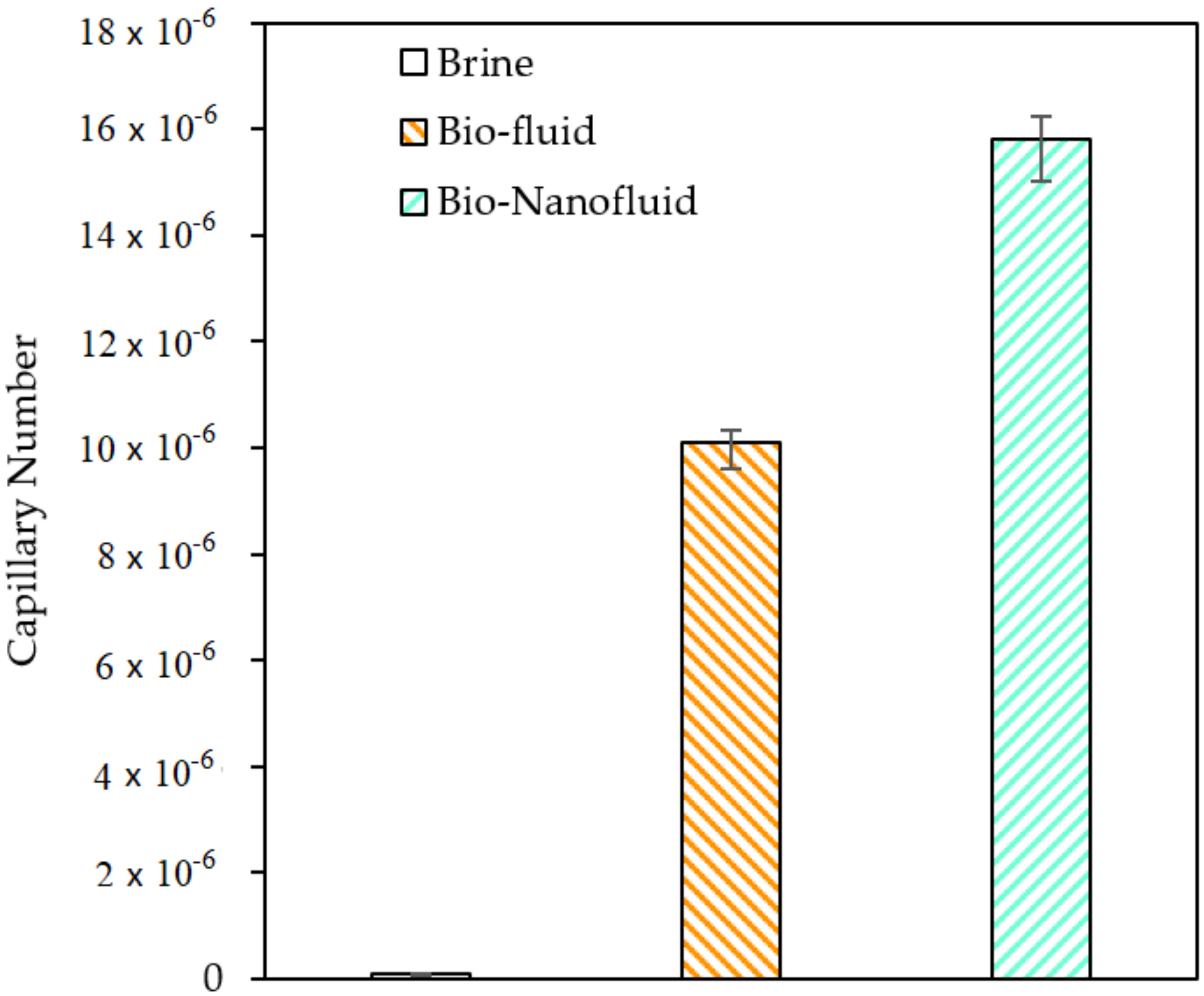
3.7. Capillary Number Estimation
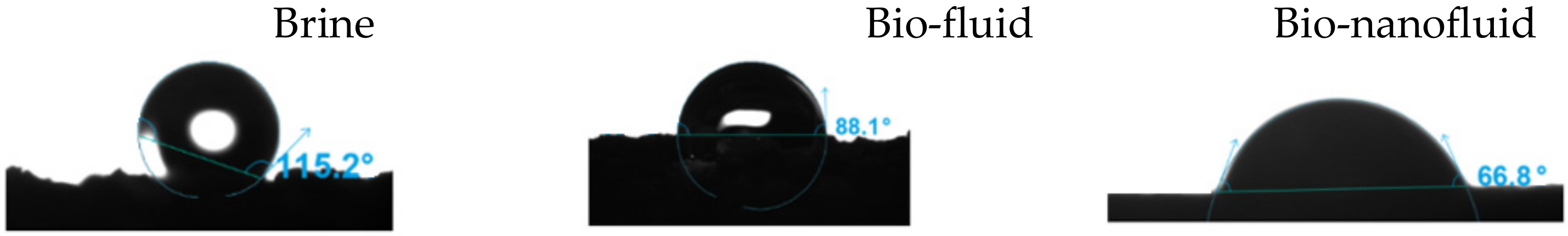
3.8. Coreflooding Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, S.; Mushtaq, M.; Banat, F.; Al Sumaiti, A.M.J.P.S. Review of surfactant-assisted chemical enhanced oil recovery for carbonate reservoirs: Challenges and future perspectives. Petrol. Sci. 2018, 15, 77–102. [Google Scholar] [CrossRef] [Green Version]
- Memon, M.K.; Elraies, K.A.; Al-Mossawy, M.I. Impact of new foam surfactant blend with water alternating gas injection on residual oil recovery. J. Pet. Explor. Prod. Technol. 2017, 7, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Patzek, T.W.J.S.R.E. Field applications of steam foam for mobility improvement and profile control. SPE Res. Eng. 1996, 11, 79–86. [Google Scholar] [CrossRef]
- Srivastava, M.; Nguyen, Q.P. Application of gas for mobility control in chemical EOR in problematic carbonate reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Nwidee, L.N.; Theophilus, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. EOR processes, opportunities and technological advancements. In Chemical Enhanced Oil Recovery (cEOR)—A Practical Overview; Books on Demand: Norderstedt, Germany, 2016; pp. 2–52. [Google Scholar]
- Petrichenko, M.; Vatin, N.; Nemova, D.; Kharkov, N.; Staritcyna, A. EOR (oil recovery enhancement) technology using shock wave in the fluid. Appl. Mech. Mater. 2014, 627, 297–303. [Google Scholar] [CrossRef]
- Samanta, A.; Ojha, K.; Sarkar, A.; Mandal, A. Surfactant and surfactant-polymer flooding for enhanced oil recovery. Adv. Pet. Explor. Dev. 2011, 2, 13–18. [Google Scholar]
- Johannessen, A.M.; Spildo, K. Enhanced oil recovery (EOR) by combining surfactant with low salinity injection. Energy Fuels 2013, 27, 5738–5749. [Google Scholar] [CrossRef]
- Amirianshoja, T.; Junin, R.; Idris, A.K.; Rahmani, O. A comparative study of surfactant adsorption by clay minerals. J. Pet. Sci. Eng. 2013, 101, 21–27. [Google Scholar] [CrossRef]
- Mehrabianfar, P.; Bahraminejad, H.; Manshad, A.K. An introductory investigation of a polymeric surfactant from a new natural source in chemical enhanced oil recovery (CEOR). J. Pet. Sci. Eng. 2021, 198, 108172. [Google Scholar] [CrossRef]
- Atta, D.Y.; Negash, B.M.; Yekeen, N.; Habte, A.D. A state-of-the-art review on the application of natural surfactants in enhanced oil recovery. J. Mol. Liq. 2020, 321, 114888. [Google Scholar] [CrossRef]
- Wu, J.; Mei, P.; Wu, J.; Fu, J.-W.; Cheng, L.; Lai, L. Surface properties and microemulsion of anionic/nonionic mixtures based on sulfonate Gemini surfactant in the presence of NaCl. J. Mol. Liq. 2020, 317, 113907. [Google Scholar] [CrossRef]
- Salager, J.-L.; Antón, R.E.; Sabatini, D.A.; Harwell, J.H.; Acosta, E.J.; Tolosa, L.I. Enhancing solubilization in microemulsions—state of the art and current trends. J. Surfactants Deterg. 2005, 8, 3–21. [Google Scholar] [CrossRef]
- Cullum, D. Surfactant types; classification, identification, separation. In Introduction to Surfactant Analysis; Springer: Berlin/Heidelberg, Germany, 1994; pp. 17–41. [Google Scholar]
- Yu, M. Development of Herding Agents for Marine Oil Spill Response. Master’s Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2021. [Google Scholar]
- Lin, W.; Chen, G.-J.; Sun, C.-Y.; Guo, X.-Q.; Wu, Z.-K.; Liang, M.-Y.; Chen, L.-T.; Yang, L.-Y. Effect of surfactant on the formation and dissociation kinetic behavior of methane hydrate. Chem. Eng. Sci. 2004, 59, 4449–4455. [Google Scholar] [CrossRef]
- Salager, J.; Morgan, J.; Schechter, R.; Wade, W.; Vasquez, E. Optimum formulation of surfactant/water/oil systems for minimum interfacial tension or phase behavior. Soc. Pet. Eng. J. 1979, 19, 107–115. [Google Scholar] [CrossRef]
- Wade, W.; Morgan, J.C.; Schechter, R.; Jacobson, J.; Salager, J. Interfacial tension and phase behavior of surfactant systems. Soc. Pet. Eng. J. 1978, 18, 242–252. [Google Scholar] [CrossRef]
- Torres, L.; Moctezuma, A.; Avendaño, J.R.; Muñoz, A.; Gracida, J. Comparison of bio-and synthetic surfactants for EOR. J. Pet. Sci. Eng. 2011, 76, 6–11. [Google Scholar] [CrossRef]
- Saxena, N.; Goswami, A.; Dhodapkar, P.; Nihalani, M.; Mandal, A. Bio-based surfactant for enhanced oil recovery: Interfacial properties, emulsification and rock-fluid interactions. J. Pet. Sci. Eng. 2019, 176, 299–311. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q.J.P. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017, 3, 197–211. [Google Scholar] [CrossRef]
- Sofla, S.J.D.; Sharifi, M.; Sarapardeh, A.H. Toward mechanistic understanding of natural surfactant flooding in enhanced oil recovery processes: The role of salinity, surfactant concentration and rock type. J. Mol. Liq. 2016, 222, 632–639. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Riahi, S. Experimental study of salinity and ionic strength effects on Critical Micelle Concentration of a cationic surfactant (HTAB). J. Appl. Chem. Res. 2020, 14, 91–107. [Google Scholar]
- Tay, A.; Mouret, A.; Mascle, M. Internal ketone sulfonate: A new bio-sourced surfactant for chemical EOR in Sea Water. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 6–9 May 2019. [Google Scholar]
- Liu, Z.; Li, Z.; Zhong, H.; Zeng, G.; Liang, Y.; Chen, M.; Wu, Z.; Zhou, Y.; Yu, M.; Shao, B. Recent advances in the environmental applications of biosurfactant saponins: A review. J. Environ. Chem. Eng. 2017, 5, 6030–6038. [Google Scholar] [CrossRef]
- Das, A.J.; Kumar, R. Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its application in oil recovery from sand matrix. Bioresour. Technol. 2018, 260, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Das, A.J.; Kumar, R. Production of biosurfactant from agro-industrial waste by Bacillus safensis J2 and exploring its oil recovery efficiency and role in restoration of diesel contaminated soil. Environ. Technol. Innov. 2019, 16, 100450. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Cheng, Y.; Xu, G. Decrease in hydrophilicity and moisture readsorption of lignite: Effects of surfactant structure. Fuel 2020, 273, 117812. [Google Scholar] [CrossRef]
- Oleszek, W.; Hamed, A. Saponin-based surfactants. In Surfactants from Renewable Resources; John Wiley & Sons, Ltd.: West Sussex, UK, 2010; pp. 239–251. [Google Scholar]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Engineering. Water-oil interfacial tension (IFT) reduction and wettability alteration in surfactant flooding process using extracted saponin from Anabasis Setifera plant. J. Pet. Sci. Eng. 2020, 189, 106901. [Google Scholar] [CrossRef]
- Bachari, Z.; Isari, A.A.; Mahmoudi, H.; Moradi, S.; Mahvelati, E.H. Application of natural surfactants for enhanced oil recovery–critical review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Prague, Czech Republic, 3–7 September 2019; p. 012039. [Google Scholar]
- Chhetri, A.; Watts, K.; Rahman, M.; Islam, M.J.E.S. Soapnut extract as a natural surfactant for enhanced oil recovery. Energy Sources A Recovery Util. Environ. Eff. 2009, 31, 1893–1903. [Google Scholar] [CrossRef]
- Deymeh, H.; Shadizadeh, S.; Motafakkerfard, R. Experimental investigation of Seidlitzia rosmarinus effect on oil–water interfacial tension: Usable for chemical enhanced oil recovery. Sci. Iran. 2012, 19, 1661–1664. [Google Scholar] [CrossRef] [Green Version]
- Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. [Google Scholar] [CrossRef]
- Seo, S.; Mastiani, M.; Mosavati, B.; Peters, D.M.; Mandin, P.; Kim, M. Performance evaluation of environmentally benign nonionic biosurfactant for enhanced oil recovery. Fuel 2018, 234, 48–55. [Google Scholar] [CrossRef]
- Betancur, S.; Giraldo, L.J.; Carrasco-Marín, F.; Riazi, M.; Manrique, E.J.; Quintero, H.; García, H.A.; Franco-Ariza, C.A.; Cortés, F.B. Importance of the nanofluid preparation for ultra-low interfacial tension in enhanced oil recovery based on surfactant–nanoparticle–brine system interaction. ACS Omega 2019, 4, 16171–16180. [Google Scholar] [CrossRef] [Green Version]
- Betancur, S.; Carrasco-Marín, F.; Pérez-Cadenas, A.n.F.; Franco, C.A.; Jiménez, J.; Manrique, E.J.; Quintero, H.; Cortés, F.B. Effect of magnetic iron core–carbon shell nanoparticles in chemical enhanced oil recovery for ultralow interfacial tension region. Energy Fuels 2019, 33, 4158–4168. [Google Scholar] [CrossRef]
- Franco, C.A.; Giraldo, L.J.; Candela, C.H.; Bernal, K.M.; Villamil, F.; Montes, D.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. Design and tuning of nanofluids applied to chemical enhanced oil recovery based on the surfactant–nanoparticle–brine interaction: From laboratory experiments to oil field application. Nanomaterials 2020, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.A.; Franco, C.A.; Zabala, R.D.; Bahamón, I.t.; Forero, A.n.; Cortés, F.B. Field Applications of nanotechnology in the oil and gas industry: Recent advances and perspectives. Energy Fuels 2021, 35, 19266–19287. [Google Scholar] [CrossRef]
- Elakkiya, V.T.; SureshKumar, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Govindarajan, M. Swift production of rhamnolipid biosurfactant, biopolymer and synthesis of biosurfactant-wrapped silver nanoparticles and its enhanced oil recovery. Saudi J. Biol. Sci. 2020, 27, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, P.; Shadizadeh, S.R.; Hayati, F.; Fattahi, M.J.P. Effects of synthesized nanoparticles and Henna-Tragacanth solutions on oil/water interfacial tension: Nanofluids stability considerations. Petroleum 2020, 6, 293–303. [Google Scholar] [CrossRef]
- Yekeen, N.; Malik, A.A.; Idris, A.K.; Reepei, N.I.; Ganie, K. Foaming properties, wettability alteration and interfacial tension reduction by saponin extracted from soapnut (Sapindus Mukorossi) at room and reservoir conditions. J. Pet. Sci. Eng. 2020, 195, 107591. [Google Scholar] [CrossRef]
- Asl, H.F.; Zargar, G.; Manshad, A.K.; Takassi, M.A.; Ali, J.A.; Keshavarz, A. Effect of SiO2 nanoparticles on the performance of L-Arg and L-Cys surfactants for enhanced oil recovery in carbonate porous media. J. Mol. Liq. 2020, 300, 112290. [Google Scholar] [CrossRef]
- Rosen, M.J.; Wang, H.; Shen, P.; Zhu, Y. Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 2005, 21, 3749–3756. [Google Scholar] [CrossRef]
- El-Batanoney, M.; Abdel-Moghny, T.; Ramzi, M. The effect of mixed surfactants on enhancing oil recovery. J. Surfactants Deterg. 1999, 2, 201–205. [Google Scholar] [CrossRef]
- Witthayapanyanon, A.; Harwell, J.; Sabatini, D. Hydrophilic–lipophilic deviation (HLD) method for characterizing conventional and extended surfactants. J. Colloid Interface Sci. 2008, 325, 259–266. [Google Scholar] [CrossRef]
- Ho, H.-O.; Hsiao, C.-C.; Sheu, M.-T. Preparation of microemulsions using polyglycerol fatty acid esters as surfactant for the delivery of protein drugs. J. Pharm. Sci. 1996, 85, 138–143. [Google Scholar] [CrossRef]
- Montes, D.; Henao, J.; Taborda, E.A.; Gallego, J.; Cortés, F.B.; Franco, C.A. Effect of textural properties and surface chemical nature of silica nanoparticles from different silicon sources on the viscosity reduction of heavy crude oil. ACS Omega 2020, 5, 5085–5097. [Google Scholar] [CrossRef] [PubMed]
- Lovato, L.; Pelegrini, B.L.; Rodrigues, J.; de Oliveira, A.J.B.; Ferreira, I.C.P. Seed oil of Sapindus saponaria L.(Sapindaceae) as potential C16 to C22 fatty acids resource. Biomass Bioenergy 2014, 60, 247–251. [Google Scholar] [CrossRef]
- Hess, H.-D.; Monsalve, L.M.; Lascano, C.E.; Carulla, J.E.; Diaz, T.; Kreuzer, M. Supplementation of a tropical grass diet with forage legumes and Sapindus saponaria fruits: Effects on in vitro ruminal nitrogen turnover and methanogenesis. Aust. J. Agric. Res. 2003, 54, 703–713. [Google Scholar] [CrossRef]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Rai, S.; Bhattarai, A. A Review on Saponin: Plant-Derived Natural Surfactants. Available online: https://encyclopedia.pub/item/revision/1aaebb7a6600912bcdb117f9977aefd1 (accessed on 4 April 2022).
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. In Application and Characterization of Surfactants; InTech Open: London, UK, 2017; pp. 184–205. [Google Scholar]
- Hernández Royero, R. Obtención de crudos de saponinas hipocolesteromizantes del Chenopodium quinoa Willd. Rev. Cuba. De Med. Mil. 1997, 26, 55–62. [Google Scholar]
- Lozsan, A.; Rivas, I.; Rodriguez, G.; Martinez, S.; Pérez, M. Determination of surface-active characteristics of a natural surfactant extracted from Sapindus saponaria. Tenside Surfactants Deterg. 2017, 54, 109–117. [Google Scholar]
- Bezerraa, K.G.; Durvala, I.J.; Silvab, I.A.; CG, F.J.C.E. Emulsifying Capacity of Biosurfactants from Chenopodium Quinoa and Pseudomonas Aeruginosa UCP 0992 with Focus of Application in the Cosmetic Industry. J. Ital. Assoc. Chem. Eng. 2020, 79, 211–216. [Google Scholar]
- Strother, T.; Lowry, S.; Bravo, B. Measurement of Dispersed Oil in Water Using an Infrared Analysis Method; Thermo Fisher Scientific: Madison, WI, USA, 2013. [Google Scholar]
- Chun, A.H.; Martin, A.N. Measurement of hydrophile-lipophile balance of surface-active agents. J. Pharm. Sci. 1961, 50, 732–736. [Google Scholar] [CrossRef]
- Fisher, M.L.; Colic, M.; Rao, M.P.; Lange, F.F. Effect of silica nanoparticle size on the stability of alumina/silica suspensions. J. Am. Ceram. Soc. 2001, 84, 713–718. [Google Scholar] [CrossRef]
- Franco, C.A.; Martínez, M.; Benjumea, P.; Patiño, E.; Cortés, F.B. Water remediation based on oil adsorption using nanosilicates functionalized with a petroleum vacuum residue. Adsorpt. Sci. Technol. 2014, 32, 197–207. [Google Scholar] [CrossRef]
- Milanova, D.; Kumar, R. Heat transfer behavior of silica nanoparticles in pool boiling experiment. J. Heat Transf. 2008, 130, 042401. [Google Scholar] [CrossRef]
- Dietrich, P.; Lerche, K.-H.; Reusch, J.; Nitzsche, R. The characterization of silica microparticles by electrophoretic mobility measurements. Chromatographia 1997, 44, 362–366. [Google Scholar] [CrossRef]
- Salager, J.-L.; Andérez, J.M.; Briceño, M.I.; De Sanchez, M.P.; de Gouveia, M.R. Emulsification yield related to formulation and composition variables as well as stirring energy. Rev. Téc. Ing. Univ. Zulia 2002, 25, 129–139. [Google Scholar]
- Salager, J.; Loaiza-Maldonado, I.; Minana-Perez, M.; Silva, F. Surfactant-oil-water systems near the affinity inversion part I: Relationship between equilibrium phase behavior and emulsion type and stability. J. Dispers. Sci. Technol. 1982, 3, 279–292. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Wantke, K.-D. Determination of the surface tension of surfactant solutions applying the method of Lecomte du Noüy (ring tensiometer). Colloid Polym. Sci. 1981, 259, 354–366. [Google Scholar] [CrossRef]
- Negm, N.A.; Morsy, S.M.; Said, M.M. Biocidal activity of some Mannich base cationic derivatives. Bioorg. Med. Chem. 2005, 13, 5921–5926. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, M.; Yu, Y.-H.; Wang, H.; Mannan, M.S.; Cheng, Z. Thermosensitive ZrP-PNIPAM Pickering emulsifier and the controlled-release behavior. ACS Appl. Mater. Interfaces 2017, 9, 7852–7858. [Google Scholar] [CrossRef]
- Elmendorp, J.; De Vos, G. Measurement of interfacial tensions of molten polymer systems by means of the spinning drop method. Polym. Eng. Sci. 1986, 26, 415–417. [Google Scholar] [CrossRef]
- Verdier, C.; Vinagre, H.T.; Piau, M.; Joseph, D.D. High temperature interfacial tension measurements of PA6/PP interfaces compatibilized with copolymers using a spinning drop tensiometer. Polymer 2000, 41, 6683–6689. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Glogowski, E.; Emrick, T.; Russell, T.P.; Dinsmore, A.D. Adsorption energy of nano-and microparticles at liquid− liquid interfaces. Langmuir 2010, 26, 12518–12522. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Gallego, J.; Villegas, J.P.; Franco, C.A.; Cortés, F.B. Enhanced waterflooding with NiO/SiO2 0-D Janus nanoparticles at low concentration. J. Pet. Sci. Eng. 2019, 174, 40–48. [Google Scholar] [CrossRef]
- Villegas, J.P.; Moncayo-Riascos, I.; Galeano-Caro, D.; Riazi, M.; Franco, C.A.; Cortés, F.B. Functionalization of γ-alumina and magnesia nanoparticles with a fluorocarbon surfactant to promote ultra-gas-wet surfaces: Experimental and theoretical approach. ACS Appl. Mater. Interfaces 2020, 12, 13510–13520. [Google Scholar] [CrossRef] [PubMed]
- Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Cortés, F.B. Development of composite materials based on the interaction between nanoparticles and surfactants for application in chemical enhanced oil recovery. Ind. Eng. Chem. Res. 2018, 57, 12367–12377. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.D.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2 nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. A novel solid–liquid equilibrium model for describing the adsorption of associating asphaltene molecules onto solid surfaces based on the “chemical theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
- Llanos, S.n.; Giraldo, L.J.; Santamaria, O.; Franco, C.A.; Cortés, F.B. Effect of sodium oleate surfactant concentration grafted onto SiO2 nanoparticles in polymer flooding processes. ACS Omega 2018, 3, 18673–18684. [Google Scholar] [CrossRef] [Green Version]
- Acosta, L.; Galeano-Caro, D.; Medina, O.E.; Cortés, F.B.; Franco, C.A. Nano-intermediate of magnetite nanoparticles supported on activated carbon from spent coffee grounds for treatment of wastewater from oil industry and energy production. Processes 2021, 9, 63. [Google Scholar] [CrossRef]
- Gupta, S.P.; Trushenski, S.P. Micellar flooding-compositional effects on oil displacement. Soc. Pet. Eng. J. 1979, 19, 116–128. [Google Scholar] [CrossRef]
- Johannesen, E.B.; Graue, A. Mobilization of remaining oil-emphasis on capillary number and wettability. In Proceedings of the International Oil Conference and Exhibition in Mexico, Veracruz, Mexico, 27–30 June 2007. [Google Scholar]
- Xia, H.; Wang, D.; Wu, W.; Jiang, H. Effect of the visco-elasticity of displacing fluids on the relationship of capillary number and displacement efficiency in weak oil-wet cores. In Proceedings of the Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 30 October–1 November 2007. [Google Scholar]
- Bashiri, A.; Kasiri, N. Properly use effect of capillary number on residual oil saturation. In Proceedings of the Nigeria Annual International Conference and Exhibition, Abuja, Nigeria, 30 July–3 August 2011. [Google Scholar]
- Moore, T.; Slobod, R. Displacement of oil by water-effect of wettability, rate, and viscosity on recovery. In Proceedings of the Fall Meeting of the Petroleum Branch of AIME, New Orleans, LA, USA, 2–5 October 1955. [Google Scholar]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability alteration of sandstone cores by alumina-based nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Franco-Aguirre, M.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Cortés, F.B. Interaction of anionic surfactant-nanoparticles for gas-Wettability alteration of sandstone in tight gas-condensate reservoirs. J. Nat. Gas Sci. Eng. 2018, 51, 53–64. [Google Scholar] [CrossRef]
- Almutairi, M.S.; Ali, M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat. Prod. Res. 2015, 29, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Kareru, P.; Keriko, J.; Gachanja, A.; Kenji, G. Direct detection of triterpenoid saponins in medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 56–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samal, K.; Das, C.; Mohanty, K. Eco-friendly biosurfactant saponin for the solubilization of cationic and anionic dyes in aqueous system. Dye. Pigment. 2017, 140, 100–108. [Google Scholar] [CrossRef]
- Magthalin, C.J.; Varadharajan, A.; Swarnalatha, S.; Sekaran, G. Utilization of chicken tallow for the production of cationic biosurfactant and thereof for decontamination of Cr (III) containing soil. Procedia Environ. Sci. 2016, 35, 895–913. [Google Scholar] [CrossRef]
- De Araujo, L.L.; Sodré, L.G.; Brasil, L.R.; Domingos, D.F.; de Oliveira, V.M.; da Cruz, G.F. Microbial enhanced oil recovery using a biosurfactant produced by Bacillus safensis isolated from mangrove microbiota-Part I biosurfactant characterization and oil displacement test. J. Pet. Sci. Eng. 2019, 180, 950–957. [Google Scholar] [CrossRef]
- Bordoloi, N.; Konwar, B. Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J. Hazard. Mater. 2009, 170, 495–505. [Google Scholar] [CrossRef]
- Fuseni, A.B.; Al-Zahrani, B.H.; AlSofi, A.M. Critical micelle concentration of different classes of EOR surfactants under representative field conditions. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017. [Google Scholar]
- Gadhave, A. Determination of hydrophilic-lipophilic balance value. IJSR 2014, 3, 573–575. [Google Scholar]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si (OR) 4 and R′′ Si (OR′) 3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Hurtado, Y.; Beltran, C.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Nassar, N.N.; Cortes, F.B. Effects of surface acidity and polarity of SiO2 nanoparticles on the foam stabilization applied to natural gas flooding in tight gas-condensate reservoirs. Energy Fuels 2018, 32, 5824–5833. [Google Scholar] [CrossRef]
- Usgodaarachchi, L.; Thambiliyagodage, C.; Wijesekera, R.; Bakker, M.G. Synthesis of mesoporous silica nanoparticles derived from rice husk and surface-controlled amine functionalization for efficient adsorption of methylene blue from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100116. [Google Scholar] [CrossRef]
- Rojas, D.F.H.; Gómez, P.P.; Rivera, A.R. Production and characterization of silica nanoparticles from rice husk. Adv. Mater. Lett 2019, 10, 67–73. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Shojaei, S.; Riazi, M.; Sharifi, M. Review on application of nanoparticles for EOR purposes: A critical review of the opportunities and challenges. Chin. J. Chem. Eng. 2019, 27, 237–246. [Google Scholar] [CrossRef]
- Franco, C.A.; Zabala, R.; Cortés, F.B. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Pet. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Medina-Erazo, O.; Castaño-Correa, J.; Caro-Vélez, C.; Zabala-Romero, R.; Bahamón-Pedrosa, J.I.; Cortés-Correa, F.; Franco-Ariza, C. Disaggregation and discretization methods for formation damage estimation in oil and gas fields: An overview. Dyna 2020, 87, 105–115. [Google Scholar] [CrossRef]
- Baviere, M.; Schechter, R.; Wade, W. The influence of alcohols on microemulsion composition. J. Colloid Interface Sci. 1981, 81, 266–279. [Google Scholar] [CrossRef]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Sharma, T. Rheological analysis and EOR potential of surfactant treated single-step silica nanofluid at high temperature and salinity. J. Pet. Sci. Eng. 2021, 196, 107704. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Vatanparast, H.; Eftekhari, M.; Javadi, A.; Miller, R.; Bahramian, A. Influence of hydrophilic silica nanoparticles on the adsorption layer properties of non-ionic surfactants at water/heptane interface. J. Colloid Interface Sci. 2019, 545, 242–250. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Azam, M.R.; Tan, I.M.; Ismail, L.; Mushtaq, M.; Nadeem, M.; Sagir, M. Static adsorption of anionic surfactant onto crushed Berea sandstone. J. Pet. Explor. Prod. Technol. 2013, 3, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, W.; Dai, C.; Huang, Y.; Li, H.; Zhao, M.; He, L.; Jiao, B. Reducing surfactant adsorption on rock by silica nanoparticles for enhanced oil recovery. J. Pet. Sci. Eng. 2017, 153, 283–287. [Google Scholar] [CrossRef]
- Suresh, R.; Kuznetsov, O.; Agrawal, D.; Darugar, Q.; Khabashesku, V. Reduction of surfactant adsorption in porous media using silica nanoparticles. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2018. [Google Scholar]
- Guzmán, J.D.; Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Importance of the adsorption method used for obtaining the nanoparticle dosage for asphaltene-related treatments. Energy Fuels 2016, 30, 2052–2059. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, F.; Wang, Y.; Ma, L.; Wu, Y.; Kerr, P.G.; Yang, L. Basic dye adsorption onto an agro-based waste material—Sesame hull (Sesamum indicum L.). Bioresour. Technol. 2011, 102, 10280–10285. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of surfactants on sand surface in enhanced oil recovery: Isotherms, kinetics and thermodynamic studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Seyyedi, M.; Sohrabi, M.; Farzaneh, A. Investigation of rock wettability alteration by carbonated water through contact angle measurements. Energy Fuels 2015, 29, 5544–5553. [Google Scholar] [CrossRef]
- Amirmoshiri, M.; Zhang, L.; Puerto, M.C.; Tewari, R.D.; Bahrim, R.Z.B.K.; Farajzadeh, R.; Hirasaki, G.J.; Biswal, S.L. Role of wettability on the adsorption of an anionic surfactant on sandstone cores. Langmuir 2020, 36, 10725–10738. [Google Scholar] [CrossRef]
- Somasundaran, P.; Zhang, L. Adsorption of surfactants on minerals for wettability control in improved oil recovery processes. J. Pet. Sci. Eng. 2006, 52, 198–212. [Google Scholar] [CrossRef]
- Imuetinyan, H.; Agi, A.; Gbadamosi, A.; Junin, R. Extraction, characterization and evaluation of saponin-based natural surfactant for enhanced oil recovery. Arab. J. Geosci. 2022, 15, 226. [Google Scholar] [CrossRef]







| Property | Core 1 | Core 2 |
|---|---|---|
| Length (cm) | 7.3 | 7.5 |
| Diameter (cm) | 3.8 | 4.0 |
| Porosity (%) | 12 | 13 |
| Permeability (mD) | 149 | 155 |
| Mineralogy | 50% Ottawa sand–50% reservoir core cuts | |
| Silica-Based Nanoparticle | Concentration (mg·L−1) | |
|---|---|---|
| SiO2-C1 | 100 | 130.05 |
| 300 | 128.64 | |
| 500 | 128.29 | |
| 1000 | 117.89 | |
| SiO2-C2 | 100 | 144.88 |
| 300 | 144.49 | |
| 500 | 142.54 | |
| 1000 | 134.73 | |
| SiO2-RH | 100 | 194.19 |
| 300 | 195.48 | |
| 500 | 194.96 | |
| 1000 | 194.45 |
| Component | Concentration in the Bio-Nanofluid |
|---|---|
| AGC:bio-ethanol (80:20) | 50% v·v−1 |
| Commercial surfactant (SB) | 50% v·v−1 |
| SiO2-RH nanoparticles | 100 mg·L−1 |
| Adsorbent | Temperature (°C) | (mg·g−1) | (g·g−1) | ||
|---|---|---|---|---|---|
| Ottawa sand | 25 | 0.42 | 0.75 | 3.77 | 2.05 |
| 40 | 0.94 | 1.58 | 3.39 | 0.01 | |
| 50 | 1.00 | 1.59 | 3.26 | 8.40 | |
| SiO2-RH nanoparticles | 25 °C | 4.40 | 1.51 | 1678.30 | 2.95 |
| 40 °C | 5.83 | 1.64 | 1382.30 | 3.79 | |
| 50 °C | 9.52 | 2.87 | 1225.20 | 7.10 |
| Adsorbent | Temperature (°C) | (J· (mol·K)−1) | (kJ· mol−1) | (J· mol−1) |
|---|---|---|---|---|
| Ottawa sand | 25 | 0.08 | 24.23 | 0.73 |
| 40 | 1.19 | |||
| 50 | 1.25 | |||
| SiO2-RH nanoparticles | 25 | 0.07 | 20.50 | 1.03 |
| 40 | 1.29 | |||
| 50 | 2.83 |
| System | Density (g·cm−3) | Viscosity (m·Pas·s−1) @ 5.6 s−1 |
|---|---|---|
| Brine | 1.011 | 1.058 |
| Bio-fluid | 1.036 | 1.064 |
| Bio-nanofluid | 1.044 | 1.165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo, L.J.; Galeano-Caro, D.; Franco, C.A.; Archila, J.; Villamil, F.; Cortés, F.B.; Franco, C.A. Development of a Novel Green Bio-Nanofluid from Sapindus Saponaria for Enhanced Oil Recovery Processes. Processes 2022, 10, 1057. https://doi.org/10.3390/pr10061057
Giraldo LJ, Galeano-Caro D, Franco CA, Archila J, Villamil F, Cortés FB, Franco CA. Development of a Novel Green Bio-Nanofluid from Sapindus Saponaria for Enhanced Oil Recovery Processes. Processes. 2022; 10(6):1057. https://doi.org/10.3390/pr10061057
Chicago/Turabian StyleGiraldo, Lady J., Dahiana Galeano-Caro, Carlos A. Franco, Jesús Archila, Fabio Villamil, Farid B. Cortés, and Camilo A. Franco. 2022. "Development of a Novel Green Bio-Nanofluid from Sapindus Saponaria for Enhanced Oil Recovery Processes" Processes 10, no. 6: 1057. https://doi.org/10.3390/pr10061057






