Abstract
Bacillus subtilis spores have important biological applications; however, high spore-cell densities and sporulation efficiencies in fermentation is poorly reported. This study systematically analyzed the spore densities and formation efficiency of B. subtilis BSNK-5 in different culture substrates. A response surface regression equation was established based on the results of single factor and Box–Behnken experimental designs. The optimal medium formulation, as predicted from the equation, consisted of soluble starch at 3 g·L−1, soybean flour at 12 g·L−1, and MgSO4 at 5 g·L−1. The spore yield reached 2.43 × 109 CFU·mL−1, and the sporulation rate was 83.3%, which was nearly three times higher than before optimization using an optimized medium at 36 °C and 200 rpm for 60 h.
1. Introduction
Bacillus subtilis is an aerobic Gram-positive probiotic which is tolerated by humans and animals. It can form endospores that have a thick cell wall, contains no built-in toxins, and is extremely resistant to all manner of harsh treatments [1]. When nutrients are limited, B. subtilis stops growing and begins sporulation, releasing mature spores through asymmetric cell division, endocytosis, and mother cell lysis. When a dormant spore encounters the right environmental stimulus, it initiates the germination process, and if sufficient nutrients are present, the spore germinates into a vegetative bacterium [2]. In recent years, research into the application of Bacillus in various aspects of life has increased all over the world. This probiotic can be used to replace antibiotics in agriculture and aquaculture or to produce new food and dietary supplements in human products [3].
As a probiotic preparation, B. subtilis is widely used in industry and is the most studied spore-forming bacterium [4]. B. subtilis can be used as a dietary supplement to maintain intestinal microecological balance. Through colonizing and consuming oxygen in the intestinal environment, it can promote the growth of anaerobic probiotics (e.g., lactic acid bacteria, bifidobacteria) and prevent the reproduction of some harmful aerobic bacteria (e.g., salmonella), resulting in the ability to both prevent gastrointestinal diseases and treat uremia [5]. In poultry farming, B. subtilis can be directly added to feed to replace antibiotics, improve the digestibility of nutrients, promote body growth, enhance immunity, and provide enzymes needed by a variety of animals [6]. In addition, with the help of fermentation technology, B. subtilis can be used to make foods, among which natto is the most prominent. Microbial metabolism can improve the nutritional composition of soybean, and enrich the bioactive ingredients of functional peptides, aglycone isoflavones, nattokinase, vitamin K, and polyglutamic acid, playing an important role in the prevention of cardiovascular disease and osteoporosis [7,8].
The aforementioned studies have shown that B. subtilis and its metabolites can affect growth, digestion, and immune performance. The concentration of B. subtilis is the main factor in determining its prebiotic effect. In the production process, complex processing technology and a harsh processing environment will reduce the survival rate of strains. The formation of spores can make the bacteria more resistant to extreme conditions, such as pH, heat, cold, and pressure and improve the survival rate of strains under different production processes, as well as improving the colonization rate in the stomach. The higher the spore content, the more suitable it is for industrial processing. During fermentation, the content of spores is also an important index to evaluate the fermentation effect [9]. Although there have been many studies on improving the spore yield of Bacillus by changing the medium components and culture conditions, different strains require their own specific culture medium components [10]. For example, for Bacillus cereus, the presence of glutamate in the medium favors its growth, but this compound inhibits the growth of other species of Bacillus [11]. Therefore, it is of great significance to study the spore-forming characteristics of B. subtilis BSNK-5, a high-yield nattokinase strain isolated and identified by our laboratory.
In order to make better use of the spores of BSNK-5, this study optimized the medium composition using response surface methodology to enhance the sporulation efficiencies of BSNK-5. Response surface analysis is a collection of mathematical and statistical techniques widely used in the food industry [12]. It uses multiple quadratic regression equations to find functional relationships between factors and response values, evaluate the relationship between independent variables and predicted values of dependent variables, and analyze and solve multivariate problems [13]. The use of response surface analysis in the optimization of Bacillus culture medium components can reduce development costs, optimize experimental conditions, and improve production efficiency [14]. Therefore, it is an effective method to solve the optimization of multiple components.
2. Materials and Methods
2.1. Strain Culture
2.1.1. Microorganism and Culture Mediums
The BSNK-5 strain was screened from fermented soybean products and identified by the 16S rDNA gene in our laboratory [15]. The bacteria were stored in Luria-Bertani (LB) broth containing 20% v/v glycerol at −80 °C and activated in an LB solid plate for 12 h at 37 °C before any experimental use.
The LB broth medium was composed of yeast extract (5 g·L−1), tryptone (10 g·L−1), and NaCl (10 g·L−1). The agar (0.1 g·L−1) was added to LB liquid medium to form the LB solid medium. The optimized medium was changed based on the composition of carbon source (yeast extract), nitrogen source (tryptone), and inorganic salt (NaCl) of the LB medium.
2.1.2. Culture Conditions
A single colony of activated BSNK-5 in the LB solid plate was first inoculated into 100 mL of LB broth medium and grown at 37 °C and 200 rpm for 12 h. An amount of 1 mL of LB broth medium was transferred to 100 mL optimized media and grown at 37 °C and 200 rpm for 60 h.
2.2. Measurement of Total Cell Density and Spore Density
The total cell density and the spore density were calculated according to the plate count method. Serial tenfold dilutions of the cell suspensions of BSNK-5 were prepared. Then, 0.1 mL of the 10−6 and 10−7 dilutions were spread in an LB solid plate. These plates were incubated at 37 °C for 12 h. Viable cell concentration was calculated and expressed as colony-forming units per milliliter (CFU·mL−1). The cell suspensions were heated at 80 °C for 20 min to harvest the spores. The spores were counted using the same method. The spore rate was calculated as the percentage of the spore count to the total number of viable cells of BSNK-5.
2.3. Single Factor Test in Culture Medium
2.3.1. Carbon Source Screening
Based on the LB medium, different carbon sources (yeast extract, glucose, sucrose, soluble starch or lactose) with a concentration of 5 g·L−1 were used to replace the yeast extract of the LB medium. The optimal carbon source was determined according to the amount of spore formation. Then, based on the best carbon source, five concentrations of 1, 3, 5, 7 and 9 g·L−1 were set to determine the optimal concentration. The total cell density, spore cell density and sporulation efficiency were calculated after being cultured for 60 h at 37 °C and 200 rpm.
2.3.2. Nitrogen Source Screening
Based on the LB medium, different nitrogen sources (tryptone, soybean flour, beef extract, (NH4)2SO4 or urea) with a concentration of 10 g·L−1 were used to replace the tryptone of the LB medium. The optimal nitrogen source was determined according to the amount of spore formation. Then, based on the best nitrogen source, five concentrations of 6, 8, 10, 12 and 14 g·L−1 were set to determine the optimal concentration. The total cell density, spore cell density and sporulation efficiency were calculated after being cultured for 60 h at 37 °C and 200 rpm.
2.3.3. Inorganic Salts Screening
Based on the LB medium, using different inorganic salts (NaCl, MgSO4, K2HPO4, CaCO3 or KCl) with a concentration of 5 g·L−1, the optimal inorganic salt was determined according to the amount of spore formation. For the best inorganic salt, five concentrations of 1, 3, 5, 7, and 9 g·L−1 were set to determine the optimal concentration. The total cell density, spore cell density and sporulation efficiency were measured after being cultured for 60 h at 37 °C and 200 rpm.
2.4. Optimize Culture Medium by Response Surface Methodology
The Box–Behnken design (BBD) of RSM demonstrates a mathematical model of the interaction between independent variables in medium composition. Important variables affecting sporulation in BSNK-5 were optimized using a BBD.
According to the results of the single factor experiments, the optimal combination of the best carbon source, nitrogen source and inorganic salt was further examined by using the zero-level center point of the response surface. High (+1) and low (−1) levels are one actual concentration higher or lower, respectively. Design Expert 12.0.7.1 software was used to design response surface experiments and perform the regression and graphic analysis. Variance (ANOVA) and LSD multiple comparison tests were performed on the obtained data.
2.5. Statistical Analysis
All treatments were performed in triplicate, and data are expressed as mean ± SD. The data of the total cell and spore cell density (CFU·mL−1) were transformed using a logarithmic scale (Log10). Graphs were generated using GraphPad Prism 8 software (GraphPad Software Inc., La Jolla, CA, USA). Statistical comparisons were made by a two-tailed Student’s t-test using IBM SPSS software (Version 22, New York, NY, USA). Differences were regarded as statistically significant for p < 0.05, p < 0.01 and p < 0.001. The combined graphs were generated using Adobe Photoshop CS5 and Adobe Illustrator CS5 (Adobe Systems Inc., San Jose, CA, USA).
3. Results
3.1. Screening the Best Carbon Source and Its Optimum Concentration
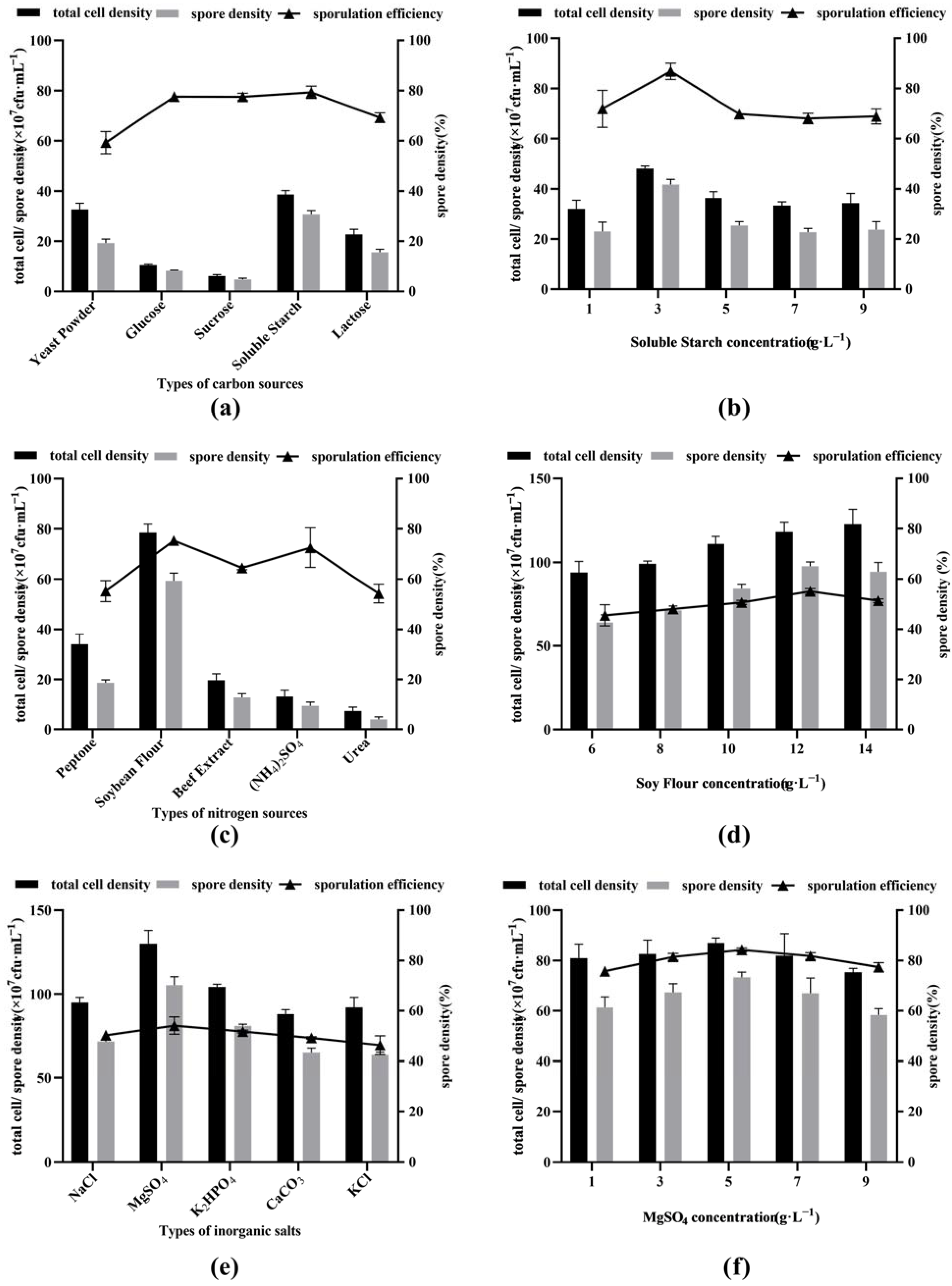
Among the five carbon sources (yeast extract, glucose, sucrose, soluble starch and lactose) with a concentration of 5 g·L−1, the total cell density and spore density of soluble starch were the highest, followed by those of yeast extract, lactose, glucose and sucrose. However, in terms of sporulation efficiency, soluble starch, glucose and lactose were the highest, followed by lactose and yeast extract (Figure 1a).
Figure 1.
Screening of different carbon sources: (a,b), nitrogen sources (c,d), and inorganic salts (e,f) in medium.
Based on the above experimental results, soluble starch was selected as the best carbon source for inducing BSNK-5 to produce spores, and its optimal concentration was further studied. When different concentrations (1, 3, 5, 7 and 9 g·L−1) of soluble starch were used as the only carbon source, the total cell density, spore cell density and sporulation efficiency showed a trend of first increasing and then decreasing with the increase in soluble starch concentration. The peak appeared at a concentration of 3 g·L−1 of soluble starch, in which the total cell density, spore cell density, and sporulation efficiency were 4.8 × 108 CFU·mL−1, 4.2 × 108 CFU·mL−1, and 86.8%, respectively (Figure 1b). Therefore, soluble starch with a concentration of 3 g·L−1 was selected as the best carbon source and the best concentration.
3.2. Screening the Best Nitrogen Source and Its Optimum Concentration
As shown in Figure 1c, among the five nitrogen sources (tryptone, soybean flour, beef extract, (NH4)2SO4 and urea) with a concentration of 10 g·L−1, on the whole, the total cell density, spore density and sporulation efficiency of the organic nitrogen sources (tryptone, soybean flour, and beef extract) were higher than those of the inorganic nitrogen sources ((NH4)2SO4 and urea). The total cell density, spore density, and sporulation efficiency of soybean flour were the highest in organic nitrogen, followed by those of tryptone and beef extract.
Then, different concentrations (6, 8, 10, 12, and 14 g·L−1) of soybean flour were used as the only nitrogen source to analyze the effect of BSNK-5 on spore formation. The total cell density, spore cell density and sporulation efficiency showed a trend of first increasing and then decreasing with the increase in soybean flour concentration. The maximum value appeared at the concentration of 12 g·L−1, at which the total cell density, spore cell density and sporulation efficiency were 1.2 × 109 CFU·mL−1, 9.8 × 108 CFU·mL−1, and 82.6%, respectively (Figure 1d). Therefore, soybean flour with a concentration of 12 g·L−1 was selected as the best nitrogen source and the optimal concentration.
3.3. Screening the Best Inorganic Salts and Their Optimum Concentrations
As shown in Figure 1e, based on five inorganic salts (NaCl, KCl, CaCO3, MgSO4, or K2HPO4) with a concentration of 5 g·L−1, the total cell density, spore cell density and sporulation efficiency were analyzed. The formation of the spores had no significant difference (p > 0.05) among NaCl, KCl and CaCO3. The number of spores of MgSO4 or K2HPO4 were higher than that of NaCl, CaCO3 or KCl. When MgSO4 was used as the only inorganic salt, the total cell density, spore density and sporulation efficiency were the highest.
When different concentrations (1, 3, 5, 7, and 9 g·L−1) of MgSO4 were used as the only inorganic salt, the total cell density, spore cell density and sporulation efficiency showed a trend of first increasing and then decreasing with the increase in MgSO4 concentration. The maximum value appeared at a concentration of 5 g·L−1, in which the total cell density, spore cell density and sporulation efficiency were 8.7 × 108 CFU·mL−1, 7.3 × 108 CFU·mL−1, and 84.3% (Figure 1f). Therefore, MgSO4 was selected as the best inorganic salt, and 5 g·L−1 was chosen as the optimal concentration.
3.4. Optimizing Combination of Medium Composition by RSM
According to the single factor test, the best components and optimal concentrations of the spore-forming medium for BSNK-5 were determined as soluble starch at 3 g·L−1, soybean flour at 12 g·L−1, and MgSO4 at 5 g·L−1. The BBD of RSM was performed to further investigate the correlation between the spore density and the three variables (A: soluble starch, B: soybean flour, C: MgSO4) of three levels (soluble starch: 2, 3 or 4 g·L−1; soybean flour: 11, 12 or 13 g·L−1; MgSO4: 4, 5 or 6 g·L−1). Response surface analysis was carried out with soluble starch at 3 g·L−1, soybean flour at 12 g·L−1, and MgSO4 at 5 g·L−1 as the center points. The levels of the respective variables are shown in Table 1.

Table 1.
Experimental factors with three levels.
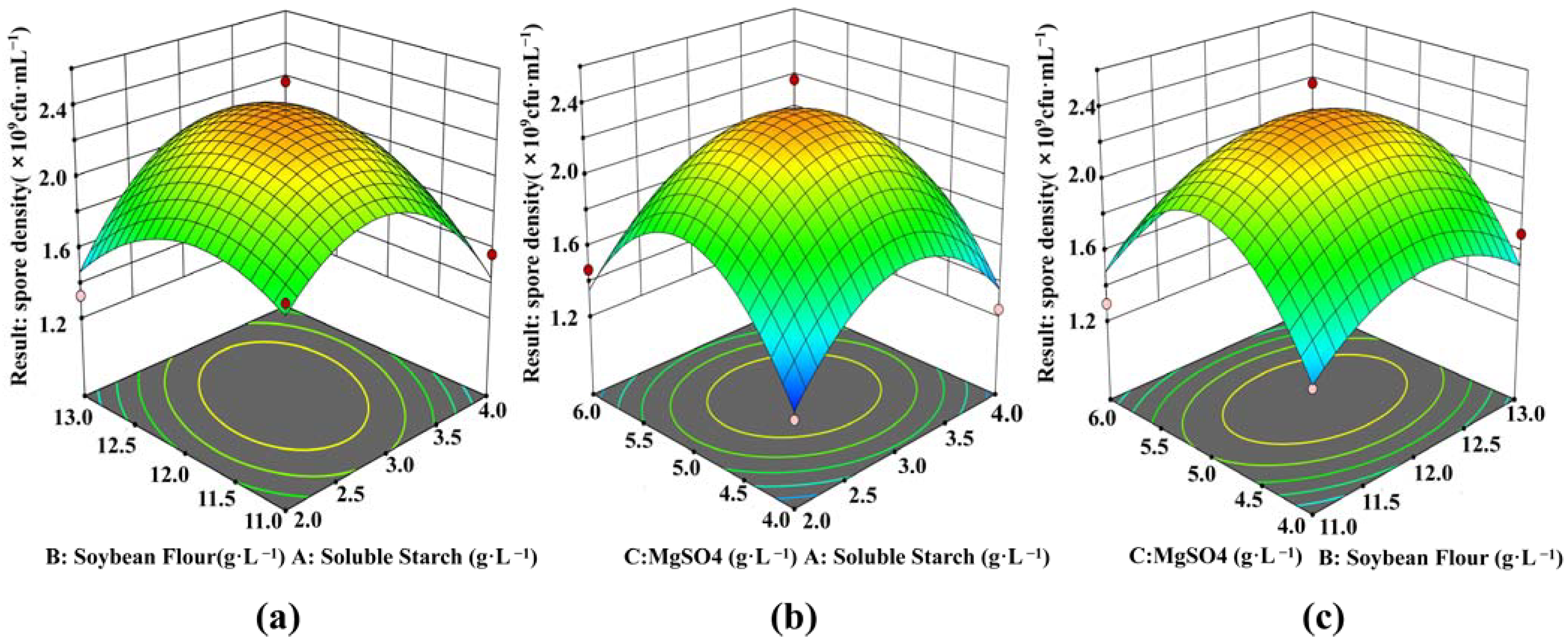
According to the BBD, a total of 15 experiments were performed, and their results are shown in Figure 2 and Table 2. Figure 2a shows the effects of soluble starch, soybean flour and their concentrations on the spore density when the addition of MgSO4 was 5 g·L−1. The minimum value of spore density was 1.33 × 109 CFU·mL−1 when the soluble starch was 2 g·L−1 and soybean flour was 13 g·L−1. When the concentrations of soluble starch increased from 2 g·L−1 to 4 g·L−1, and soybean flour increased from 11 g·L−1 to 13 g·L−1, the spore density in the fermentation broth showed a trend of first increasing and then decreasing. When soluble starch was at 3 g·L−1 and soybean flour at 12 g·L−1, the spore density reached the maximum. The surfaces in Figure 2b, c reflect the similar interactions between soluble starch and MgSO4, and soybean flour and MgSO4, respectively.
Figure 2.
Response surface interaction influence surface diagram. Effects of soybean flour and soluble starch on spore formation (a); Effects of MgSO4 and soluble starch on spore formation (b); Effects of MgSO4 and soybean on spore formation (c).

Table 2.
Central composite design of actual factors and responses based on actual values.
The results of ANOVA for the response surface quadratic model are shown in Table 3. The multivariate correlation coefficient of the quadratic model was 0.9146, indicating that only 8.54% of the variance could not be explained using this model. The regression model p = 0.0319 indicated that the model was significant. The lack of fit item p-value is 0.3930, indicating that the lack of fit was not significant, and the model had no lack of fit phenomenon. The linear and square effects of the model were significant, and the interaction effect was not significant.

Table 3.
Analysis of variance (ANOVA) for response surface quadratic model.
After regression fitting, the quadratic polynomial equation was obtained: Y = 2.34 + 0.0087A + 0.025B + 0.0063C + 0.17AB − 0.0425AC − 0.025BC − 0.4412A2 − 0.2787B2 − 0.5863C2. According to the quadratic regression equation, the spore density reached a maximum value of 2.34 × 109 CFU·mL−1 when the soluble starch concentration was 3 g·L−1, soybean flour was 12 g·L−1, and MgSO4 was 5 g·L−1. To confirm the predicted results, repeated experiments were performed at these concentrations. The results obtained from three repetitions of the experiments, are shown in Table 4. The spore density was 2.43 × 109 CFU·mL−1 which was close to the expected value of 2.34 × 109 CFU·mL−1. The good fit of the two values confirmed the validity of the model.

Table 4.
Numerical optimization and validation of pretreatment based on the regression models.
4. Discussion
Spores are a naturally occurring form of B. subtilis. The formation of spores can keep bacteria highly active in extreme environments and industrial production processes, which can be used in the production and application of probiotics. It has been confirmed that the composition of the culture medium is a key factor affecting the spore formation of B. subtilis [16]. BSNK-5 is a lab-bred strain with a high nattokinase yield and excellent application prospects [17]. In this research, the influence of the sporulation of a carbon source, nitrogen source and inorganic salt on BSNK-5 were studied for the first time. The results indicated that the best components of the spore medium for BSNK-5 were soluble starch, soybean flour, and MgSO4. With the concentration of soluble starch at 3 g·L−1, soybean flour at 12 g·L−1, and MgSO4 at 5 g·L−1, the maximum spore density of 2.43 × 109 CFU·mL−1 was obtained.
In this study, single carbon sources (glucose, sucrose and lactose) and compound carbon sources (yeast extract and soluble starch) were used to screen for the best carbon source for the spore production of BSNK-5. In general, the total colony density and spore density of compound carbon sources were higher than those of single carbon sources. Soluble starch showed the best advantage in promoting spore formation. This may be related to the differences in basic composition and absorption and utilization efficiency of the two kinds of carbon sources [18]. A single carbon source is composed of one component and can be absorbed directly, while a compound carbon source is composed of several components and needs to be degraded before absorption and utilization [19]. It can be seen that proper nutrient supply is favorable for spore formation. Studies have shown that glucose has a negative effect on spore formation [20], and excess glucose inhibits sporulation by repressing the transcription of the spo0A gene. This gene is responsible for encoding the Spo0A protein, a response regulator activated by phosphorylation in response to several internal and external stimuli, and is the master regulator of entry into sporulation [21,22].
Inorganic nitrogen sources ((NH4)2SO4 and urea) and organic nitrogen sources (tryptone, soybean flour and beef extract) were used to screen for the best nitrogen source for the spore production of BSNK-5. Nitrogen sources also showed the same trend as those of carbon sources in this study. The results of nitrogen source optimization showed that the total cell density and spore density of BSNK-5 were relatively low when (NH4)2SO4 and urea were used as nitrogen sources, which indicated that inorganic nitrogen was the only nitrogen source that was not conducive to the growth and spore formation of BSNK-5. When soybean flour was used as the sole carbon source, the total colony density and spore density were highest, probably because soybean flour contains limited and complex nutrient components, which are more difficult to digest and absorb than those of the other nitrogen sources, and this resulted in BSNK-5 forming more spores under starvation stress.
Numerous studies have confirmed that inorganic salts can influence spore formation. Adding inorganic salts to the medium, such as high concentrations of Na+, Mg2+, K+ and Ca2+, affected the formation of spores [23]. The addition of NaCl significantly increased the total number of bacteria, but had little effect on the number of spores, and CaCO3 played a role in buffering pH and affecting spore formation [24]. In our experiment, Na+, Mg2+, K+ and Ca2+ all promoted the total cell density and spore density, and the addition of MgSO4 had the most obvious improvement in the total density of colonies and spores. This may be related to the fact that Mg2+ can stabilize the ribosome complex, which can change the sensitivity of bacteria to antibiotics [25].
The response surface analysis is clearly accurate and widely used. At present, it is commonly used to optimize medium composition and fermentation conditions to improve various enzymes, exopolysaccharides, amino acids and other metabolites produced by B. subtilis [26,27]. In this experiment, the center points and factor levels of the response surface were determined by the single factor experiment, and then the response surface analysis chart was obtained through BBD analysis. This can directly reflect the influence of various factors on the response value, so as to find the optimal process parameters and the interaction between them [28]. The greater the curvature of the convex graph, the more significant the interaction between the two factors [29]. The response surface analysis graphs in this experiment were all convex graphs, indicating that the interaction between the soluble starch, soybean flour and MgSO4 were significant.
Spores are not an indispensable part of bacterial life history; they are simply a stress-resistant dormant body formed by spore-producing bacteria during the growth process. The formation of spores is affected by many factors. In addition to the characteristics of the bacteria and the composition of the medium studied in this paper, some fermentation process parameters, such as temperature, pH value, and ventilation, etc., also need to be focused on [30]. At the same time, the effects and mechanisms of different media components on the spore formation of BSNK-5 still need to be further explored.
5. Conclusions
High cell density and sporulation efficiency are critical for Bacillus-based products. In this study, the BSNK-5 sporulation medium was optimized using the single factor test and response surface methodology. Based on the single factor test, the best components of the spore-forming medium for BSNK-5 were determined as soluble starch, soybean flour, and MgSO4. After composite analysis by response surface, the optimized component concentrations were 3 g·L−1 of soluble starch, 12 g·L−1 of soybean flour, and 5 g·L−1 of MgSO4. With this combination, the maximum spore density of 2.43 × 109 CFU·mL−1 for BSNK-5 was obtained, which was nearly three times higher than before. Through this study, the production of bacterial spores was not only improved, but the amount of nutrients required for fermentation was also determined, laying a theoretical foundation for the application of BSNK-5 in industrial production.
Author Contributions
Conceptualization, F.W. and S.L.; Writing—original draft preparation, Z.T.; Writing—review and editing, supervision, Z.T., D.L., M.H., Y.G. and S.L.; Investigation, Y.G., D.L., Z.T. and L.H.; Validation, Y.G., S.L. and Z.T.; Funding acquisition, F.W., B.F. and S.L.; Project administration, F.W., B.F. and S.L.; Data curation, Z.T., Y.G. and D.L.; Formal analysis, L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by China Agriculture Research System (CARS-04), Central Public-interest Scientific Institution Basal Research Fund (S2021JBKY-02) and Central Public-interest Scientific Institution Basal Research Incremental Fund (Y2020PT10).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the funds from the Knowledge Innovation Program Funding of the Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, Y.M.; Lee, K.S.; Kim, W.M.; Kim, M.; Park, H.O.; Choi, C.W.; Han, J.S.; Park, S.Y.; Lee, K.S. Hydrochloric Acid-Treated Bacillus subtilis Ghosts Induce IL-1 beta, IL-6, and TNF-alpha in Murine Macrophage. Mol. Cell. Toxicol. 2022, 18, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Dworkin, J. Recent Progress in Bacillus subtilis Sporulation. FEMS Microbiol. Rev. 2012, 36, 131–148. [Google Scholar] [CrossRef] [Green Version]
- Idiyatov, I.I.; Eroshin, A.I.; Yusupov, S.A.; Zdoroveva, E.V.; Tremasova, A.M. Endophytic Isolates of Bacillus Subtilis: Prospects of Application for Improving the Quality of Food Raw Materials. IOP Conf. Ser. Earth Environ. Sci. 2022, 953, 012024. [Google Scholar] [CrossRef]
- Peng, Q.; Wu, J.; Chen, X.; Qiu, L.; Zhang, J.; Tian, H.; Song, F. Disruption of Two-component System LytSR Affects Forespore Engulfment in Bacillus thuringiensis. Front. Cell Infect. Microbiol. 2017, 7, 468. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.H.; Li, Q.Y.; Zhao, W.; Yang, G.; Zhang, R.R.; Chen, H.L.; Li, Y.; Wan, D.; Zhao, D.D.; Huang, H.B.; et al. Bacillus Subtilis BSH has a Protective Effect on Salmonella Infection by Regulating the Intestinal Flora Structure in Chickens. Microb. Pathog. 2021, 155, 104898. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. EFfects of Bacillus Subtilis and Bacillus Licheniformis on Growth Performance, Immunity, Short Chain Fatty Acid Production, Antioxidant Capacity, and Cecal Microflora in Broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Q.; Nie, Y.; Wu, J.; Xu, Y. Construction of Synthetic Microbiota for Reproducible Flavor Compound Metabolism in Chinese Light-Aroma-Type Liquor Produced by Solid-State Fermentation. Appl. Environ. Microbiol. 2019, 85, e03090-18. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Mei, L.H.; Hu, S.; Sheng, Q.; Xu, J.; Wu, H.; Yao, S.J. Screening of Bacillus subtilis Natto from Traditional Japanese Food Natto and Separation of Nattokinase. J. Chem. Eng. Chin. Univ. 2005, 19, 518–522. [Google Scholar] [CrossRef]
- Gu, X.B.; Zheng, Z.M.; Yu, H.Q.; Wang, J.; Liang, F.L.; Liu, R.L. Optimization of Medium Constituents for a Novel Lipopeptide Production by Bacillus subtilis MO-01 by a Response Surface Method. Process Biochem. 2005, 40, 3196–3201. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, Y.B.; Kim, E.K. Optimization of Culture Media for Bacillus Species by Statistical Experimental Design Methods. Korean J. Chem. Eng. 2009, 26, 754–759. [Google Scholar] [CrossRef]
- Buhr, T.L.; McPherson, D.C.; Gutting, B.W. Analysis of Broth-Cultured Bacillus atrophaeus and Bacillus cereus Spores. J. Appl. Microbiol. 2008, 105, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Iqbal, S.; Anwar, S. Optimization and Modeling of Glyphosate Biodegradation by a Novel Comamonas odontotermitis P2 Through Response Surface Methodology. Pedosphere 2017, 30, 618–627. [Google Scholar] [CrossRef]
- Korondi, P.; Marchi, M.; Poloni, C. Response Surface Methodology. In Optimization Under Uncertainty with Applications to Aerospace Engineering; Springer: Cham, Switzerland, 2021; pp. 387–409. [Google Scholar]
- Xu, Q.; Shen, Y.; Wang, H.; Zhang, N.; Xu, S.; Zhang, L. Application of Response Surface Methodology to Optimise Extraction of Flavonoids from Fructus Sophorae. Food Chem. 2013, 138, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.L.; Li, S.Y.; Nie, Y.; Li, Y.; Yuan, C.; Tang, X.M. Screening of Nattokinase Producing Strain and Characterazation of Nattokinase. Biotechnol. Bull. 2015, 31, 161–164. [Google Scholar]
- Chen, Z.M.; Li, Q.; Liu, H.M.; Yu, N.; Xie, T.J.; Yang, M.Y.; Shen, P.; Chen, X.D. Greater Enhancement of Bacillus Subtilis Spore Yields in Submerged Cultures by Optimization of Medium Composition through Statistical Experimental Designs. Appl. Microbiol. Biotechnol. 2010, 85, 1353–1360. [Google Scholar] [CrossRef]
- Gao, Y.X.; Xu, B.; Fan, H.R.; Zhang, M.R.; Zhang, L.J.; Lu, C.; Zhang, N.N.; Fan, B.; Wang, F.Z.; Li, S. 1H NMR-Based Chemometric Metabolomics Characterization of Soymilk Fermented by Bacillus subtilis BSNK-5. Food Res. Int. 2020, 138, 109686. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.T.; Eiteman, M.A.; Adang, M.J. Bacillus thuringiensis Subsp. Kurstaki Spore Production in Batch Culture Using Broiler Litter Extracts as Complex Media. Bioresour. Technol. 1999, 67, 83–87. [Google Scholar] [CrossRef]
- Huang, C.T.; Xu, K.D.; McFeters, G.A.; Stewart, P.S. Spatial Patterns of Alkaline Phosphatase Expression within Bacterial Colonies and Biofilms in Response to Phosphate Starvation. Appl. Environ. Microbiol. 1998, 64, 1526–1531. [Google Scholar] [CrossRef] [Green Version]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 739. [Google Scholar] [CrossRef] [Green Version]
- Sonenshein, A.L. Control of Sporulation Initiation in Bacillus subtilis. Curr. Opin. Microbiol. 2000, 3, 561–566. [Google Scholar] [CrossRef]
- Grimshaw, C.E.; Huang, S.; Hanstein, C.G.; Strauch, M.A.; Burbulys, D.; Wang, L.; Hoch, J.A.; Whiteley, J.M. Synergistic Kinetic Interactions between Components of the Phosphorelay Controlling Sporulation in Bacillus subtilis. Biochemistry 1998, 37, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Hageman, J.H.; Shankweiler, G.W.; Wall, P.R.; Franich, K.; McCowan, G.W.; Cauble, S.M.; Grajeda, J.; Quinones, C. Single, Chemically Defined Sporulation Medium for Bacillus subtilis: Growth, Sporulation, and Extracellular Protease Production. J. Bacteriol. 1984, 160, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Tavares, M.B.; Souza, R.D.; Luiz, W.B.; Cavalcante, R.C.M.; Casaroli, C.; Martins, E.G.; Ferreira, R.C.C.; Ferreira, L.C.S. Bacillus subtilis Endospores at High Purity and Recovery Yields: Optimization of Growth Conditions and Purification Method. Curr. Microbiol. 2013, 66, 279–285. [Google Scholar] [CrossRef]
- Nierhaus, K. Mg2+, K+, and the Ribosome. J. Bacteriol. 2014, 196, 3817–3819. [Google Scholar] [CrossRef] [Green Version]
- Sreekumar, G.; Krishnan, S. Enhanced Biomass Production Study on Probiotic Bacillus subtilis SK09 by Medium Optimization Using Response Surface Methodology. Afr. J. Biotechnol. 2010, 9, 8078–8084. [Google Scholar]
- Saxena, R.; Singh, R. Contemporaneous Production of Amylase and Protease through CCD Response Surface Methodology by Newly Isolated Bacillus megaterium Strain B69. Enzyme Res. 2014, 2014, 601046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wang, H.; Cai, X. Optimization of the Extraction of Total Flavonoids from Scutellaria Baicalensis Georgi Using the Response Surface Methodology. J. Food Sci. Technol. 2015, 52, 2336–2343. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Cui, S.W.; Tang, J.; Gu, X. Optimization of Extraction Process of Crude Polysaccharides from Boat-Fruited Sterculia Seeds by Response Surface Methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Li, Y.; Tao, L.; Li, T. Optimization of pH Conditions to Improve the Spore Production of Clostridium butyricum NN-2 during Fermentation Process. Arch. Microbiol. 2020, 202, 1251–1256. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).