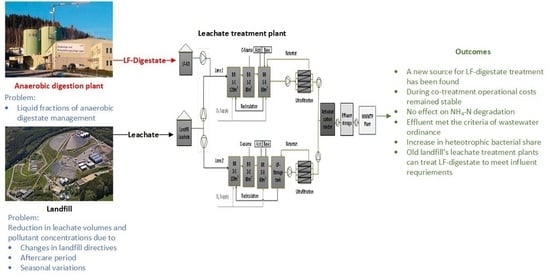
Co-Treatment of Landfill Leachate and Liquid Fractions of Anaerobic Digestate in an Industrial-Scale Membrane Bioreactor System
Abstract
1. Introduction
2. Materials and Methods
2.1. Estimating the Potential Additional Inflow Rate through LF-AD
2.2. Experimental Plant Operation
2.3. Physico-Chemical and Statistical Data Analysis
2.4. Selective Oxygen Uptake Rate (SOUR)
2.5. Operational Resources
3. Results and Discussion
- Phase 0: both lanes were provided with LL.
- Phase 1: Preliminary phase for the LF-AD in the LLTP; the substitution of 10% of the NH4-N load (v/v) with LF-AD.
- Phase 2: An increase in the volume percentage of the NH4-N load from the LF-AD; the substitution of 20% of the NH4-N load (v/v) with LF-AD.
3.1. Nitrification Activity in the Bioreactors of the Leachate Treatment Plant
3.2. Selective Oxygen Uptake Rate in the Nitrification Bioreactors
3.3. Performance Analysis of Long-Term Nitrogen and COD Removal
3.4. Performance of the Leachate Treatment Plant, Leppe
3.5. Particle Size Distribution of the Biomass in the LLTP
3.6. Annual Specific Consumption of the Operational Resources of the LLTP in Leppe
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestate |
| AOO | Ammonia oxidizing organisms |
| C/N | Carbon/nitrogen |
| COD | Chemical oxygen demand |
| COD-LR | Chemical oxygen demand load rate |
| COD-RE | Chemical oxygen demand removal efficiency |
| GAC | Granular activated carbon |
| L-1 | Lane-1 |
| L-2 | Lane-2 |
| LF-AD | Liquid fractions of anaerobic digestate |
| LL | Landfill leachate |
| LLTP | Landfill leachate treatment plant |
| NLR | Nitrogen loading rate |
| NOO | Nitrite oxidizing organisms |
| NRE | Nitrogen removal efficiency |
| NRR | Nitrogen removal rate |
| P1 COD | COD in permeate of Lane-1 |
| P2 COD | COD in permeate of Lane-2 |
| SOUR | Selective oxygen uptake rate |
| UF | Ultrafiltration |
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development; World Bank: Washington, DC, USA, 2018; ISBN 9781464813290. [Google Scholar]
- Tałałaj, I.A.; Biedka, P.; Bartkowska, I. Treatment of landfill leachates with biological pretreatments and reverse osmosis. Environ. Chem. Lett. 2019, 17, 1177–1193. [Google Scholar] [CrossRef]
- The Landfill Situtation in the EU-28, European Enhanced Landfill Minning Consortium. 2018. Available online: https://eurelco.org/2018/09/30/data-launched-on-the-landfill-situation-in-the-eu-28/ (accessed on 23 May 2022).
- Eggen, T.; Moeder, M.; Arukwe, A. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci. Total Environ. 2010, 408, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- John, D. Keenan, R. Lee Steiner, A.A.F. Landfill Leachate Treatment. Curr. Dev. Biotechnol. Bioeng. Solid Waste Manag. 2017, 56, 391–406. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Treating Contaminated Groundwater and Leachate. Groundw. Remediat. Treat. Technol. 1997, 259–308. [Google Scholar] [CrossRef]
- Brennan, R.B.; Healy, M.G.; Morrison, L.; Hynes, S.; Norton, D.; Clifford, E. Management of landfill leachate: The legacy of European Union Directives. Waste Manag. 2016, 55, 355–363. [Google Scholar] [CrossRef]
- European Parliament and Council Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 amending Directive 1999/31/EC on the landfill of waste. Off. J. Eur. Union 2018, 2018, 100–108.
- Lotti, T.; Burzi, O.; Scaglione, D.; Ramos, C.A.; Ficara, E.; Pérez, J.; Carrera, J. Two-stage granular sludge partial nitritation/anammox process for the treatment of digestate from the anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2019, 100, 36–44. [Google Scholar] [CrossRef]
- Di Maria, F.; Sisani, F. A sustainability assessment for use on land or wastewater treatment of the digestate from bio-waste. Waste Manag. 2019, 87, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Baek, S.R.; Kim, J.I.; Choi, J.W.; Hur, J.; Lee, T.U.; Park, C.J.; Lee, B.J. Characteristics and biodegradability of wastewater organic matter in municipal wastewater treatment plants collecting domestic wastewater and industrial discharge. Water 2017, 9, 409. [Google Scholar] [CrossRef]
- Di Maria, F.; Sisani, F.; Lasagni, M.; Borges, M.S.; Gonzales, T.H. Replacement of energy crops with bio-waste in existing anaerobic digestion plants: An energetic and environmental analysis. Energy 2018, 152, 202–213. [Google Scholar] [CrossRef]
- Światczak, P.; Cydzik-Kwiatkowska, A.; Zielińska, M. Treatment of liquid phase of digestate from agricultural biogas plant in a system with aerobic granules and ultrafiltration. Water 2019, 11, 104. [Google Scholar] [CrossRef]
- Chiumenti, A.; Da Borso, F.; Teri, F.; Chiumenti, R.; Piaia, B. Full-scale membrane filtration system for the treatment of digestate from a co-digestion plant. Appl. Eng. Agric. 2013, 29, 985–990. [Google Scholar] [CrossRef]
- Camilleri-Rumbau, M.S.; Briceño, K.; Søtoft, L.F.; Christensen, K.V.; Roda-Serrat, M.C.; Errico, M.; Norddahl, B. Treatment of manure and digestate liquid fractions using membranes: Opportunities and challenges. Int. J. Environ. Res. Public Health 2021, 18, 3107. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Fang, J. Applied sciences Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710. [Google Scholar] [CrossRef]
- Annepogu, N.B.; Steiner, C.; Rehorek, A. Process development and process monitoring of landfill leachate treatment in combination with complementary long- term addition of process water from fermenter. In Book of Proceedings of STEPsCON; Rosenwinkel: Leverkusen, Germany, 2018; Volume 150, p. 2018. [Google Scholar]
- Steiner, C. Entwicklung Einer Halbtechnischen Deponiesickerwasserreinigungsanlage und Analyse des Metaproteoms in Belebtschlamm. Ph.D. Thesis, Universität Duisburg-Essen, Duisburg, Germany, 2019; pp. 1–192. [Google Scholar]
- Debouny, T.; Catherina, D.; Isunza Manrique, I.; Beese-Vasbender, P.; Nguyen, F. Landfill Characterization by Multi-Method Geophysical Investigation: The Case Study of Leppe (Germany). EGU General Assembly 2020, Online, 4–8 May 2020; EGU2020-18151. Available online: https://doi.org/10.5194/egusphere-egu2020-18151. [CrossRef]
- Gallenkemper, B.; Lohse, M.; Böning, T.; Rosenwinkel, K.-H.; Weichgrebe, D.; Märker, S. Prozesswassermanagement auf MBA-Anlagen am Beispiel der MBA-Gescher. 2007, p. 107. Available online: https://www.lanuv.nrw.de/fileadmin/forschung/wasser/klaeranlage_abwasser/Abschlussberichtprozesswassermanagement.pdf (accessed on 5 March 2022).
- Dahm, W.; Kollback, J.S.; Gebel, J. Sickerwasserreinigung, Stand der Technik 1993/94, Zukünftige Entwicklungen, 1st ed.; EF-Verlag für Energie-und Umwelttechnik GmbH: Berlin, Germany, 1994. [Google Scholar]
- Bundesministerium der Justiz. Verordnung über Anforderungen an das Einleiten von Abwasser in Gewässer (Abwasserverordnung - AbwV). [Gesetz]. 1997. Available online: https://www.gesetze-im-internet.de/abwv/BJNR056610997.html (accessed on 3 January 2022).
- You, S.J.; Tsai, Y.P.; Huang, R.Y. Effect of heavy metals on nitrification performance in different activated sludge processes. J. Hazard. Mater. 2009, 165, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Zhang, Y.; Ma, H.; He, S.; Li, Y.-Y. Reactor kinetics evaluation and performance investigation of a long-term operated UASB-anammox mixed culture process. Int. Biodeterior. Biodegradation 2016, 108, 24–33. [Google Scholar] [CrossRef]
- Steiner, C.; Annepogu, N.; Schluckebier, L.; Rehorek, A. Nitrification monitoring—Determination of bacterial groups in a biocoenosis with selective inhibition and oxygen uptake rate measurements. In Book of Proceedings of STEPsCON 2018; International Scientific Conference on Sustainability and Innovation: Leverkusen, Germany; p. 2018.
- Ginestet, P.; Audic, J.M.; Urbain, V.; Block, J.C. Estimation of nitrifying bacterial activities by measuring oxygen uptake in the presence of the metabolic inhibitors allylthiourea and azide. Appl. Environ. Microbiol. 1998, 64, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.; Lazarova, V.; Manem, J.; Melo, L.F. Influence of dissolved oxygen on the nitrification kinetics in a circulating bed biofilm reactor. Bioprocess Eng. 1998, 19, 441–449. [Google Scholar] [CrossRef]
- Daigger, G.T. Oxygen and Carbon Requirements for Biological Nitrogen Removal Processes Accomplishing Nitrification, Nitritation, and Anammox. Water Environ. Res. 2014, 86, 204–209. [Google Scholar] [CrossRef]
- Harrison, W.; Flotard, R.D.; Ford, D. Assessment of Activated Carbon for Environmental Control of Trace Organics in Petroleum Refinery Wastewater; Argonne National Lab.: Lemont, IL, USA, 1979. [Google Scholar]
- HU, J.; LI, D.; LIU, Q.; TAO, Y.; HE, X.; WANG, X.; LI, X.; GAO, P. Effect of organic carbon on nitriffication efficiency and community composition of nitrifying bioffilms. J. Environ. Sci. 2009, 21, 387–394. [Google Scholar] [CrossRef]
- Chuda, A.; Ziemiński, K. Challenges in treatment of digestate liquid fraction from biogas plant. Performance of nitrogen removal and microbial activity in activated sludge process. Energies 2021, 14, 7321. [Google Scholar] [CrossRef]
- Carrera, J.; Vicent, T.; Lafuente, J. Effect of influent COD/N ratio on biological nitrogen removal (BNR) from high-strength ammonium industrial wastewater. Process Biochem. 2004, 39, 2035–2041. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.C.; Cheng, Z.; Li, Y.; Tang, J. Effect of fermentation liquid from food waste as a carbon source for enhancing denitrification in wastewater treatment. Chemosphere 2016, 144, 689–696. [Google Scholar] [CrossRef]
- Choubert, J.M.; Marquot, A.; Stricker, A.E.; Racault, Y.; Gillot, S.; Héduit, A. Anoxic and aerobic values for the yield coefficient of the heterotrophic biomass: Determination at full-scale plants and consequences on simulations. Water SA 2009, 35, 103–110. [Google Scholar] [CrossRef][Green Version]
- Quan, Z.X.; Jin, Y.S.; Yin, C.R.; Lee, J.J.; Lee, S.T. Hydrolyzed molasses as an external carbon source in biological nitrogen removal. Bioresour. Technol. 2005, 96, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Obaja, D.; Macé, S.; Costa, J.; Sans, C.; Mata-Alvarez, J. Nitrification, denitrification and biological phosphorus removal in piggery wastewater using a sequencing batch reactor. Bioresour. Technol. 2003, 87, 103–111. [Google Scholar] [CrossRef]
- Dosta, J.; Galí, A.; Benabdallah El-Hadj, T.; Macé, S.; Mata-Álvarez, J. Operation and model description of a sequencing batch reactor treating reject water for biological nitrogen removal via nitrite. Bioresour. Technol. 2007, 98, 2065–2075. [Google Scholar] [CrossRef]
- Dinçer, A.R.; Kargi, F. Kinetics of sequential nitrification and denitrification processes. Enzym. Microb. Technol. 2000, 27, 37–42. [Google Scholar] [CrossRef]
- Xu, J.; Wang, P.; Li, Y.; Niu, L.; Xing, Z. Shifts in the microbial community of activated sludge with different COD/N ratios or dissolved oxygen levels in Tibet, China. Sustainability 2019, 11, 2284. [Google Scholar] [CrossRef]
- Wu, G.; Rodgers, M.; Zhan, X. Nitrification in sequencing batch reactors with and without glucose addition at 11 °C. Biochem. Eng. J. 2008, 40, 373–378. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Durowoju, O.S.; Odiyo, J.O. Assessment of Heavy Metals in Landfill Leachate: A Case Study of Thohoyandou Landfill, Limpopo Province, South Africa. Heavy Met. 2018, 16, 214–227. [Google Scholar] [CrossRef]
- Flyhammar, P. Heavy Metals in Municipal Solid Waste Deposits; Lund University: Lund, Sweden, 1997. [Google Scholar]
- Afangideh, C.; Nnaji, C.; Onuora, C.; Okafor, C. Comparative Study of the Leachability of Heavy Metals from Sewage Sludge, Sawdust and Organic Fraction of Municipal Solid Waste. Br. J. Appl. Sci. Technol. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Kapoor, V.; Li, X.; Elk, M.; Chandran, K.; Impellitteri, C.A.; Santo Domingo, J.W. Impact of Heavy Metals on Transcriptional and Physiological Activity of Nitrifying Bacteria. Environ. Sci. Technol. 2015, 49, 13454–13462. [Google Scholar] [CrossRef]
- Sun, M.; Wang, H.; Zhang, H. Effect of Ca2+ and Fe3+ addition on nitrification-denitrification process in a membrane bioreactor. Adv. Mater. Res. 2013, 610–613, 422–427. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Zhu, C.; Liu, S.; Wang, Q.; Wu, Z. Analysis of Nitrification Efficiency and Microbial Community in a Membrane Bioreactor Fed with Low COD/N-Ratio Wastewater. PLoS ONE 2013, 8, e63059. [Google Scholar] [CrossRef]
- Xie, H.; Ji, D.; Zang, L. Effects of Inhibition Conditions on Anammox process. IOP Conf. Ser. Earth Environ. Sci. 2017, 100, 012149. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Wu, P.; Wang, H.; Deng, L.; Wang, W. Nitrification performance evaluation of activated sludge under high potassium ion stress during high-ammonia nitrogen organic wastewater treatment. J. Environ. Sci. 2022, 111, 84–92. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Tan, F.; Wu, D. Treatment of landfill leachate using activated sludge technology: A review. Archaea 2018, 2018, 1039453. [Google Scholar] [CrossRef]
- European Environment Agency. Industrial Waste Water Treatment—Pressures on Europes’s Environment; European Environment Agency: Maastricht, The Netherlands, 2019. [Google Scholar]
- Rico, C.; Rico, J.L.; Lasa, C. Anaerobic digestion of the liquid fraction of dairy manure separated by screw pressing and centrifugation in a upflow anaerobic sludge blanket reactor at 25 °C. Biosyst. Eng. 2012, 112, 344–351. [Google Scholar] [CrossRef]
- Gao, J.L.; Oloibiri, V.; Chys, M.; De Wandel, S.; Decostere, B.; Audenaert, W.; He, Y.L.; Van Hulle, S.W.H. Integration of autotrophic nitrogen removal, ozonation and activated carbon filtration for treatment of landfill leachate. Chem. Eng. J. 2015, 275, 281–287. [Google Scholar] [CrossRef]
- Im, J.; Kim, D.; Gil, K. COD fraction analysis of anaerobic digester supernatant and piggery wastewater, using respirometric method. Environ. Earth Sci. 2016, 75, 47. [Google Scholar] [CrossRef]
- Pajooh, E.M.; Weichgrebe, D.; Cuff, G. Municipal landfill leachate characteristics and feasibility of retrofitting existing treatment systems with deammonification—A full scale survey. J. Environ. Manag. 2016, 187, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Steiner, C.; Beese-vasbender, P.; Annepogu, N.; Steensen, M.; Rehorek., A. Kombinierte Behandlung von Deponiesickerwasser und Vergärungsabwasser Membranbelebungsreaktoren im halbtechnischen und betrieblichen Maßstab. Müll und Abfall 2019, 500–504. [Google Scholar] [CrossRef]
- Yue, C.; Dong, H.; Chen, Y.; Shang, B.; Wang, Y.; Wang, S.; Zhu, Z. Direct purification of digestate using ultrafiltration membranes: Influence of pore size on filtration behavior and fouling characteristics. Membranes 2021, 11, 179. [Google Scholar] [CrossRef] [PubMed]











| Analysis Parameters | Landfill Leachate | LF-AD |
|---|---|---|
| Conductivity [μS/cm] | 500–21,400 | 18,010–19,110 |
| Total organic carbon (TOC) [mg/L] | 61–1250 | – |
| Total suspended solids [mg/L] | 1310–8410 | 1210–2000 |
| Chemical oxygen demand [mg/L] | 560–3760 | 7200–12,510 |
| Biochemical oxygen demand (BOD5) [mg/L] | 3.8–1300 | 2000–2860 |
| Total phosphor [mg/L] | 0.45–6.23 | 38–107 |
| Total nitrogen [mg/L] | 4102–2300 | 2110–3760 |
| Organic nitrogen [mg/L] | 0.4–165 | – |
| Ammonium nitrogen [mg/L] | 323–2280 | 1200–2870 |
| pH | 7.5–8.5 | 8.0–8.5 |
| Influent Capacity [m3/d] | Average Influent Rate (LL) [m3/d] | Available Influent Capacity [m3/d] | Max. Possible COD Load [kg/d] | Average COD-Load (LL) [kg/d] | Available COD Load [kg/d] | COD in LF-AD [kg/m3] | Max Treatable LF-AD Quantity [m3/d] | LL:LF-AD Ratio [%] |
|---|---|---|---|---|---|---|---|---|
| 580 | 300 | 280 | 787 | 229 | 558 | 9.5 | 59 | 20 |
| Chemicals | Ginestet et al. [26] | Own Tests | |
|---|---|---|---|
| Concentration in the Measuring Volume | Concentration in the Measuring Volume | Added Volume [mL] | |
| Acetic acid | 10 mg C/litre | 10 mg C/litre | 10 |
| Ammonium, Nitrite | 10 mg N/litre | 10 mg N/litre | 10 |
| Allylthiourea | 86 μM | 120 μM | 14 |
| Azide | 24 μM | 86 μM | 3.6 |
| Phase 0 | Influent Volume [m3/d] | NH4-N Load from LF-AD % (v/v) | N-Load [kg/d] | COD-Load [kg/d] | C/N Ratio |
|---|---|---|---|---|---|
| Phase 0 | |||||
| L-1 (LL) | 116.9 | 0 | 50 | 435 | 8.7 |
| L-2 (LL) | 235.3 | 0 | 96 | 588 | 6.1 |
| Phase 1 | |||||
| L-1 (LL + LF-AD) | 123.4 | 10 | 61 | 548 | 8.9 |
| L-2 (LL) | 225.8 | 0 | 104 | 652 | 6.2 |
| Phase 2 | |||||
| L-1 (LL + LF-AD) | 94 | 20 | 54 | 510 | 9.4 |
| L-2 (LL) | 200 | 0 | 94 | 680 | 7.2 |
| Source | Copper (mg/L) | Zinc (mg/L) | Lead (mg/L) | Calcium (mg/L) | Nickel (mg/L) | Silver (mg/L) |
|---|---|---|---|---|---|---|
| LL | 0 | 0 | 2.65 | 41.20 | 0 | 0 |
| LF-AD | 0 | 0 | 2.31 | 12.27 | 1.85 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annepogu, N.B.; Beese-Vasbender, P.F.; Himanshu, H.; Wolf, C.; Rehorek, A. Co-Treatment of Landfill Leachate and Liquid Fractions of Anaerobic Digestate in an Industrial-Scale Membrane Bioreactor System. Processes 2022, 10, 1140. https://doi.org/10.3390/pr10061140
Annepogu NB, Beese-Vasbender PF, Himanshu H, Wolf C, Rehorek A. Co-Treatment of Landfill Leachate and Liquid Fractions of Anaerobic Digestate in an Industrial-Scale Membrane Bioreactor System. Processes. 2022; 10(6):1140. https://doi.org/10.3390/pr10061140
Chicago/Turabian StyleAnnepogu, Nitesh Babu, Pascal F. Beese-Vasbender, Himanshu Himanshu, Christian Wolf, and Astrid Rehorek. 2022. "Co-Treatment of Landfill Leachate and Liquid Fractions of Anaerobic Digestate in an Industrial-Scale Membrane Bioreactor System" Processes 10, no. 6: 1140. https://doi.org/10.3390/pr10061140
APA StyleAnnepogu, N. B., Beese-Vasbender, P. F., Himanshu, H., Wolf, C., & Rehorek, A. (2022). Co-Treatment of Landfill Leachate and Liquid Fractions of Anaerobic Digestate in an Industrial-Scale Membrane Bioreactor System. Processes, 10(6), 1140. https://doi.org/10.3390/pr10061140