Optimization of Hemp Bast Microfiber Production Using Response Surface Modelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Pre-Treatment
2.3. Acid Hydrolysis
2.4. RSM Experimental Design
2.5. Characterization
3. Results and Discussion
3.1. Characterization of Acid Hydrolysed Fibers
3.1.1. Morphology of Cellulose Microfibrils
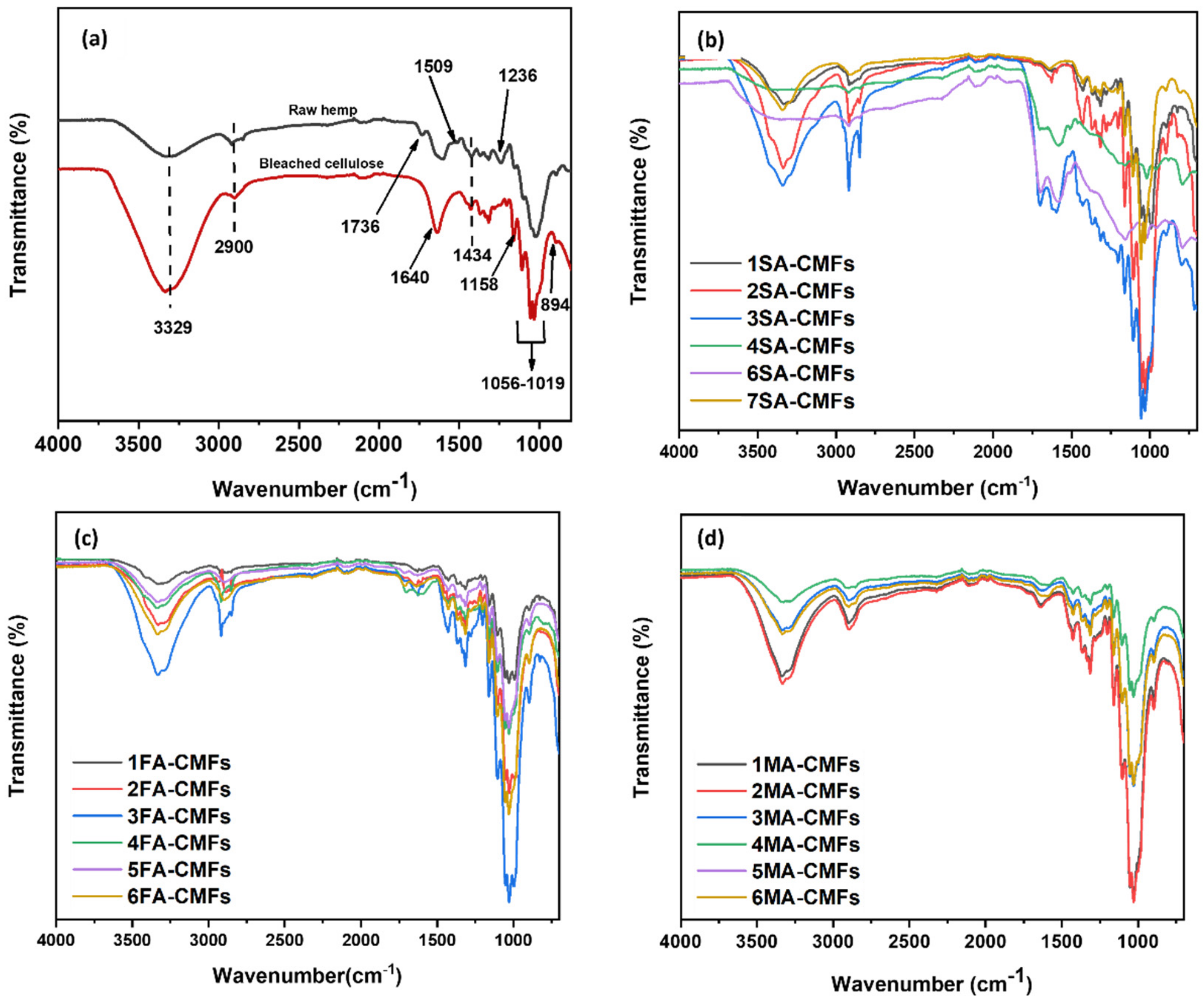
3.1.2. Surface Chemical Groups of CMFs
3.1.3. Crystallinity
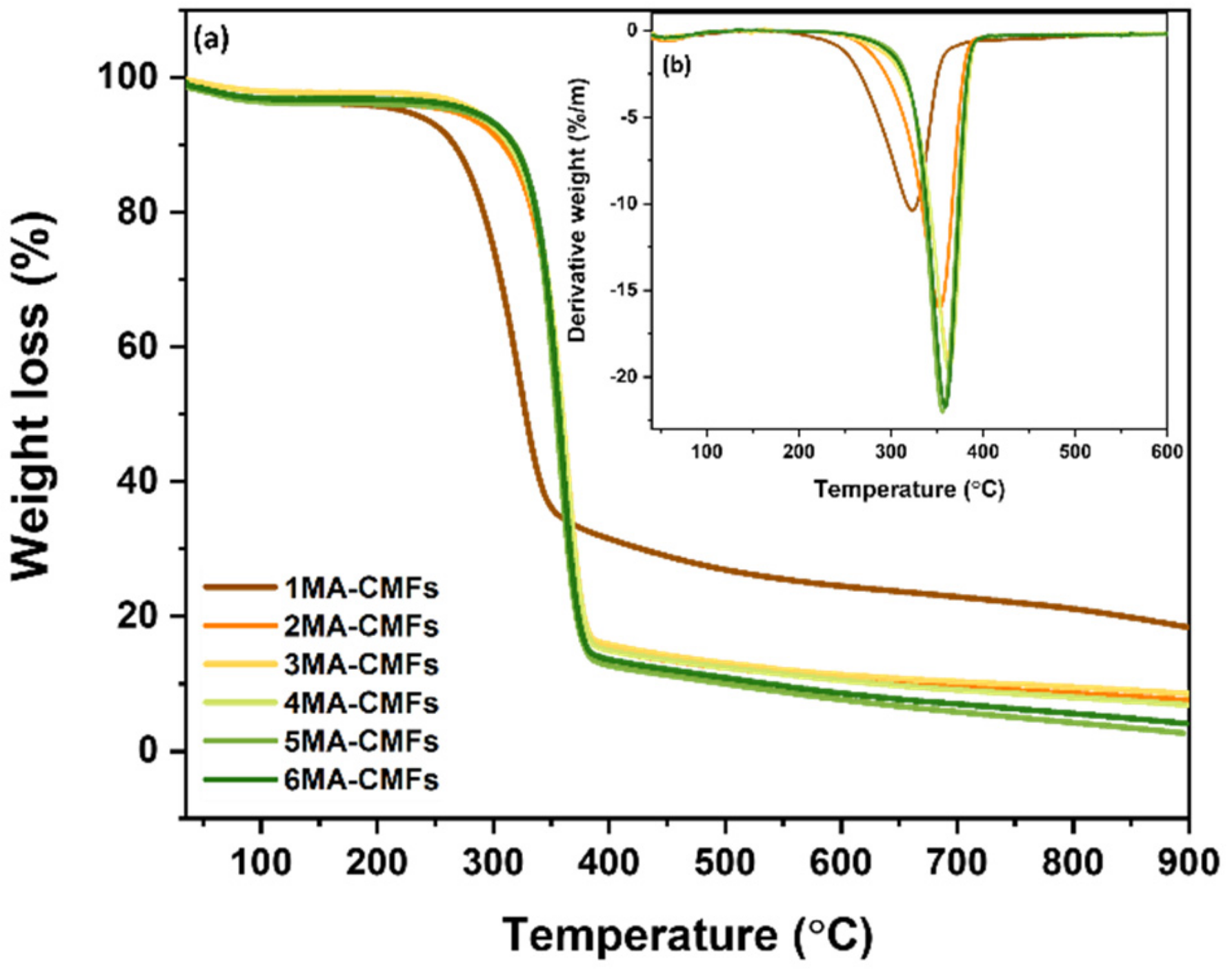
3.1.4. Thermogravimetric Analysis
4. Model Fit
4.1. Regression Model and Coefficients Plot
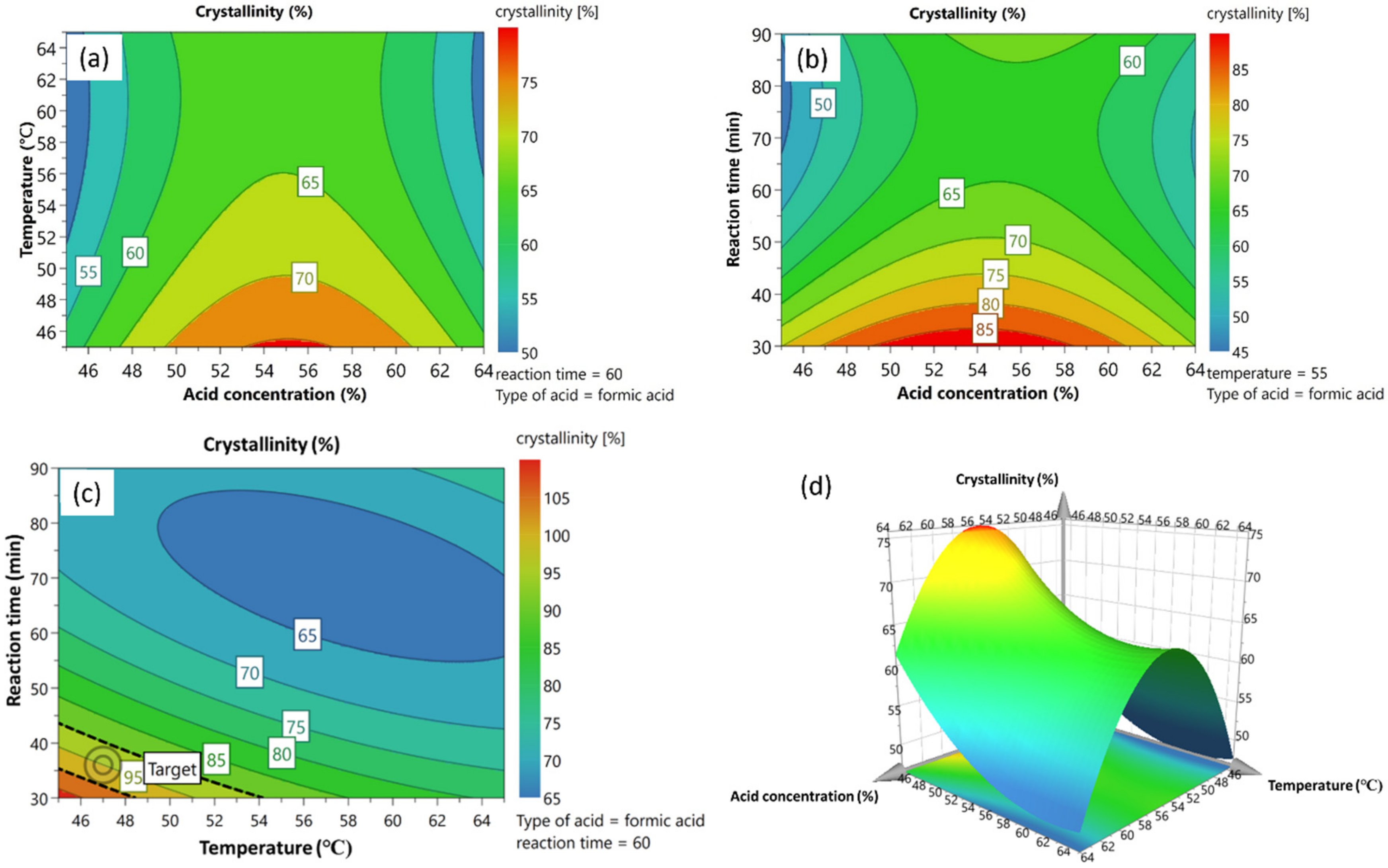
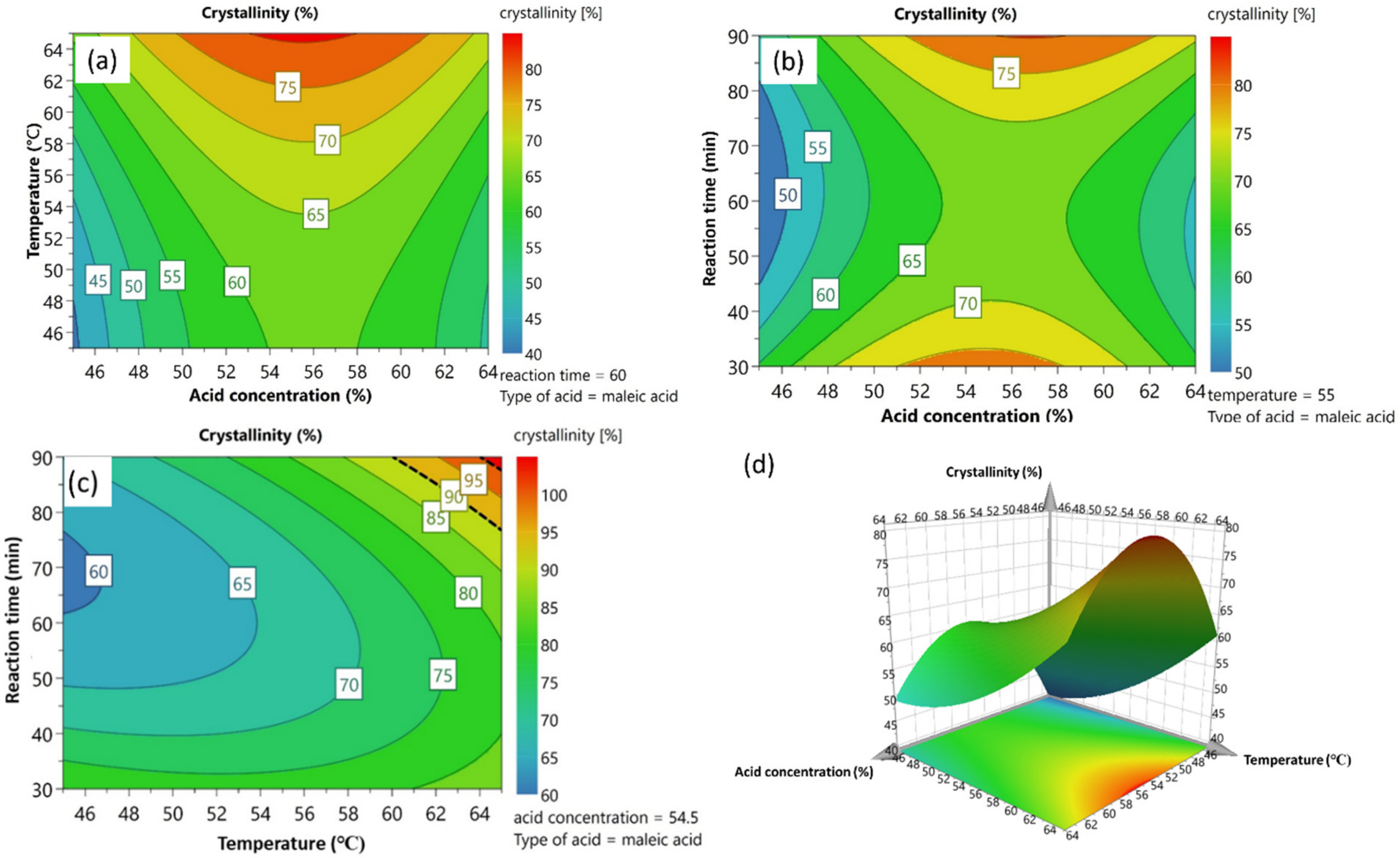
4.2. Response Contour and Surface Plot Analysis
4.3. Optimization and Model Verification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample ID | Crystallinity Degree (%) | |
|---|---|---|
| Predicted | Observed | |
| 1SA-CMFs | 72.8 | 75 |
| 1FA-CMFs | 57.2 | 55 |
| 1MA-CMFs | 40 | 40 |
| 2SA-CMFs | 54.2 | 52 |
| 2FA-CMFs | 47.8 | 50 |
| 2MA-CMFs | 55 | 55 |
| 3FA-CMFs | 66 | 66 |
| 3MA-CMFs | 61 | 61 |
| 3SA-CMFs | 53 | 53 |
| 4MA-CMFs | 65 | 65 |
| 4SA-CMFs | 72.1 | 71 |
| 4FA-CMFs | 57.9 | 59 |
| 5FA-CMFs | 70 | 70 |
| 5MA-CMFs | 54 | 54 |
| 5SA-CMFs | 76 | 76 |
| 6MA-CMFs | 67 | 67 |
| 6SA-CMFs | 57.9 | 59 |
| 6FA-CMFs | 62.1 | 61 |
| 7SA-CMFs | 66 | 66 |
| 7SA-CMFs | 66 | 66 |
| 7SA-CMFs | 66 | 66 |
| 62.1 | 61 | |
References
- Sachelaru, A. Cellulose Fibers from Hemp for Textile Industry; Bachelors Research Project; University of Groningen: Groningen, The Netherlands, 2019; Available online: https://fse.studenttheses.ub.rug.nl/20256/1/Andreea%20-%20Report%20bachelor%20research%20project%20-%20Isolation%20of%20cellulose%20fibers%20from%20hemp%20for%20textile%20industry.pdf (accessed on 10 May 2022).
- Morin-Crini, N.; Loiacono, S.; Placet, V.; Torri, G.; Bradu, C.; Kostić, M.; Cosentino, C.; Chanet, G.; Martel, B.; Lichtfouse, E.; et al. Hemp-Based Materials for Metal Removal. In Green Adsorbents for Pollutant Removal, Environmental Chemistry for a Sustainable World; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–34. ISBN 9783319921624. [Google Scholar]
- Väisänen, T.; Batello, P.; Lappalainen, R.; Tomppo, L. Modification of hemp fibers (Cannabis Sativa L.) for composite applications. Ind. Crops Prod. 2018, 111, 422–429. [Google Scholar] [CrossRef]
- Salami, A.; Raninen, K.; Heikkinen, J.; Tomppo, L.; Vilppo, T.; Selenius, M.; Raatikainen, O.; Lappalainen, R.; Vepsäläinen, J. Complementary chemical characterization of distillates obtained from industrial hemp hurds by thermal processing. Ind. Crops Prod. 2020, 155, 112760. [Google Scholar] [CrossRef]
- Abraham, R.E.; Wong, C.S.; Puri, M. Enrichment of cellulosic waste hemp (Cannabis sativa) hurd into non-toxic microfibres. Materials 2016, 9, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarabo, R.; Fuente, E.; Monte, M.C.; Savastano, H.; Mutjé, P.; Negro, C. Use of cellulose fibers from hemp core in fiber-cement production. Effect on flocculation, retention, drainage and product properties. Ind. Crops Prod. 2012, 39, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Pejić, B.M.; Kramar, A.D.; Obradović, B.M.; Kuraica, M.M.; Žekić, A.A.; Kostić, M.M. Effect of plasma treatment on chemical composition, structure and sorption properties of lignocellulosic hemp fibers (Cannabis sativa L.). Carbohydr. Polym. 2020, 236, 116000. [Google Scholar] [CrossRef]
- Kassab, Z.; Abdellaoui, Y.; Salim, M.H.; Bouhfid, R.; Qaiss, A.E.K.; El Achaby, M. Micro- and nano-celluloses derived from hemp stalks and their effect as polymer reinforcing materials. Carbohydr. Polym. 2020, 245, 116506. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Witayakran, S.; Boonmahitthisud, A. Study on structural and thermal properties of cellulose microfibers isolated from pineapple leaves using steam explosion. J. Environ. Chem. Eng. 2019, 7, 102836. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Kommula, V.P. Exploration on the characteristics of cellulose microfibers from Palmyra palm fruits. Int. J. Polym. Anal. Charact. 2016, 21, 286–295. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Wang, S.; Wang, F.; Song, Z.; Song, X.; Yang, X.; Wang, Q. Preparation of cellulose nanocrystals using highly recyclable organic acid treated softwood pulp. BioResources 2019, 14, 9331–9351. [Google Scholar] [CrossRef]
- Frost, B.A.; Johan Foster, E. Isolation of thermally stable cellulose nanocrystals from spent coffee grounds via phosphoric acid hydrolysis. J. Renew. Mater. 2020, 8, 187–203. [Google Scholar] [CrossRef]
- Sherif, N.; Gadalla, M.; Kamel, D. Acid–hydrolysed furfural production from rice straw bio-waste: Process synthesis, simulation, and optimisation. S. Afr. J. Chem. Eng. 2021, 38, 34–40. [Google Scholar] [CrossRef]
- Rehman, N.; Alam, S.; Amin, N.U.; Mian, I.; Ullah, H. Ecofriendly isolation of cellulose from eucalyptus lenceolata: A novel approach. Int. J. Polym. Sci. 2018, 2018, 8381501. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Liu, C.; Wang, D.; Zhang, Y.; Yu, G.; Si, C.; Li, B.; Mu, X.; Peng, H. Sustainable preparation and characterization of thermally stable and functional cellulose nanocrystals and nanofibrils via formic acid hydrolysis. J. Bioresour. Bioprod. 2017, 2, 10–15. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose-Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [Green Version]
- Amenaghawon, A.N.; Balogun, A.A.; Agbonghae, E.E.; Ogbeide, S.E.; Okieimen, C.O. Statistical Optimisation of Dilute Acid Pre-Treatment of Corn Stover using Response Surface Methodology. J. Environ. 2013, 2, 34–40. [Google Scholar]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Mota, A.T.; Andrade, P.B. Optimization of the recovery of high-value compounds from pitaya fruit by-products using microwave-assisted extraction. Food Chem. 2017, 230, 463–474. [Google Scholar] [CrossRef]
- Chang, C.W.; Yen, C.C.; Wu, M.T.; Hsu, M.C.; Wu, T.Y. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: Optimization and comparative study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, L.; Kasra Kermanshahi, R.; Jahed Khaniki, G. Response Surface Design for Removal of Lead by Different Lactic Acid Bacteria. Health Scope 2020, 9, e101049. [Google Scholar] [CrossRef]
- Murray, L.; Mason, R.L.; Gunst, R.F.; Hess, J.L. Statistical Design and Analysis of Experiments: With Applications to Engineering and Science; John Wiley & Sons: Hoboken, NJ, USA, 1990; Volume 85, ISBN 3175723993. [Google Scholar]
- Dean, A.; Morris, M.; Stufken, J.; Bingham, D. Handbook of Design and Analysis of Experiments; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466504349. [Google Scholar]
- Guo, Y.; Zhang, Y.; Zheng, D.; Li, M.; Yue, J. Isolation and characterization of nanocellulose crystals via acid hydrolysis from agricultural waste-tea stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. [Google Scholar] [CrossRef]
- Akhabue, C.E.; Osubor, N.T. Optimization of extraction of microcrystalline cellulose from orange peel waste using response surface methodology. Ife J. Sci. 2017, 19, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, Z.Z.; Chandran, R.R.R.; Jahan, A.; Khalid, K.; Rahman, M.M.; Al-Amin, M.; Akbarzadeh, O.; Badruddin, I.A.; Khan, T.M.Y.; Kamangar, S.; et al. Extraction of cellulose nano-whiskers using ionic liquid-assisted ultra-sonication: Optimization and mathematical modelling using Box-Behnken design. Symmetry 2019, 11, 1148. [Google Scholar] [CrossRef] [Green Version]
- Kandhola, G.; Djioleu, A.; Rajan, K.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.W. Maximizing production of cellulose nanocrystals and nanofibers from pre-extracted loblolly pine kraft pulp: A response surface approach. Bioresour. Bioprocess. 2020, 7, 19. [Google Scholar] [CrossRef]
- Abiaziem, C.V.; Williams, A.B.; Inegbenebor, A.I.; Onwordi, C.T.; Ehi-Eromosele, C.O.; Petrik, L.F. Isolation and Characterisation of Cellulose Nanocrystal Obtained From Sugarcane Peel. Rasayan J. Chem. 2020, 13, 177–187. [Google Scholar] [CrossRef]
- Malucelli, L.C.; Matos, M.; Jordão, C.; Lomonaco, D.; Lacerda, L.G.; Carvalho Filho, M.A.S.; Magalhães, W.L.E. Influence of cellulose chemical pretreatment on energy consumption and viscosity of produced cellulose nanofibers (CNF) and mechanical properties of nanopaper. Cellulose 2019, 26, 1667–1681. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef]
- Abd Hamid, S.B.; Chowdhury, Z.Z.; Karim, M.Z. Catalytic extraction of microcrystalline cellulose (MCC) from Elaeis guineensis using central composite design (CCD). BioResources 2014, 9, 7403–7426. [Google Scholar] [CrossRef] [Green Version]
- Akgün, D. Optimization and Characterization of Cellulose Nanocrystal Production from Aseptic Tetra Pak Food Packaging Waste. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 131–148. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and characterization of thermally stable cellulose nanocrystals via a sustainable approach of FeCl3-catalyzed formic acid hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Matebie, B.Y.; Tizazu, B.Z.; Kadhem, A.A.; Venkatesa Prabhu, S. Synthesis of Cellulose Nanocrystals (CNCs) from Brewer’s Spent Grain Using Acid Hydrolysis: Characterization and Optimization. J. Nanomater. 2021, 2021, 7133154. [Google Scholar] [CrossRef]
- Ventura-Cruz, S.; Tecante, A. Extraction and characterization of cellulose nanofibers from Rose stems (Rosa spp.). Carbohydr. Polym. 2019, 220, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, F.; Ma, T.; Zhang, B.; Manyande, A.; Du, H. Preparation and characterization of cellulose nanofibers isolated from lettuce peel. Cellul. Chem. Technol. 2019, 53, 677–684. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Sobral, P.J.D.A.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from banana peels. Cellulose 2014, 21, 417–432. [Google Scholar] [CrossRef]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT-Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Dominic, M.C.D.; Joseph, R.; Begum, P.M.S.; Joseph, M.; Padmanabhan, D.; Morris, L.A.; Kumar, A.S.; Formela, K. Cellulose nanofibers isolated from the Cuscuta Reflexa plant as a green reinforcement of natural rubber. Polymers 2020, 12, 814. [Google Scholar] [CrossRef] [Green Version]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Chen, J.; Ji, X.; Wang, Q.; Yang, G.; Fatehi, P. Dilute sulfuric acid hydrolysis of Pennisetum (sp.) Hemicellulose. BioResources 2017, 12, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Syafri, E.; Kasim, A.; Abral, H.; Sudirman; Sulungbudi, G.T.; Sanjay, M.R.; Sari, N.H. Synthesis and characterization of cellulose nanofibers (CNF) ramie reinforced cassava starch hybrid composites. Int. J. Biol. Macromol. 2018, 120, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, S.; Zhang, N.; Zhang, J. Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose 2014, 21, 335–346. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.J.; Li, D.; Cheng, Y.L.; Adhikari, B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.; Malik, S.; Joshi, G.; Rajput, N.K.; Gupta, P.K. Preparation of alpha cellulose from sugarcane bagasse and its cationization: Synthesis, characterization, validation and application as wet-end additive. Int. J. Biol. Macromol. 2021, 170, 793–809. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Mustapha, A.; Masri, M.M.; Owolabi, A.F.; Syakir, M.I.; Dungani, R.; Paridah, M.T.; Jawaid, M.; Abdul Khalil, H.P.S. Isolation and characterization of cellulose nanofibers from gigantochloa scortechinii as a reinforcement material. J. Nanomater. 2016, 2016, 4024527. [Google Scholar] [CrossRef] [Green Version]
- Widiarto, S.; Pramono, E.; Suharso; Rochliadi, A.; Arcana, I.M. Cellulose nanofibers preparation from cassava peels via mechanical disruption. Fibers 2019, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef]
- Yang, X.; Han, F.; Xu, C.; Jiang, S.; Huang, L.; Liu, L.; Xia, Z. Effects of preparation methods on the morphology and properties of nanocellulose (NC) extracted from corn husk. Ind. Crops Prod. 2017, 109, 241–247. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Balaji, A.N.; Ramanujam, N.R. Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment. Carbohydr. Polym. 2019, 212, 312–322. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Joy, J.; Mathew, L.; Mozetic, M.; Koetz, J.; Thomas, S. Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind. Crops Prod. 2014, 59, 27–34. [Google Scholar] [CrossRef]
- Wu, J.; Du, X.; Yin, Z.; Xu, S.; Xu, S.; Zhang, Y. Preparation and characterization of cellulose nanofibrils from coconut coir fibers and their reinforcements in biodegradable composite films. Carbohydr. Polym. 2019, 211, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Revati, R.; Majid, M.S.A.; Ridzuan, M.J.M.; Nasir, N.F.M. Characterisation of structural and physical properties of cellulose nanofibers from Pennisetum purpureum. IOP Conf. Ser. Mater. Sci. Eng. 2019, 670, 012043. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Zhang, Y.; Yu, G.; Si, C.; Li, B. Preparation and characterization of functional cellulose nanofibrils via formic acid hydrolysis pretreatment and the followed high-pressure homogenization. Ind. Crops Prod. 2016, 94, 736–745. [Google Scholar] [CrossRef]
- Santmarti, A.; Lee, K. Crystallinity and Thermal Stability of Nanocellulose. In Nanocellulose and Sustainability: Production, Properties, Applications, and Case Studies; CRC Press: Boca Raton, FL, USA, 2018; pp. 67–86. ISBN 9781351262927. [Google Scholar]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Alam, M.A. Isolation and characterization of alpha and nanocrystalline cellulose from date palm (Phoenix dactylifera L.) trunk mesh. Polymers 2021, 13, 1893. [Google Scholar] [CrossRef]
- Lv, D.; Du, H.; Che, X.; Wu, M.; Zhang, Y.; Liu, C.; Nie, S.; Zhang, X.; Li, B. Tailored and Integrated Production of Functional Cellulose Nanocrystals and Cellulose Nanofibrils via Sustainable Formic Acid Hydrolysis: Kinetic Study and Characterization. ACS Sustain. Chem. Eng. 2019, 7, 9449–9463. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nano fi bres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.; Yao, S.; Cao, H.; Xu, Q.; et al. Preparation and characterization of high yield cellulose nanocrystals (CNC) derived from ball mill pretreatment and maleic acid hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef]
- Peretz, R.; Sterenzon, E.; Gerchman, Y.; Kumar, V.; Luxbacher, T.; Mamane, H. Nanocellulose production from recycled paper mill sludge using ozonation pretreatment followed by recyclable maleic acid hydrolysis. Carbohydr. Polym. 2019, 216, 343–351. [Google Scholar] [CrossRef]
- Bian, H.; Luo, J.; Wang, R.; Zhou, X.; Ni, S.; Shi, R.; Fang, G.; Dai, H. Recyclable and Reusable Maleic Acid for Efficient Production of Cellulose Nanofibrils with Stable Performance. ACS Sustain. Chem. Eng. 2019, 7, 20022–20031. [Google Scholar] [CrossRef]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mofarrah, E.; Mehranian, M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J. Hazard. Mater. 2005, 123, 187–195. [Google Scholar] [CrossRef]
- Sartika, D.; Syamsu, K.; Warsiki, E.; Fahma, F. Optimization of Sulfuric Acid Concentration and Hydrolysis Time on Crystallinity of Nanocrystalline Cellulose: A Response Surface Methodology Study. In Proceedings of the IOP Conference Series: Earth and Environmental Science, South Sulawesi, Indonesia, 6–8 August 2019; Volume 355, pp. 1–9. [Google Scholar]
- Shet, V.B.; Sanil, N.; Bhat, M.; Naik, M.; Mascarenhas, L.N.; Goveas, L.C.; Rao, C.V.; Ujwal, P.; Sandesh, K.; Aparna, A. Acid hydrolysis optimization of cocoa pod shell using response surface methodology approach toward ethanol production. Agric. Nat. Resour. 2018, 52, 581–587. [Google Scholar] [CrossRef]
- Yadav, S.P.; Ray, A.K.; Ghosh, U.K. Optimization of Rice Straw Acid Hydrolysis Using Response Surface Methodology. Am. J. Environ. Eng. 2016, 6, 174–183. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Z.; Zhu, L.; Yuan, X.; Wei, D.; Peng, W.; Wu, C. Efficient approach for the extraction and identification of red pigment from zanthoxylum bungeanum maxim and its antioxidant activity. Molecules 2018, 23, 1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.K.; Leng Chew, I.M.; Yaw Choong, T.S.; Tan, J.; Tan, K.W. Isolation of Nanocrystalline Cellulose from oil palm empty fruit bunch—A response surface methodology study. MATEC Web Conf. 2016, 60, 04009. [Google Scholar] [CrossRef]










| Exp No | Temperature (°C) | Hydrolysis Time (minutes) | Acid Concentration (%) | Acid | Sample ID |
|---|---|---|---|---|---|
| 1 | 45 | 30 | 45 | Sulfuric acid | 1SA-CMFs |
| 2 | 45 | 60 | 45 | Formic acid | 1FA-CMFs |
| 3 | 45 | 90 | 45 | Maleic acid | 1MA-CMFs |
| 4 | 55 | 30 | 45 | Sulfuric acid | 2SA-CMFs |
| 5 | 55 | 60 | 45 | Formic acid | 2FA-CMFs |
| 6 | 55 | 90 | 45 | Maleic acid | 2MA-CMFs |
| 7 | 65 | 30 | 45 | Formic acid | 3FA-CMFs |
| 8 | 65 | 60 | 45 | Maleic acid | 3MA-CMFs |
| 9 | 65 | 90 | 45 | Sulfuric acid | 3SA-CMFs |
| 10 | 45 | 30 | 64 | Maleic acid | 4MA-CMFs |
| 11 | 45 | 60 | 64 | Sulfuric acid | 4SA-CMFs |
| 12 | 45 | 90 | 64 | Formic acid | 4FA-CMFs |
| 13 | 55 | 30 | 64 | Formic acid | 5FA-CMFs |
| 14 | 55 | 60 | 64 | Maleic acid | 5MA-CMFs |
| 15 | 55 | 90 | 64 | Sulfuric acid | 5SA-CMFs |
| 16 | 65 | 30 | 64 | Maleic acid | 6MA-CMFs |
| 17 | 65 | 60 | 64 | Sulfuric acid | 6SA-CMFs |
| 18 | 65 | 90 | 64 | Formic acid | 6FA-CMFs |
| 19 | 55 | 60 | 55 | Sulfuric acid | 7SA-CMFs |
| 20 | 55 | 60 | 55 | Sulfuric acid | 7SA-CMFs |
| 21 | 55 | 60 | 55 | Sulfuric acid | 7SA-CMFs |
| Sample ID | CrI (%) | Main Thermal Degradation | Char Residue Amount at 900 °C (%) | ||
|---|---|---|---|---|---|
| Tonset (°C) | Tmax (°C) | Weight Loss (%) | |||
| Raw hemp fiber | 60 | 207 | 386 | 72 | 14 |
| Bleached cellulose fiber | 73 | 232 | 359 | 82 | 10 |
| 1SA-CMFs | 75 | 268 | 387 | 78 | 11 |
| 2SA-CMFs | 52 | 178 | 410 | 70 | 18 |
| 3SA-CMFs | 53 | 152 | 489 | 74 | 19 |
| 4SA-CMFs | 71 | 256 | 377 | 85 | 5 |
| 5SA-CMFs | 76 | 244 | 373 | 85 | 4 |
| 6SA-CMFs | 59 | 190 | 522 | 48 | 48 |
| 7SA-CMFs | 66 | 244 | 323 | 53 | 22 |
| 1FA-CMFs | 55 | 208 | 364 | 72 | 18 |
| 2FA-CMFs | 50 | 247 | 378 | 81 | 9 |
| 3FA-CMFs | 66 | 252 | 380 | 83 | 8 |
| 4FA-CMFs | 59 | 238 | 387 | 86 | 7 |
| 5FA-CMFs | 70 | 235 | 389 | 81 | 9 |
| 6FA-CMFs | 61 | 262 | 370 | 85 | 5 |
| 1MA-CMFs | 40 | 203 | 368 | 67 | 18 |
| 2MA-CMFs | 55 | 244 | 386 | 84 | 8 |
| 3MA-CMFs | 61 | 253 | 385 | 84 | 8 |
| 4MA-CMFs | 65 | 245 | 389 | 84 | 7 |
| 5MA-CMFs | 54 | 261 | 385 | 87 | 3 |
| 6MA-CMFs | 67 | 265 | 384 | 86 | 4 |
| Source | DF | Sum of Squares | Mean Square | F | p |
|---|---|---|---|---|---|
| Regression | 17 | 1564.09 | 92.0051 | 11.41 | 0.034 * |
| Residual | 3 | 24.2 | 8.8.67 | ||
| Lack of fit | 1 | 24.2 | 24.2 | - | - |
| Pure error | 2 | 0.00 | 0 | ||
| Total corrected | 21 | 1588.21 | |||
| R2 | 0.985 | Coefficient of variance (CV) (%) | 4.07 | ||
| Adjusted R2 | 0.961 | Adequate precision | 9.59 | ||
| Std.Dev | 2.84 |
| Observed Results | Predicted Results | |
|---|---|---|
| Acid type | Formic acid | Formic acid |
| Acid concentrations | 62% | 62.1% |
| Hydrolysis temperature | 47 °C | 47 °C |
| Reaction time | 36 min | 36 min |
| Maximum crystallinity | 82% | 83.69% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhlongo, J.T.; Nuapia, Y.; Tlhaole, B.; Mahlangu, O.T.; Etale, A. Optimization of Hemp Bast Microfiber Production Using Response Surface Modelling. Processes 2022, 10, 1150. https://doi.org/10.3390/pr10061150
Mhlongo JT, Nuapia Y, Tlhaole B, Mahlangu OT, Etale A. Optimization of Hemp Bast Microfiber Production Using Response Surface Modelling. Processes. 2022; 10(6):1150. https://doi.org/10.3390/pr10061150
Chicago/Turabian StyleMhlongo, Jessica Tsakani, Yannick Nuapia, Boitumelo Tlhaole, Oranso Themba Mahlangu, and Anita Etale. 2022. "Optimization of Hemp Bast Microfiber Production Using Response Surface Modelling" Processes 10, no. 6: 1150. https://doi.org/10.3390/pr10061150





