Microwave Pre-Treatment of Model Food Waste to Produce Short Chain Organic Acids and Ethanol via Anaerobic Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Microwave Treatments
2.3. AF Batch Tests
2.4. Analytical Methods
2.5. Statistical Analysis
2.6. Energy Applied and Product Market Value
3. Results and Discussion
3.1. Microwave Pre-Treatments at Different Temperatures and Times
3.2. Anaerobic Fermentation Tests
3.3. Scanning Electron Microscopy Images
3.4. Energy and Economic Considerations
- Higher production of SCOAs with longer run time. The length of our experiments was fixed to 21 d. From the experimental profiles in Figure 2a, SCOA production had not stopped by the end of the experiment in the pre-treated fermentation runs, therefore it is likely that longer run times could give higher SCOA production and higher revenue from the products;
- Scale-up factors for energy consumption. Our measured electricity consumption was obtained for 200 mL volumes, which is very different from the size and geometry of a full-scale installations. The energy consumption per unit of volatile solids may benefit from scale-up effects;
- Use of excess or “curtailed” electricity form peaks of renewable energy production. The cost of electricity from peaks of generation from solar and wind farms has been assumed to be zero in a recent report by the UK Government [14]. The MW pre-treatment could therefore be designed to be operated only when excess electricity becomes available, which would significantly reduce the operating costs of the pre-treatment.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organisation of the United Nations (FAO). Global Food Losses and Food Waste: Extent, Causes and Prevention; FAO: Dü sseldorf, Germany, 2011. [Google Scholar]
- Quested, T.E.; Parry, A.D.; Easteal, S.; Swannell, R. Food and drink waste from households in the UK. Nutr. Bull. 2011, 36, 460–467. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations (FAO). Post 2015 and SDGs: Nourishing People, Nurturing the Planet; E-bulletin 6; FAO: Roma, Italy, 2015. [Google Scholar]
- Eriksson, M.; Strid, I.; Hansson, P. Carbon footprint of food waste management options in the waste hierarchy e a Swedish case study. J. Clean. Prod. 2015, 93, 115–125. [Google Scholar] [CrossRef]
- Papargyropoulou, E.; Lozano, R.; Steinberger, J.K.; Wright, N.; Ujang, Z. The food waste hierarchy as a framework for the management of food surplus and food waste. J. Clean. Prod. 2014, 76, 106–115. [Google Scholar] [CrossRef]
- Gao, A.; Tian, Z.; Wang, Z.; Wennersten, R.; Sun, Q. Comparison between the technologies for food waste treatment. Energy Procedia 2017, 105, 3915–3921. [Google Scholar] [CrossRef]
- Dionisi, D.; Silva, I.M.O. Production of ethanol, organic acids and hydrogen: An opportunity for mixed culture biotechnology? Rev. Environ. Sci. Biotechnol. 2016, 15, 213–242. [Google Scholar] [CrossRef]
- Ma, C.; Liu, J.; Ye, M.; Zou, L.; Qian, G.; Li, Y. Towards utmost bioenergy conversion efficiency of food waste: Pretreatment, co-digestion, and reactor type. Renew. Sustain. Energy Rev. 2018, 90, 700–709. [Google Scholar] [CrossRef]
- Posmanik, R.; Labatut, R.A.; Kim, A.H.; Usack, J.G.; Tester, J.W.; Angenent, L.T. Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Bioresour. Technol. 2017, 233, 134–143. [Google Scholar] [CrossRef]
- Yue, L.; Cheng, J.; Tang, S.; An, X.; Hua, J.; Dong, H.; Zhou, J. Ultrasound and microwave pretreatments promote methane production potential and energy conversion during anaerobic digestion of lipid and food wastes. Energy 2021, 228, 120525. [Google Scholar] [CrossRef]
- Mudhoo, A.; Moorateeah, P.R.; Mohee, R. Effects of Microwave Heating on Biogas Production, Chemical Oxygen Demand and Volatile Solids S Solubilization of Food Residues. World Acad. Sci. Eng. Technol. Int. J. Environ. Ecol. Eng. 2012, 69, 805–810. [Google Scholar]
- Ormaechea, P.; Castrillón, L.; Marañón, E.; Fernández-Nava, Y.; Negral, L.; Megido, L. Influence of the ultrasound pretreatment on anaerobic digestion of cattle manure, food waste and crude glycerine. Environ. Technol. 2017, 38, 682–686. [Google Scholar] [CrossRef]
- Scherzinger, M.; Kaltschmitt, M. Thermal pre-treatment options to enhance anaerobic digestibility—A review. Ren. Sustain. Energy Rev. 2021, 137, 110627. [Google Scholar] [CrossRef]
- Department for Business, Energy & Industrial Strategy. Hydrogen Production Costs 2021. Available online: https://www.gov.uk/government/publications/hydrogen-production-costs-2021 (accessed on 26 April 2022).
- Szmidt, R.A.K. Pretreatment Equipment and Food Waste Preparation for Anaerobic Digestion; Wong, M.H., Purchase, D., Dickinson, N., Eds.; Food Waste Valorisation; World Scientific: Singapore, 2023; In press. [Google Scholar]
- Kingman, S.; Dodds, C.; Buttress, A.; Groszek, D. Microwave Processing—Beyond Food. Chem. Eng. 2021, 965. Available online: https://www.thechemicalengineer.com/features/microwave-processing-beyond-food/ (accessed on 30 April 2022).
- Fan, J.; Santomauro, F.; Budarin, V.L.; Whiffin, F.; Abeln, F.; Chantasuban, T.; Gore-Lloyd, D.; Henk, D.; Scott, R.J.; Clark, J.; et al. The additive free microwave hydrolysis of lignocellulosic biomass for fermentation to high value products. J. Clean. Prod. 2018, 198, 776–784. [Google Scholar] [CrossRef]
- Marin, J.; Kennedy, K.J.; Eskicioglu, C. Effect of microwave irradiation on anaerobic degradability of model kitchen waste. Waste Manag. 2010, 30, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, Z.M.A. Effects of microwave and ultrasound irradiations on dark fermentative bio-hydrogen production from food and yard wastes. Int. J. Hydrog. Energy 2017, 42, 4040–4050. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K.J. Evaluation of single vs. staged mesophilic anaerobic digestion of kitchen waste with and without microwave pretreatment. J. Environ. Manag. 2013, 125, 74–84. [Google Scholar] [CrossRef]
- Simonetti, S.; Martín, C.F.; Dionisi, D. Anaerobic fermentation for the production of short chain organic acids: Product concentration, yield and productivity in batch experiments at high feed concentration. J. Environ. Chem. Eng. 2021, 9, 106311. [Google Scholar] [CrossRef]
- Quested, T.; Johnson, H. Household Food and Drink Waste in the UK; final report; WRAP: Banbury, UK, 2009. [Google Scholar]
- Liu, J.; Yin, J.; He, X.; Chen, T.; Shen, D. Optimizing food waste hydrothermal parameters to reduce Maillard reaction and increase volatile fatty acid production. J. Environ. Sci. 2021, 103, 43–49. [Google Scholar] [CrossRef]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Biores. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef]
- Cheng, Y.; Mu, D.; Jiao, Y.; Xu, Z.; Chen, M. Microwave-assisted maillard reaction between rice protein and dextran induces structural changes and functional improvements. J. Cereal Sci. 2021, 97, 103134. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Cacho, J.A.; Mumme, J.; Mohedano, Á.F.; Battimelli, A.; Bolzonella, D.; Schuit, A.D.; Noguerol-Arias, J.; Frigon, J.; et al. Harmonization of the quantitative determination of volatile fatty acids profile in aqueous matrix samples by direct injection using gas chromatography and high-performance liquid chromatography techniques: Multi-laboratory validation study. J. Chromatogr. A 2015, 1413, 94–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Public Health Association, APHA. Standard Methods for the Examination of Water and Wastewater; Report No. 18th edition; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Koehler, L.H. Differentiation of Carbohydrates by Anthrone Reaction Rate and Color Intensity. Anal. Chem. 1952, 24, 1576–1579. [Google Scholar] [CrossRef]
- Business Electricity Prices. 2022. Available online: https://www.businesselectricityprices.org.uk/ (accessed on 6 May 2022).
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Ren. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, C.; Gabriel, S.; Grant, E.H.; Halstead, B.S.J.; Mingos, D.M.P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 1998, 27, 213–224. [Google Scholar] [CrossRef]
- Gezahegn, Y.A.; Tang, J.; Sablani, S.S.; Pedrow, P.D.; Hong, Y.; Lin, H.; Tang, Z. Dielectric properties of water relevant to microwave assisted thermal pasteurization and sterilization of packaged foods. Innov. Food Sci. Emerg. Technol. 2021, 74, 102837. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Biores. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Srinivasan, A.; Saha, M.; Caufield, K.; Abida, O.; Liao, P.H.; Lo, K.V. Microwave-enhanced advanced oxidation treatment of lipids and food wastes. Water Air Soil Pollut. 2018, 229, 227. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K.J. Anaerobic digestion of organic fraction of municipal solid waste combining two pretreatment modalities, high temperature microwave and hydrogen peroxide. Waste Manag. 2012, 32, 41–52. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Li, X.; Ye, T.; Gan, Y.; Zhang, A.; Chen, H.; Xue, G.; Liu, Y. Production of lactic acid from thermal pretreated food waste through the fermentation of waste activated sludge: Effects of substrate and thermal pretreatment temperature. Biores. Technol. 2018, 247, 890–896. [Google Scholar] [CrossRef]
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated-Batch Fermentation of Cheese Whey for Semi-Continuous Lactic Acid Production Using Mixed Cultures at Uncontrolled pH. Sustainability 2019, 11, 3330. [Google Scholar] [CrossRef] [Green Version]
- Warnecke, T.; Gill, R.T. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb. Cell Factories 2005, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravber, M.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Fats, Carbohydrates and Proteins in Sunflower Seeds after Treatment with Subcritical Water. Chem. Biochem. Eng. Q. 2015, 29, 351–355. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Terzian, N.; Kennedy, K.J.; Droste, R.L.; Hamoda, M. Athermal microwave effects for enhancing digestibility of waste activated sludge. Water Res. 2007, 41, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Toreci, I.; Kennedy, K.J.; Droste, R.L. Effect of High-Temperature Microwave Irradiation on Municipal Thickened Waste Activated Sludge Solubilization. Heat Transf. Eng. 2010, 31, 766–773. [Google Scholar] [CrossRef]
- Eswari, P.; Kavitha, S.; Kaliappan, S.; Yeom, I.; Banu, J.R. Enhancement of sludge anaerobic biodegradability by combined microwave-H2O2 pretreatment in acidic condition. Environ. Sci. Pollut. Res. 2016, 23, 13467–13479. [Google Scholar] [CrossRef]
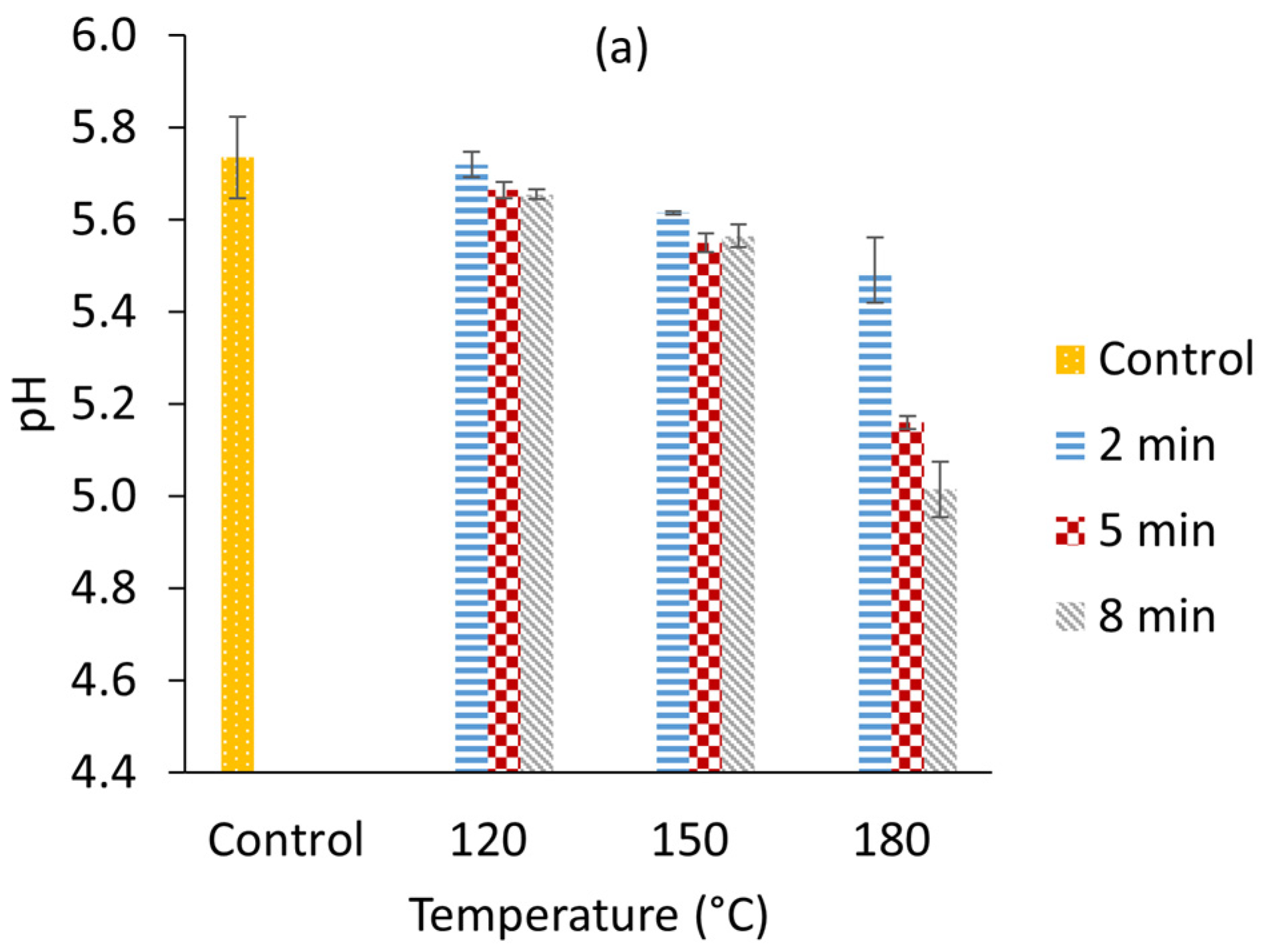
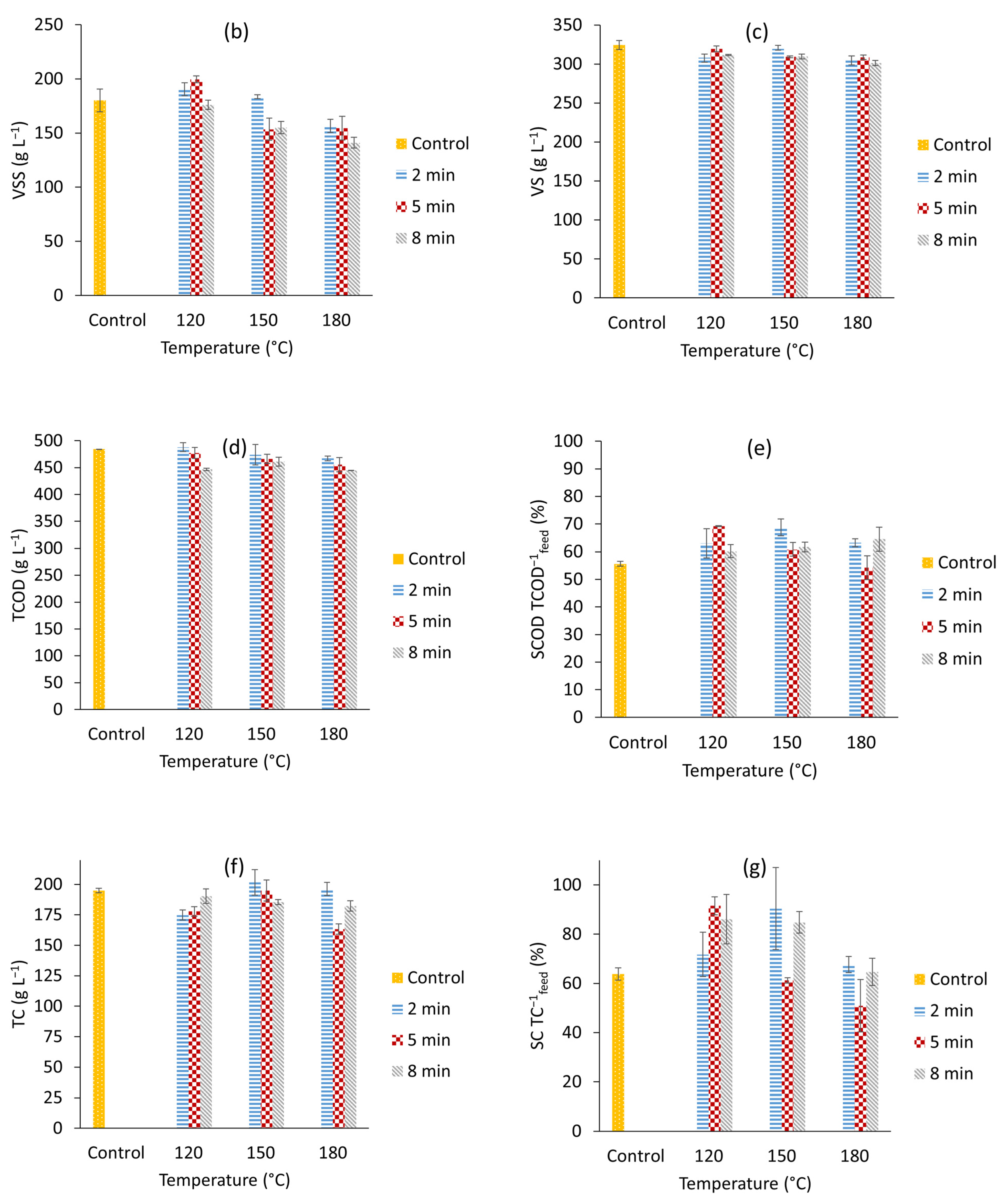
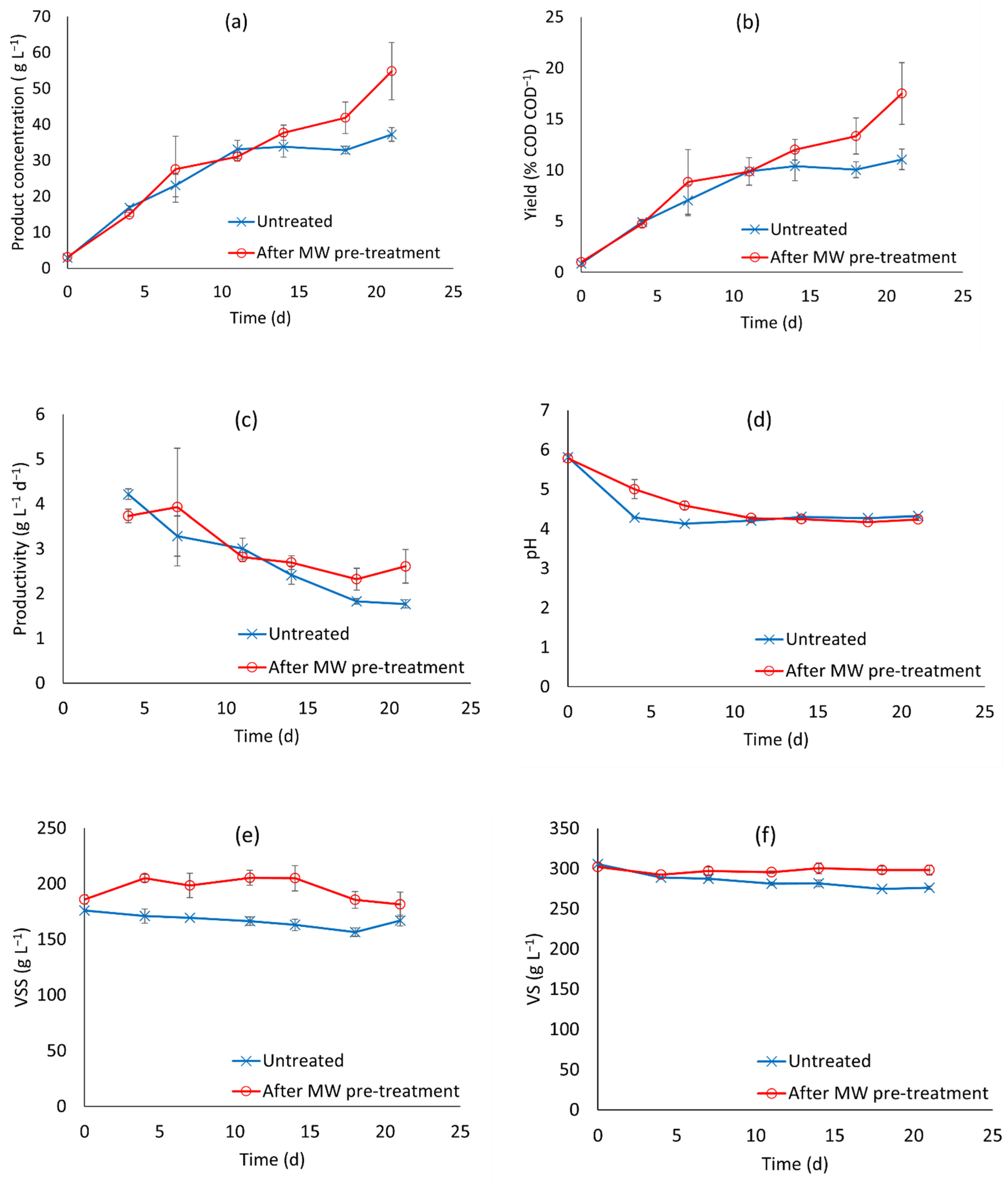
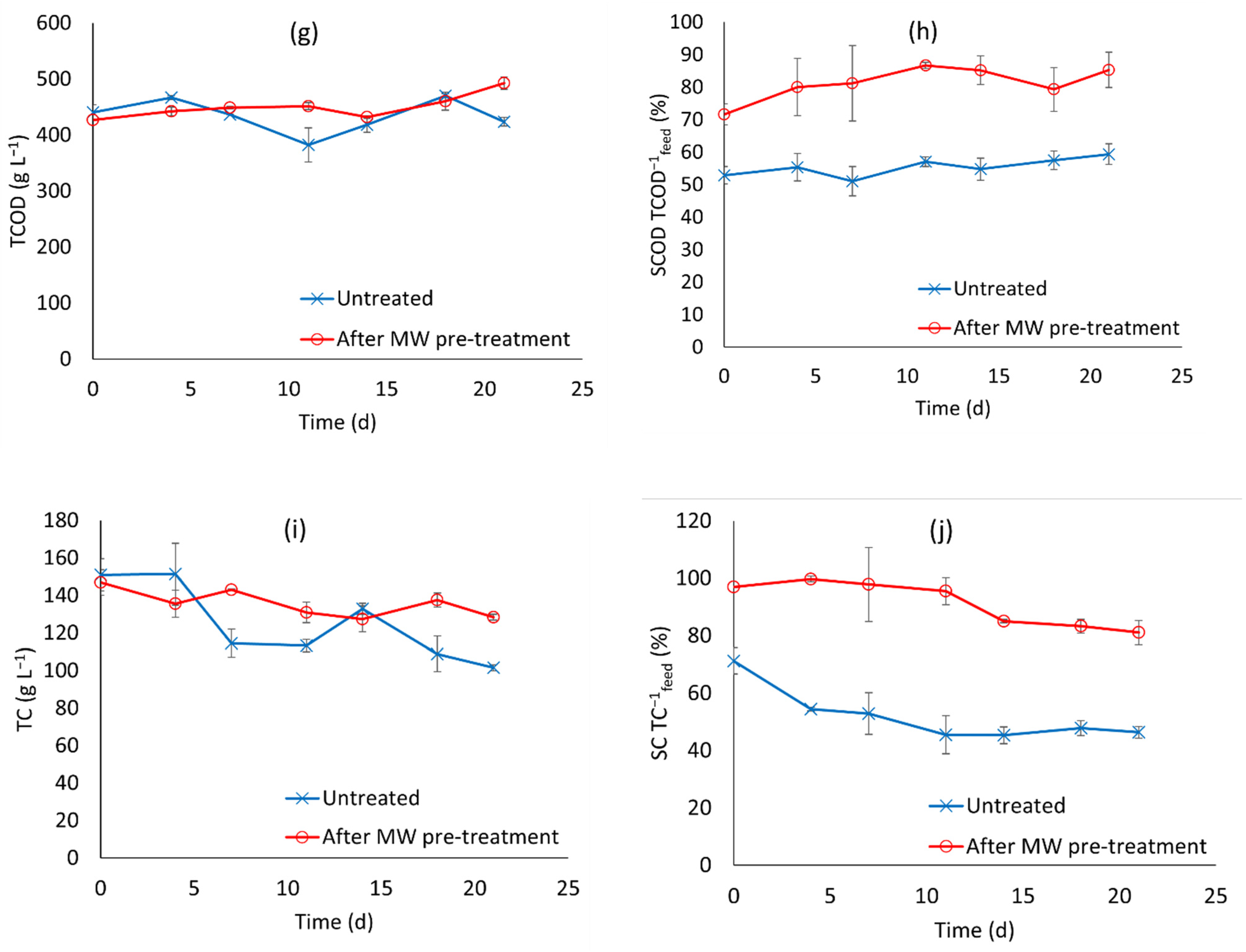


| Treatment Name | Temperature (°C) | Time (Min) |
|---|---|---|
| MW0 | - | - |
| MW1 | 120 | 2 |
| MW2 | 120 | 5 |
| MW3 | 120 | 8 |
| MW4 | 150 | 2 |
| MW5 | 150 | 5 |
| MW6 | 150 | 8 |
| MW7 | 180 | 2 |
| MW8 | 180 | 5 |
| MW9 | 180 | 8 |
| Fermentation Product | Market Price (GBP kg−1) 1 |
|---|---|
| Lactic acid | 1.28 |
| Butyric acid | 0.96 |
| Ethanol | 0.40 |
| Acetic acid | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetti, S.; Fernández Martín, C.; Dionisi, D. Microwave Pre-Treatment of Model Food Waste to Produce Short Chain Organic Acids and Ethanol via Anaerobic Fermentation. Processes 2022, 10, 1176. https://doi.org/10.3390/pr10061176
Simonetti S, Fernández Martín C, Dionisi D. Microwave Pre-Treatment of Model Food Waste to Produce Short Chain Organic Acids and Ethanol via Anaerobic Fermentation. Processes. 2022; 10(6):1176. https://doi.org/10.3390/pr10061176
Chicago/Turabian StyleSimonetti, Serena, Claudia Fernández Martín, and Davide Dionisi. 2022. "Microwave Pre-Treatment of Model Food Waste to Produce Short Chain Organic Acids and Ethanol via Anaerobic Fermentation" Processes 10, no. 6: 1176. https://doi.org/10.3390/pr10061176








