Intensification Insights from Chemical Looping Combustion Using Coal–Biomass Mixtures with Fe-Based Oxygen Carrier
Abstract
:1. Introduction
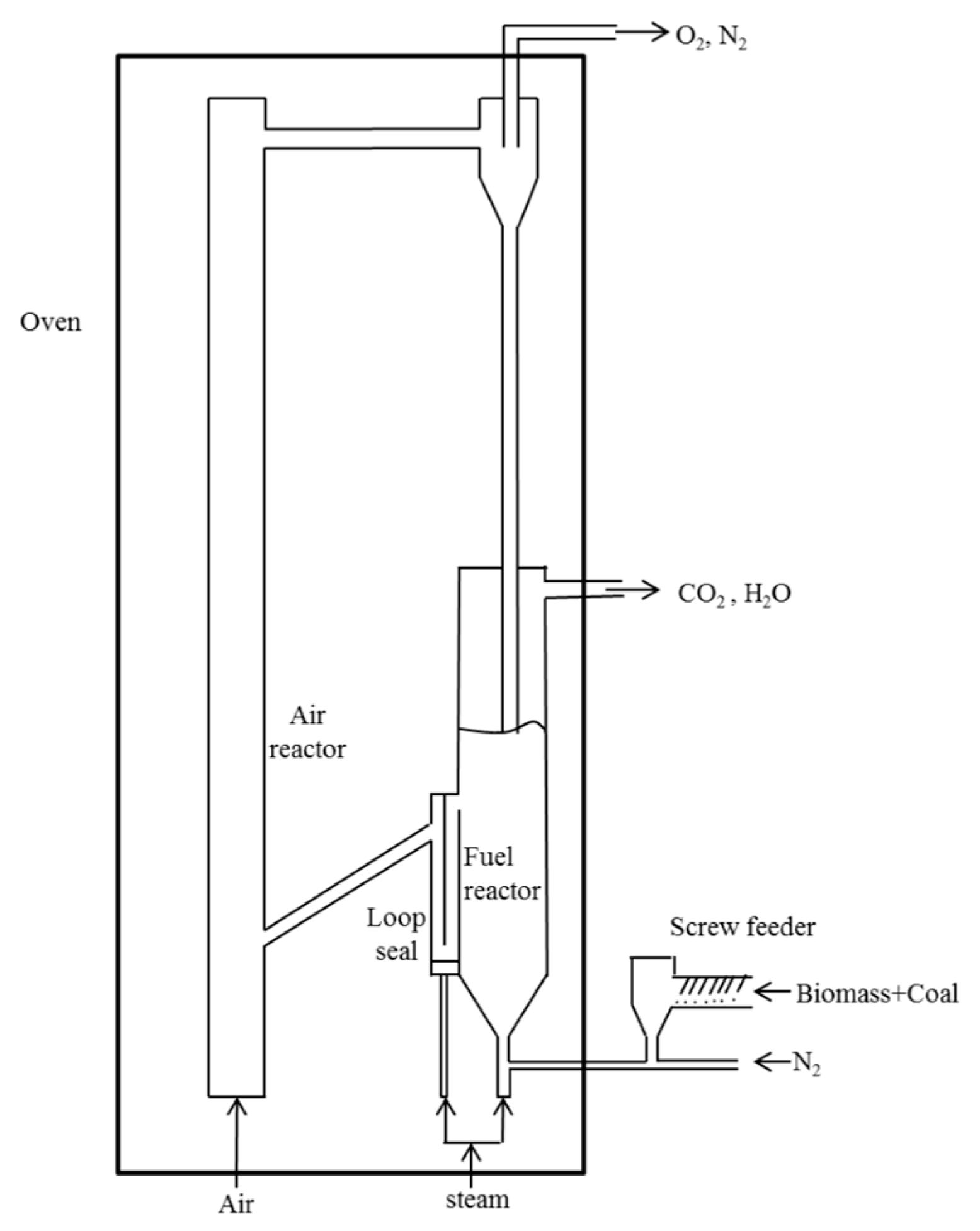
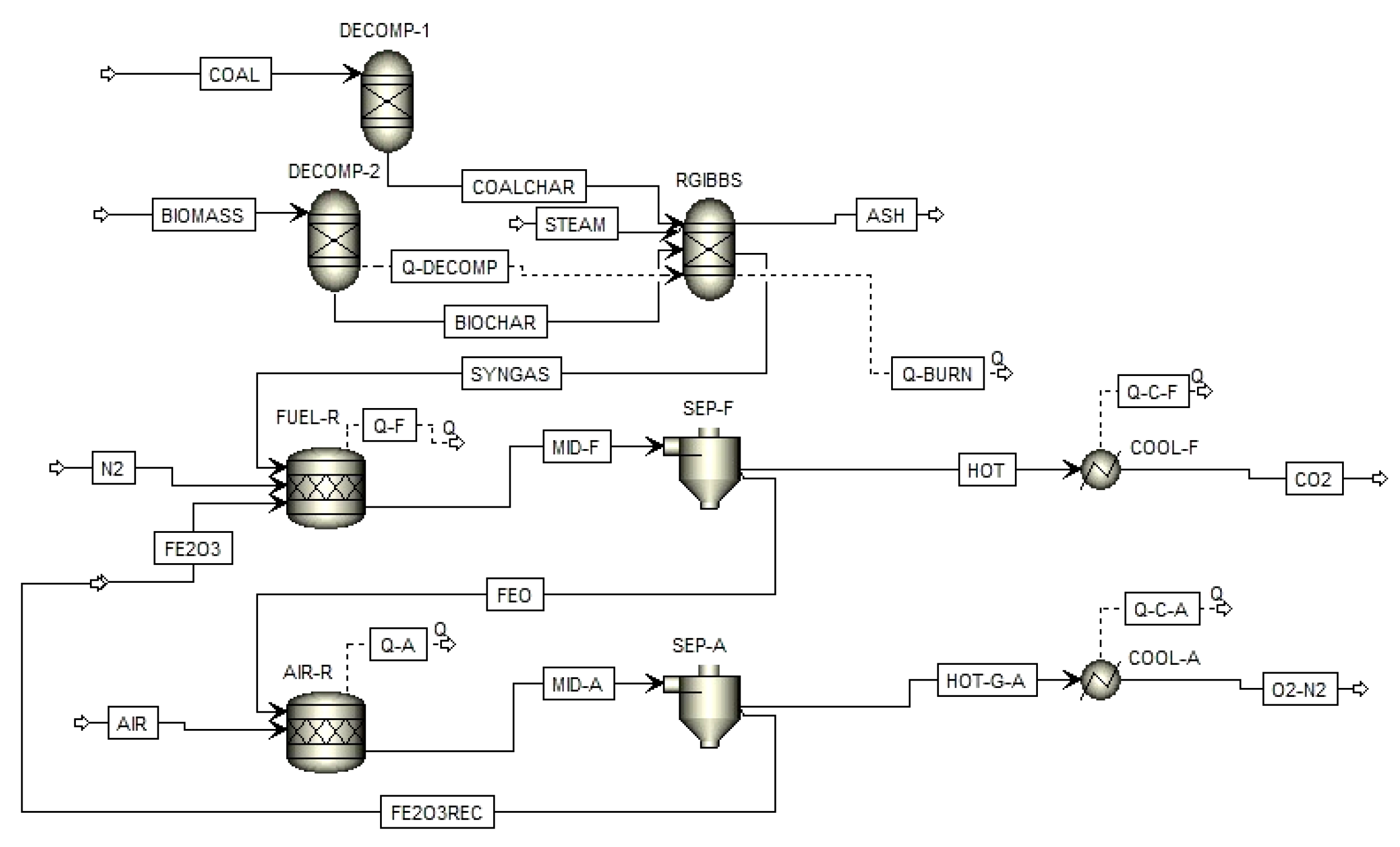
2. Process Simulation Setup
3. Results and Discussions
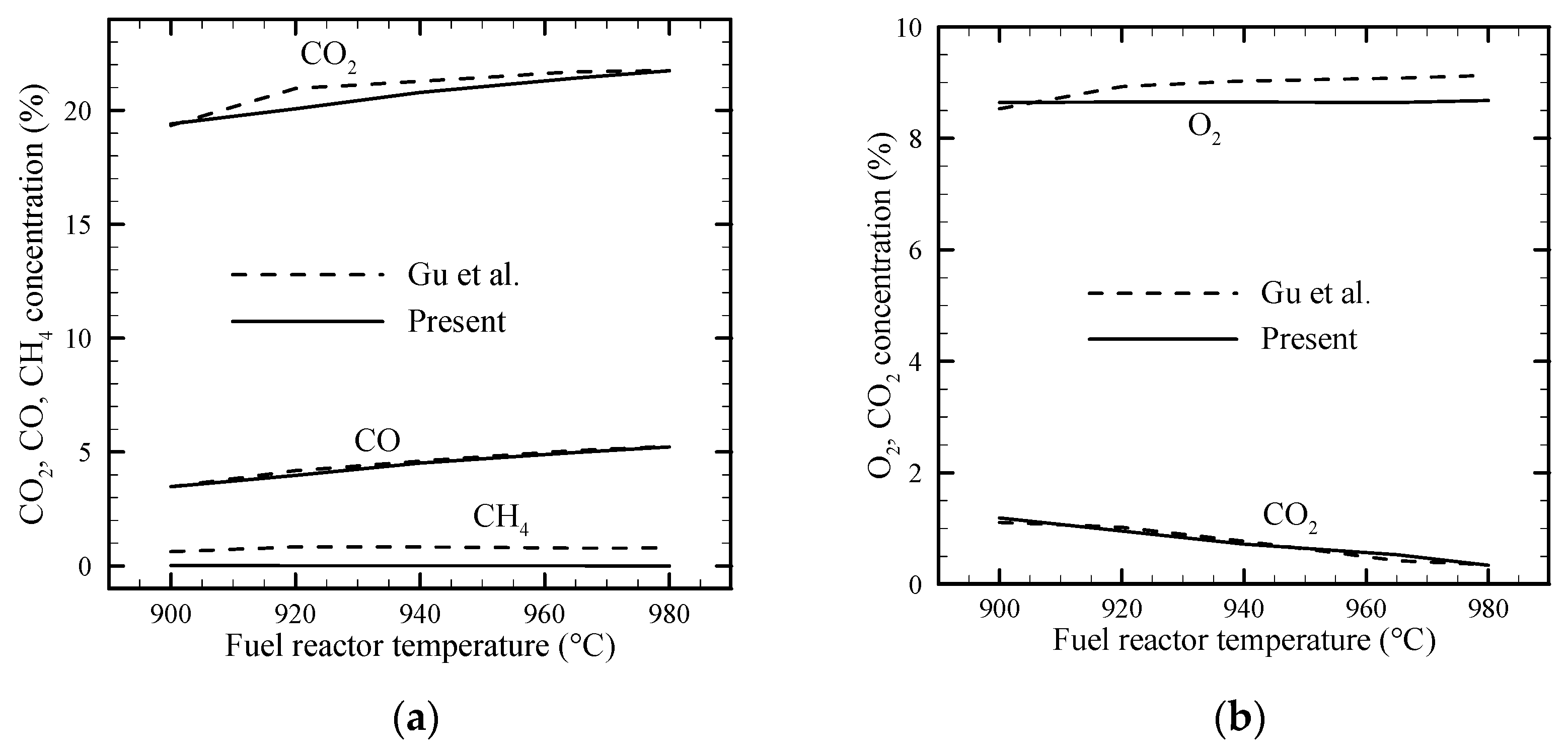
3.1. Reactor Gas Concentration
3.2. Conversion Efficiency
3.3. Carbon Capture Efficiency and Oxide Oxygen Fraction
3.4. Effect of Gasification Agent
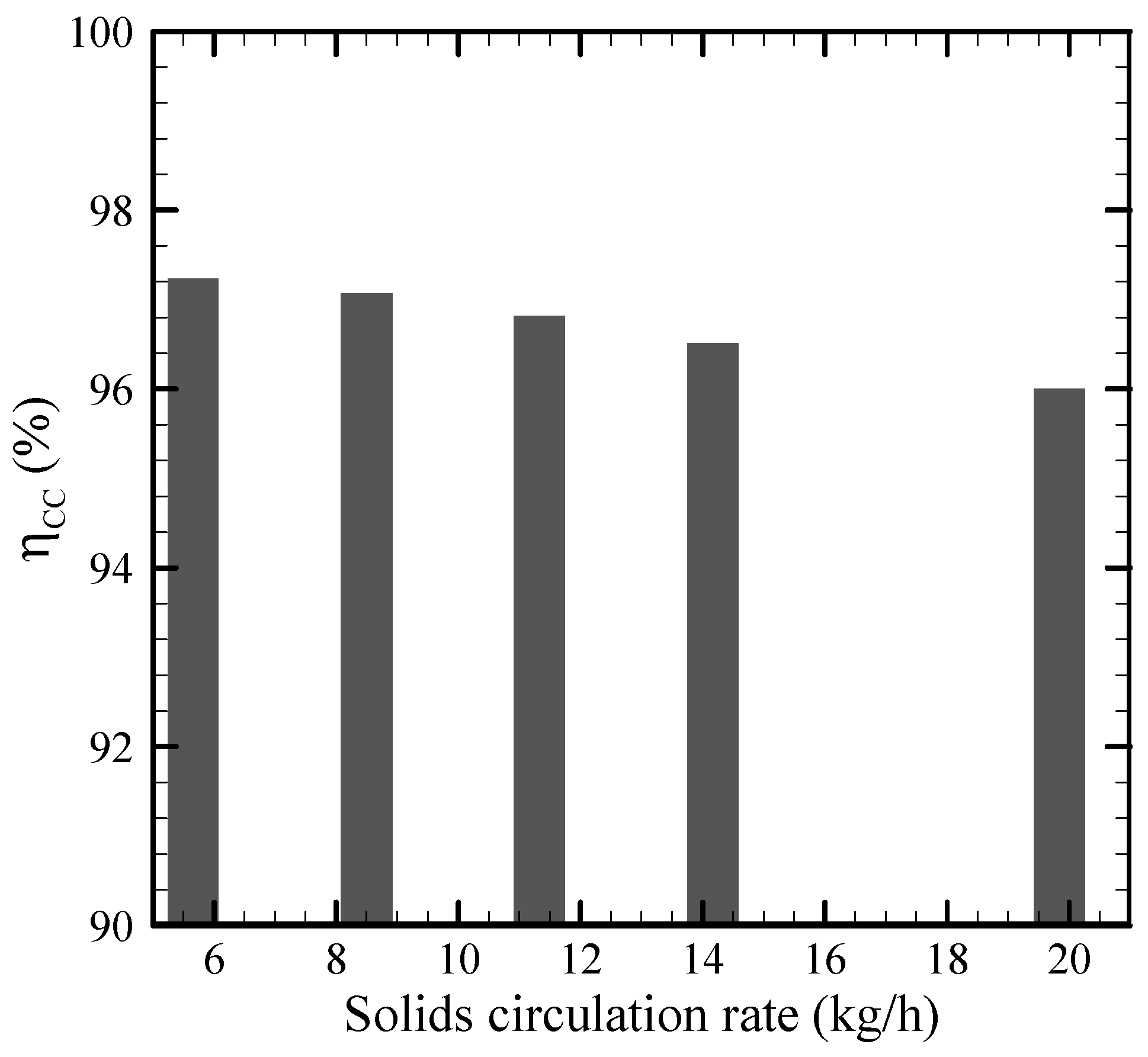
3.5. Effect of the Solid Circulation Rate on the Performance Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Paris Climate Agreement. 2015. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 10 May 2021).
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef] [Green Version]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical looping combustion of solid fuels in a laboratory fluidized-bed reactor. Oil Gas Sci. Technol. Rev. IFP Energ. Nouv. 2011, 66, 201–208. [Google Scholar]
- Mendiara, T.; García-Labiano, F.; Abad, A.; Gayán, P.; de Diego, L.F.; Izquierdo, L.F.; Adánez, J. Negative CO2 emissions through the use of biofuels in chemical looping technology: A Review. Appl. Energy 2018, 232, 657–684. [Google Scholar] [CrossRef]
- Gu, H.; Wu, J.; Hao, J.; Shen, L.H.; Xiao, T. Experiments on chemical looping combustion of coal in interconnected fluidized bed using hematite as oxygen carrier. Proc. CSEE 2010, 30, 51–56. [Google Scholar]
- Zhang, B.; Wu, Z.; Zhang, J.; Guo, W.; Yang, B. Chemical-looping with oxygen uncoupling of the Lignocellulosic Biomass main model compound: Product distribution and kinetic analysis on Lignin. Energy Fuels 2020, 34, 10968–10979. [Google Scholar] [CrossRef]
- Adánez, J.; Gayán, P.; Adánez-Rubio, I.; Cuadrat, A.; Mendiara, T.; Abad, A.; García-Labiano, F.; de Diego, L.F. Use of chemical looping processes for coal combustion with CO2 capture. Energy Procedia 2013, 37, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Lyngfelt, A. Chemical-looping combustion of solid fuels–Status of development. App. Energy 2014, 113, 1869–1873. [Google Scholar] [CrossRef] [Green Version]
- Shen, T.; Ge, H.; Shen, L. Characterization of combined Fe-Cu oxides as oxygen carrier in chemical looping gasification of biomass. Int. J. Greenh. Gas Control 2018, 75, 63–73. [Google Scholar] [CrossRef]
- Gogolev, I.; Linderholm, C.; Gall, D.; Schmitz, M.; Mattisson, T.; Pettersson, J.B.C.; Lyngfelt, A. Chemical looping combustion in a 100kW unit using a ixture of synthetic and natural oxygen carriers-operational results and fate of biomass fuel alkali. Int. J. Greenh. Gas Control 2019, 88, 371–382. [Google Scholar] [CrossRef]
- Kuang, C.; Wang, S.; Luo, M.; Cai, J.; Zhao, J. Investigation of Cuo-based oxygen carriers modified by three different ores in chemical looping combustion with solid fuels. Renew. Energy 2020, 154, 937–948. [Google Scholar] [CrossRef]
- Abad, A.; Adanez-Rubio, I.; Gayan, P.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Demonstration of chemical-looping with oxygen uncoupling (CLOU) process in a 1.5 kWth continuously operating unit using a Cu-based oxygen-carrier. Int. J. Greenh. Gas Control 2012, 6, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, X.; Pawlak-Kruczek, H.; Yang, W.P.; Blasiak, W. Process simulation of co-firing torrefied biomass in a 220 MWe coal-fired power plant. Energy Conv. Manag. 2014, 84, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Ohlemuller, P.; Strohle, J.; Epple, B. Chemical looping combustion of hard coal and torrefied biomass in a 1 MWth pilot plant. Int. J. Greenh. Gas Control 2017, 65, 149–159. [Google Scholar] [CrossRef]
- Shen, L.H.; Wu, J.H.; Xiao, J.; Song, Q.L.; Xiao, R. Chemical-looping combustion of biomass in a 10 kWth reactor with iron oxide as an oxygen carrier. Energy Fuels 2009, 23, 2498–2505. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. Int. J. Greenh. Gas Control 2013, 19, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Biomass combustion with CO2 capture by chemical looping with oxygen uncoupling (CLOU). Fuel Proc. Tech. 2014, 124, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Sarvaramini, A.; Larachi, F. Integrated biomass torrefaction–Chemical looping combustion as a method to recover torrefaction volatiles energy. Fuel 2014, 116, 158–167. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Linderholm, C. Chemical-looping combustion of solid fuels-status and recent progress. Energy Procedia 2017, 114, 371–386. [Google Scholar] [CrossRef]
- Jerndal, E.; Leion, H.; Axelsson, L.; Ekvall, T.; Hedberg, M.; Johansson, K.M.; Svensson, R.; Mattisson, T.; Lyngfelt, A. Using low-cost iron-based materials as oxygen carriers for chemical looping combustion. Oil Gas Sci. Tech. Rev. IFP Energ. Nouv. 2011, 66, 235–248. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. Effect of operating conditions in Chemical-Looping Combustion of coal in a 500Wth unit. Int. J. Greenh. Gas Control 2012, 6, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.G.; Harris, D.J. Char gasification in mixtures of CO2 and H2O: Competition and inhibition. Fuel 2007, 86, 2672–2678. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.H.; Xiao, J.; Zhang, S.; Song, T. Chemical looping combustion of biomass/coal with natural iron ore as oxygen carrier in a continuous reactor. Energy Fuel 2011, 25, 446–455. [Google Scholar] [CrossRef]
- Nandy, A.; Loha, C.; Gu, S.; Sarkar, P.; Karmakar, M.K.; Chatterjee, P.K. Present status and overview of chemical looping combustion technology. Renew. Sustain. Energy Rev. 2016, 59, 597–619. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F.; Adanez, J. The use of ilmenite as oxygen-carrier in a 500Wth Chemical-Looping Coal Combustion unit. Int. J. Greenh. Gas Control 2011, 5, 1630–1642. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Butler, J.; Leon, M.A. Biomass co-firing options on the emission reduction and electricity generation costs in coal-fired power plants. Renew. Energy 2011, 36, 282–288. [Google Scholar] [CrossRef]
- Khorshidi, Z.; Hoa, M.T.; Wiley, D.E. The impact of biomass quality and quantity on the performance and economics of co-firing plants with and without CO2 capture. Int. J. Greenh. Gas Control 2014, 21, 191–202. [Google Scholar] [CrossRef]
- Pragadeesh, K.S.; Regupathi, I.; Sudhakar, D.R. Study of devolatilization during chemical looping combustion of large coal and biomass particles. J. Energy Inst. 2020, 93, 1460–1472. [Google Scholar] [CrossRef]
- Bhui, B.; Prabu, V. Chemical looping based co-combustion of high ash Indian coal and rice straw operating under CO2 in-situ gasification mode. J. Energy Inst. 2021, 94, 176–190. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Liu, A.; Wang, L.; Yu, G. Co-pyrolysis characteristic of biomass and bituminous coal. Bioresour. Tech. 2015, 179, 414–420. [Google Scholar] [CrossRef]
- Jimin, Z.; Jun, Y.; Lihui, J.; Shihe, C.; Shaonan, Z.; Zhichao, L. Kinetics of solid reactions with gas intermediates in chemical looping combustion. Chem. Eng. J. 2021, 420, 127695. [Google Scholar]
- Luo, M.; Wang, S.; Wang, L.; Lv, M.; Qian, L.; Fu, H. Experimental investigation of co-combustion of coal and biomass using chemical looping technology. Fuel Proc. Tech. 2013, 110, 258–267. [Google Scholar] [CrossRef]
- Li, F.; Zeng, L.; Fan, L.S. Biomass direct chemical looping process: Process simulation. Fuel 2010, 89, 3773–3784. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Chivetta, C.; Agarwal, R. Process simulation and validation of chemical-looping with oxygen uncoupling (CLOU) process using Cu-based oxygen carrier. Energy Fuels 2013, 27, 6906–6912. [Google Scholar] [CrossRef]
- Sahir, A.H.; Dansie, J.K.; Cadore, A.L.; Lighty, J.S. A comparative process study of chemical-looping combustion (CLC) and chemical-looping with oxygen uncoupling (CLOU) for solid fuels. Int. J. Greenh. Gas Control 2014, 2, 237–243. [Google Scholar] [CrossRef]
- Meng, W.X.; Banerjee, S.; Zhang, X.; Agarwal, R.K. Process simulation of multi-stage chemical-looping combustion using Aspen Plus. Energy 2015, 90, 1869–1877. [Google Scholar] [CrossRef]
- Gopaul, S.G.; Dutta, A.; Clemmer, R. Chemical looping gasification for hydrogen production: A comparison of two unique processes simulated using Aspen Plus. Int. J. Hydrog. Energy 2014, 39, 5804–5817. [Google Scholar] [CrossRef]
- Peltola, P.; Tynjälä, T.; Ritvanen, J.; Hyppänen, T. Mass, energy, and exergy balance analysis of chemical looping with oxygen uncoupling (CLOU) process. Energy Conv. Manag. 2014, 87, 483–494. [Google Scholar] [CrossRef]




| Sawdust | Bituminous Coal | |
|---|---|---|
| Proximate analysis (wt %) | ||
| Ash | 1.01 | 4.76 |
| Fixed carbon | 10.1 | 54.13 |
| Volatile matter | 74.61 | 35.1 |
| Moisture | 14.28 | 6.01 |
| Ultimate analysis (wt %) | ||
| Oxygen | 40.55 | 13.81 |
| Nitrogen | 1.02 | 1.03 |
| Hydrogen | 5.61 | 4.3 |
| Carbon | 37.43 | 69.57 |
| Sulphur | 0.1 | 0.52 |
| LHV (MJ·kg−1) | 14.5 | 27.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kevat, M.D.; Banerjee, T. Intensification Insights from Chemical Looping Combustion Using Coal–Biomass Mixtures with Fe-Based Oxygen Carrier. Processes 2022, 10, 1242. https://doi.org/10.3390/pr10071242
Kevat MD, Banerjee T. Intensification Insights from Chemical Looping Combustion Using Coal–Biomass Mixtures with Fe-Based Oxygen Carrier. Processes. 2022; 10(7):1242. https://doi.org/10.3390/pr10071242
Chicago/Turabian StyleKevat, Mayur D., and Tamal Banerjee. 2022. "Intensification Insights from Chemical Looping Combustion Using Coal–Biomass Mixtures with Fe-Based Oxygen Carrier" Processes 10, no. 7: 1242. https://doi.org/10.3390/pr10071242
APA StyleKevat, M. D., & Banerjee, T. (2022). Intensification Insights from Chemical Looping Combustion Using Coal–Biomass Mixtures with Fe-Based Oxygen Carrier. Processes, 10(7), 1242. https://doi.org/10.3390/pr10071242






