Abstract
In this study, we aimed to select the optimal solvents for the removal of asphaltene–resin–paraffin deposits. The effectiveness of various solvents was determined based on the asphaltene–resin–paraffin deposits (ARPDs) of the Zhanaozen (Ozen) crude oil field. These deposits affect the geological, physical, and technological conditions of the oil field, thus influencing its development. According to the results, we found that the most effective composite solvent is a composition comprising a 50% gasoline fraction and a 50% kerosene fraction. This composition showed mass loss of deposits of 97.7% and a dissolving power of 93.5 g/cm3 after 5 h. We confirmed the effectiveness of this composition by the paraffinic type of the deposits, which is explained by the high content of paraffin in the oil from the Zhanaozen field. Aromatic solvents showed a relatively low dissolving power compared with aliphatic solvents, which also confirms the low content of resins and asphaltenes in the ARPD.
1. Introduction
The efficiency of oil and gas production and transportation is reduced by the formation of asphaltene–resin–paraffin deposits (ARPDs) in pipelines, underground equipment, and the bottomhole zone of productive formation. Such deposits considerably affect the geological, physical, and technological conditions during oil field development; they reduce the flow rate of wells, increase water cut production, and result in the production of organic depositions. ARPDs are mainly composed of 40–60% solid paraffin and 10–56% resins, asphaltenes, water, sand, and inorganic salts. They form as a result of changes in thermobaric conditions. When the temperature in the wellbore drops to the temperature at which crystallization is initiated, paraffin crystals begin to form in the oil, which are the centers of crystallization [1,2,3,4,5].
ARPDs are divided into three types according to their asphaltene, resin, and paraffin contents: asphaltene, P/(A + R) < 1; paraffin, P/(A + R) > 1; mixed, P/(A + R) ~ 1. Here, P, A, and R are the mass contents of paraffins, asphaltenes, and resins, respectively.
ARPDs are managed using two strategies: prevention or inhibition of formation and removal of formed deposits [6]. To prevent the formation of ARPDs, chemical and physical methods and smooth coatings are used. Chemical methods include the injection of various types of inhibitors: dispersants, depressants, wetting agents, and modifiers.
To remove deposits, thermal, chemical, and mechanical methods are used [7]. Chemical methods involve the use of solvents as reagents for the removal of paraffin deposits. Solvents are conditionally divided into several groups: individual solvents, natural solvents, products and wastes of oil-refining and petrochemical processes, and solvents and their mixtures with surfactant additives.
Physical and chemical approaches to the choice of effective solvents for ARPDs have been previously considered [8]. Methods have been developed for evaluating the effectiveness of reagents used for dissolving paraffin deposits based on determining the kinetics of ongoing processes at various temperature conditions and evaluating the change in paraffin melting points after treatment with various reagents. The regularities of the influence of the chemical nature of the compounds in composite aliphatic–naphthenic–aromatic solvents on the temperatures of crystallization and melting processes were revealed as well as the degree of crystallinity of commercial paraffins.
Of the individual solvents, aromatic compounds such as benzene, toluene, and xylenes show the highest removal efficiency. However, these reagents are highly toxic and flammable. Therefore, natural solvents, such as gas condensate, natural gasoline, a mixture of liquefied petroleum gases, hexane fraction, etc., are most widely used in the field. While they are widely available, their efficiency is low because resins and asphaltenes are poorly soluble in low-boiling aliphatic hydrocarbons. This is addressed by introducing various additives into natural solvents, such as alcohols, ethers, and phenol and its derivatives, at concentrations of 0.1–3%.
The main factor that determines the solubility of ARPD components in a particular class of hydrocarbons is the possibility of favorable solvation of the solute by the solvent, which occurs at the molecular level [9]. Considering this factor, paraffins are highly soluble in light alkanes due to their favorable solvation and the high energy of solvent molecules. Naphthenic hydrocarbons, which are widely distributed in oil, are undesirable components of solvents, as they are not able to solvate paraffins and resin–asphaltene substances.
Aliphatic, naphthenic, and aromatic hydrocarbons affect the content of the crystalline phase and the melting point of paraffins in composite solvents [10]. The degree of crystallinity of paraffins is known to depend on the degree of solvent aliphaticity, which decreases in the sequence aliphatic → aliphatic–aromatic → aliphatic–naphthenic–aromatic solvent, and their melting points increase in the series aliphatic–aromatic → aliphatic → aliphatic–naphthenic–aromatic solvent.
In the published paper, the formation of stable stratification, rather than a uniformly mixed state is discussed. Also, it is shown to depend critically on the equation of state (EOS) or mixing rule for the density as a function of the solution constitution [11]. The proposition that the additional heat flux was controlled by the phase fraction of microbubbles was also tested by steady state solutions of the canonical hot wall/cold wall buoyant convection problem. These conclusions of article hold qualitatively for a variant of the model which simply treats the gas exchange between the microbubble phase and dissolved gases in the liquid, resulting in variation of the microbubble phase fraction with temperature, and hence with position in the domain [12].
Chlorinated hydrocarbons of various types are effective solvents because they are relatively inexpensive and have a high specific gravity [13]. High specific gravity is an important factor that promotes solvent penetration and thus the dissolution of paraffin deposits, which are usually found at the bottom of the wet end. However, chlorinated hydrocarbons are toxic, and aromatic solvents have a low specific gravity, are difficult to use in downhole processing, have a low flash point, and are difficult to handle, whereas carbon disulfide is effective but also flammable and has toxic fumes.
Thus, a review of the literature has shown that no universal solvent for removing paraffin deposits is available. The optimal ARPD solvent in wells is typically chosen by considering the operating conditions and well design features as well as the properties and composition of the product.
In this study, we selected an effective solvent for the removal of paraffin deposits from the Zhanaozen field. We also investigated the mechanisms of paraffin crystal growth from the content of ethylene-vinyl acetate (EVA) copolymer and solvent. We studied paraffin deposition inhibition for various reagent compositions.
2. Materials and Methods
The solubility of ARPD from oil of the Zhanaozen field was determined under static conditions at a given temperature. In static mode, the cleaning technology was simulated by pumping solvent into a stopped oil pipeline or well, followed by holding for several hours to allow a reaction [14].
More specifically, paraffin deposits weighing 2.5 g were compacted to a homogeneous state into metal mesh baskets and preliminarily weighed to an accuracy of 0.001 g. We poured a given amount of solvent into the vessel, and the ratio of deposits and reagent varied from 1:5 to 1:50. Baskets with deposits were then placed in containers with solvent. The time of contact between ARPDs and the solvent ranged from 3 to 24 h. The tests were conducted at room temperature.
At the end of the experiment, the baskets were removed from the solvent and left to dry at room temperature for several hours until a constant weight was reached.
The weight loss of the deposits for each experiment (P) in weight percent was calculated according to:
where m1 is the mass of the studied sediment sample before contact with the reagent, g, and m2 is the mass of the insoluble sediment residue after contact with the reagent, g.
P = [(m1 − m2)/m1] × 100
The dissolving power of the reagent (C) in g/cm3 was calculated as:
where V is the solvent volume, cm3.
C = [(m1 − m2)/V] × 1000
Chromatographic analysis of the liquid fraction was performed using a Khromatek Crystal instrument with a flame ionization detector, nitrogen carrier gas, stainless steel column (3 m long and 3 mm in diameter), g-A12O3 sorbent, and temperatures ranging from 90 to 180 °C. We conducted the studies on a UNICO 2100 spectrophotometer. An inhibitor (from 0.5 to 2 wt %) was added to the oil samples, which were heated to 60 °C to accelerate the dissolution of additive in the oil.
The dispersing ability of the synthesized solvent with respect to asphaltene particles was studied using the photocolorimetric method. The optical density of oil is linearly dependent on the solvent content up to 1% of the mass. Above 2 wt % solvent, no changes in the optical density values were observed.
Chemical composition of natural bitumen was identified by a Fourier transform infrared spectroscopy Spectrum-65 in cells of KBr and tablets with KBr. It is used to obtain an infrared spectrum of absorption, emission, photoconductivity, or Raman scattering of a solid, liquid, or gas. A spectrometer simultaneously collects spectral data in a wide spectral range. The spectra with 500–5000 cm−1 range were obtained.
3. Results
We investigated the effectiveness of several types of solvents which were divided into two groups, aliphatic and aromatic, because paraffins are highly soluble in aliphatic and aromatic solvents, whereas resins and asphaltenes are highly soluble in aromatic solvents. Hexane, white spirit, gasoline, and kerosene were chosen as aliphatic solvents, and benzene, toluene, and o-xylene were chosen as the aromatic solvents.
The results of our tests with aliphatic solvents are shown in Table 1. The results for almost all aliphatic solvents showed similar weight loss of deposits after 5 h, ranging from 96% to 98.9%. However, the dissolving power of the solvents differed. The gasoline fraction shows the maximum dissolving power of 78 g/cm3 after 3 h. The kerosene and white spirit fractions were inferior in terms of dissolving power; hexane, despite the high sediment mass loss, had the lowest dissolving power. Therefore, we chose gasoline, kerosene, and white spirit for use in composite solvents for further testing. The results of testing the mixtures containing various compositions of solvents showed that the composite consisting of 50% gasoline and 50% kerosene had the highest paraffin weight loss and dissolving power of those tested. This mixture, after 5 h, produced a deposit weight loss of 97.7% and a dissolving power of 93.5 g/cm3.

Table 1.
Test results for the effectiveness of aliphatic solvents in relation to paraffin deposits of the Zhanaozen field.
The results of tests of the efficiencies of aromatic solvents are presented in Table 2. Here, too, the mass loss of deposits ranged from 93% to 98%; however, the dissolving power of the solvents differed. o-Xylene was the most effective based on the value of the corresponding indicator (76.48 g/cm3). Benzene and toluene each produced higher deposit weight loss, but their dissolving power was low.

Table 2.
Results of tests of the effectiveness of aromatic solvents in relation to the ARPDs of the Zhanaozen field.
As ARPDs not only contain paraffinic hydrocarbons but also resinous–asphaltene substances, we tested a combination of solvents of 75% white spirit and 25% o-xylene. The use of this mixture resulted in a slight increase in the dissolving power up to 77.6 g/cm3.
We studied the pour point in the presence of EVA in Zhanaozen and Karazhanbas crude oils. ARPD formation is influenced by the water cut of oil. As water cut increases, the ARPD crystal formation intensity and the oil viscosity decrease. We studied the influence of the inhibition of paraffin deposition by an ethylene and vinyl acetate copolymer using oil samples from the Zhanaozen field. The ability of the ethylene and vinyl acetate copolymer to inhibit crystallization growth and reduce the oil pour point was then determined by varying the ratio of the copolymer of ethylene and vinyl acetate and characterizing the resulting oil pour point based on tests with the Zhanaozen oil. Oil-containing reagents were also characterized for comparison. Analysis of the results showed that crude oil from Zhanaozen had a pour point of up to −5 °C, losing fluidity. We found that 12% ethylene-vinyl acetate copolymer (EVA-12) and 15% ethylene-acrylic acid copolymer (EAA-15) reached the test temperature limit for crude oil. For crude oils, 25% ethylene-vinyl acetate copolymer (EVA-25) performed the best as a paraffin inhibitor, lowering the pour point. The order of performance of the tested additives was as follows: EVA-25 < EAA-15 < EVA-12. According to the results, we found that the vinyl acetate content in the EVA copolymer significantly affects the growth mechanism of ARPDs and their rheological characteristics as a means of lowering the pour point.
The results of chromatographic analysis of ARPD samples with a solvent and with the addition of EVA showed that the component composition of ARPD is represented by high saturated hydrocarbons C15–C44 and their isomers. Their chromatograms are shown in Figure 1 and tabulated data are presented in Table 3. Naphthenes and polycyclic aromatic hydrocarbons, derivatives of cyclohexane and phenanthrene were found in small amounts. Comparison of the percentage of alkanes and their isomers shows a decrease in the amount of all detected alkanes and their isomers in ARPD samples with a solvent upon addition of EVA, which confirms the effectiveness of the inhibitor. The addition of EVA leads to the dissolution of solid paraffins and their isomers. At the same time, when EVA was added to the ARPD composition, new hydrocarbons were identified, such as octadecane, pentacosane, heptacosane, nonacosan, hexatriacontane, the presence of which in the ARPD composition with the solvent was not confirmed by the chromatogram.
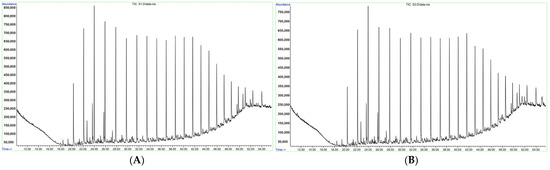
Figure 1.
Chromatogram of ARPD solvent (A) and chromatogram of ARPD + solution + EVA sample (B).

Table 3.
Results of chromatographic analysis of samples.
We established the inhibitory ability of copolymers EVA-12, EAA-15, and EVA-25 with a predominance of asphaltenes. When 3% EVA-12 was introduced into the oil, the rheological properties changed, and the dynamic viscosity decreased.
When the oil temperature drops to the saturation temperature for oil with paraffin or lower, the formation of ARPD microcrystals begins. The saturation temperature of oil with paraffin, the value of which is strongly affected by the gas saturation of reservoir oil and its degassing, determines the depth within the well at which there is ARPD crystallization onset in addition to the sediment interval of this formation. The chemical composition of oil and, in particular, its solid hydrocarbon content, strongly impacts ARPD formation. The presence of polar compounds in oil can change the nature of crystallization of high-molecular-weight paraffins. The dependence of paraffin crystallization temperature on the ratio of EVA-25 and paraffin concentrations is linear: as the proportion of EVA-25 increases, the temperature for crystallization onset decreases.
We studied the mechanism of paraffin crystal growth for various ethylene and vinyl acetate (EVA) copolymer contents in Zhanaozen oil. We determined that adding EVA-15 to the oil can prevent the process of sedimentation by 15–35%, depending on its mass concentration in the oil.
We developed a hydrocarbon solvent from oil sludge by direct distillation of oil at temperatures of 150–180 °C. Table 4 shows the yield and overall composition of the products of the light fractions depending on the distillation temperature of the oil sludge.

Table 4.
Composition of light fraction isolated from Zhanaozen oil sludge depending on distillation temperature.
When EVA-25 and solvent were added to the oil, a precipitate with lower viscosity was formed. When we introduced EVA-25 at concentrations of 0.5 and 1 wt %, we noted the largest reduction in viscosity. An increase in the EVA-15 and aromatic hydrocarbons contents in oil up to 2 wt % led to a deterioration in rheological properties. When kerosene was added to oil, we observed 2–3-fold decreases in both effective viscosity and ultimate shear stress for all sediment samples. Thus, the most effective dissolution was observed with EVA-25 and solvent, with EVA-25 and kerosene being the most effective of those tested combinations.
The chromatographic analysis of ARPD samples with the addition of a solvent and EVA showed that the ARPD component composition was represented by highly saturated C15–C44 hydrocarbons and their isomers. We found small amounts of naphthenes and polycyclic aromatic hydrocarbons, which are derivatives of cyclohexane and phenanthrene. A comparison of the percentage content of alkanes and their isomers showed a decrease in the amount of all detected alkanes and their isomers in the ARPD samples with a solvent when we added EVA, which confirmed its effectiveness as an inhibitor of formation. The addition of EVA led to the dissolution of solid paraffins and their isomers. When we added EVA to the ARPD composition, we identified new hydrocarbons, such as octadecane, pentacosan, heptacosan, nonacosane, and hexatriacontane; the chromatogram did not confirm the presence in the ARPD composition with the solvent.
Changes in the group composition affect the structure of sediments, characterized by rheological properties. We noted the highest effective viscosity for the precipitate isolated from the paraffin solution, due to the formation of a sufficiently strong crystal lattice from fine paraffin crystals solvated by the dispersion medium.
At the Zhanaozen (Ozen) fields, the oils, which have high paraffin and asphaltene contents, predominate; at a 10 °C, they lose fluidity. Increasing the EVA content to 5% reduced the pour point.
The composition of the Karazhanbas oil as ARPD samples and its sample with a solvent the addition of EVA was studied by IR spectroscopic analysis (Figure 2). The infrared spectra of the samples showed the presence of absorption bands of saturated structures in the form of -CH2, -CH3 groups in the region of 2923, 2854, 1457, and 1376 cm−1 in both spectra. An absorption band characteristic of alcohols and phenols structures as O-H stretching vibrations (at 3383 cm−1) is observed in the oil of Karazhanbas. There is an increase in the intensity of amine salts (at 2308 cm−1) in the ARPD sample with the solvent and the addition of EVA. We can see aromatic compounds (O-H stretching vibrations and other vibrations associated with the C-O-H group), alkenes (in the form of HRC = CR’H and RR′C = CR″H), and alkanes ((CH2)x) in the wavelength range from 667 to 873 cm−1 in the two IR-spectrum. At the same time acid amides, lactams, and urethanes (carbamates) appeared at the intensity band ~ 620 cm−1. It should be noted that the intensity of the absorption bands characteristic of alkanes (at 728 cm−1) and alkenes (at 693 cm−1) is significantly increased in the sample of hydrocarbon with a solvent the addition of EVA.
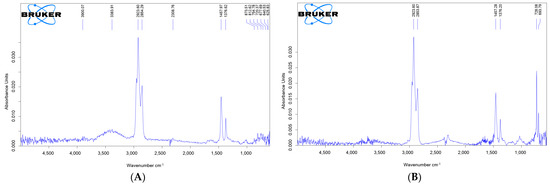
Figure 2.
IR spectrum of ARPD solvent (A) and spectrum of ARPD + solution + EVA sample (B).
We found that an increase in the content of EVA from 5% to 10% did not have a notable effect on fluidity or viscosity, because vinyl acetate groups react with paraffins and asphaltenes [1]. As such, we introduced EVA-25 into Karazhanbas oil together with the solvent that we synthesized. We selected the composition of the solvent with the optimal low-molecular-weight paraffin and aromatic hydrocarbon contents. Introduction of 0.1% EVA into Karazhanbas oil resulted in decreased dynamic viscosity decreased and an improvement in rheological properties.
4. Conclusions
As a result of the conducted studies, we determined the effectiveness of the use of solvents for treating the asphaltene–resin–paraffin deposits (ARPDs) of the Zhanaozen (Ozen) oil field. The test results showed that the most effective solvent was a mixture consisting of 50% gasoline and 50% kerosene. This composition achieved a deposit mass loss of 97.7% in 5 h and had a dissolving power of 93.5 g/cm3. We confirmed that the effectiveness of this composition is related to the paraffin type of the deposits, where the oil Zhanaozen field contains a high content of paraffins. Aromatic solvents showed a relatively low dissolving power compared with aliphatic solvents, which also confirmed the low resin and asphaltene contents in the ARPDs.
Thus, the use of two components, EAK-15 and EVA-25, in the ARPD solvent increased its solubility and reduced its dissolution time. The resulting composition was able to reduce the strength of the armor shells of water–oil emulsions stabilized by asphaltenes and thus affect the rheological characteristics of oil. Additionally, the developed composition lowered the pour point of oil and reduced the precipitation of paraffin crystals.
Author Contributions
Project administration and funding acquisition, S.T.; methodology and experimental data analysis, E.K.; investigation, A.Z.; writing—original draft preparation, Y.O.; writing—review and editing, Y.T.; supervision, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan, grant number and title: “OR11465430 Development of new composite and structural materials for the development of the innovative industry of the Republic of Kazakhstan” under the subprogram “Development of new types of reagents with improved rheological properties for the oil industry”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was acknowledge the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan; Project of “Development of new types of reagents with improved rheological properties for the oil industry”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abilmagzhanov, A.Z.; Ivanov, N.S.; Kozhabekov, S.S.; Abdikalykov, E.N.; Kydyrbayeva, U.O.; Nurtazina, A.E. The Investigation of the Depressor Additives Application Efficiency for Asphaltene-Resin-Paraffin Deposits Inhibition and Stripping in Petroleum Storage Tanks. Orient. J. Chem. 2019, 35, 711–717. [Google Scholar] [CrossRef]
- Akbarzadeh, K.; Hammami, A.; Kharrat, A.; Zhan, D.; Allenso, S.; Creek, J.L.; Kabir, S.; Jamaluddin, A.; Marshall, A.G.; Rodgers, R.P.; et al. Asphaltenes—Problematic but rich in potential. Oilfield Rev. 2007, 19, 22–43. [Google Scholar]
- Sultanov, F.R.; Tileuberdi, Y.; Ongarbayev, Y.K.; Mansurov, Z.A.; Khaseinov, K.A.; Tuleutaev, B.K.; Behrendt, F. Study of Asphaltene Structure Precipitated from Oil Sands. Euras. Chem. Tech. J. 2013, 15, 77–81. [Google Scholar] [CrossRef]
- Mansoori, G.A. Phase behavior in petroleum fluids. In Encyclopedia of Life Support Systems (EOLSS); UNESCO, 2009; Available online: https://www.eolss.net (accessed on accessed on 12 May 2022).
- Khaibullina, K. Technology to Remove Asphaltene, Resin and Paraffin Deposits in Wells Using Organic Solvents. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 26–28 September 2016. [Google Scholar] [CrossRef]
- Sousa, A.L.; Matos, H.A.; Guerreiro, L.P. Preventing and removing wax deposition inside vertical wells: A review. J. Pet. Exp. Prod. Tech. 2019, 9, 2091–2107. [Google Scholar] [CrossRef]
- Ivanova, L.V.; Burov, Y.A.; Koshelev, V.N. Asphalt-resin-paraffin deposits in the processes of extraction, transport and storage. J. Neftegazov. Delo 2011, 1, 268–284. (In Russian) [Google Scholar]
- Ivanova, I.K. Physical and Chemical Approaches to the Choice of Effective Solvents for Asphalt, Resin and Paraffin Deposits. Ph.D. Thesis, North-Eastern Federal University in Yakutsk, Yakutsk, Russia, 2019; 266p. (In Russian). [Google Scholar]
- Turukalov, M.B.; Stroganov, V.M.; Yasian, Y.P. Formation of asphalt-resin-paraffin deposits in oil production: An alternative view of the mechanism. J. Neftepererab. Neftehim. 2007, 7, 31–34. (In Russian) [Google Scholar]
- Ivanova, I.K.; Kashircev, V.A.; Semenov, M.E.; Glyaznecova, Y.S.; Chalaya, O.N.; Zueva, I.N.; Portnyagin, A.S. Influence of the composition of the solvent on the content of the crystalline phase and the melting point of paraffins. Zhurnal Prikladnoi Himii 2020, 93, 600–608. (In Russian) [Google Scholar] [CrossRef]
- Zimmerman, W.B. The effect of chemical equilibrium on the formation of stable stratification. Appl. Sci. Res. 1997, 59, 283–298. [Google Scholar] [CrossRef]
- Zimmerman, W.B. Mediating heat transport by microbubble dispersions: The role of dissolved gases and phase change dynamics. Appl. Therm. Engin. 2022, 213, 118720. [Google Scholar] [CrossRef]
- Theyab, M.A. A Review of Wax Mitigation Methods through Hydrocarbon Production. J. Pet. Environ. Biotechnol. 2020, 9, 412. [Google Scholar]
- Nasybullina, A.S.; Galieva, A.M.; Sabitov, R.F. Development of a composite solvent for cleaning the main oil pipeline of the Nozhovskoye field. Vestnik Tehnol. Univ. 2016, 19, 83–85. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).