The Influence of Reservoir Clay Composition on Heavy Oil In Situ Combustion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NMR Analysis of the Initial Oil-Saturated Samples
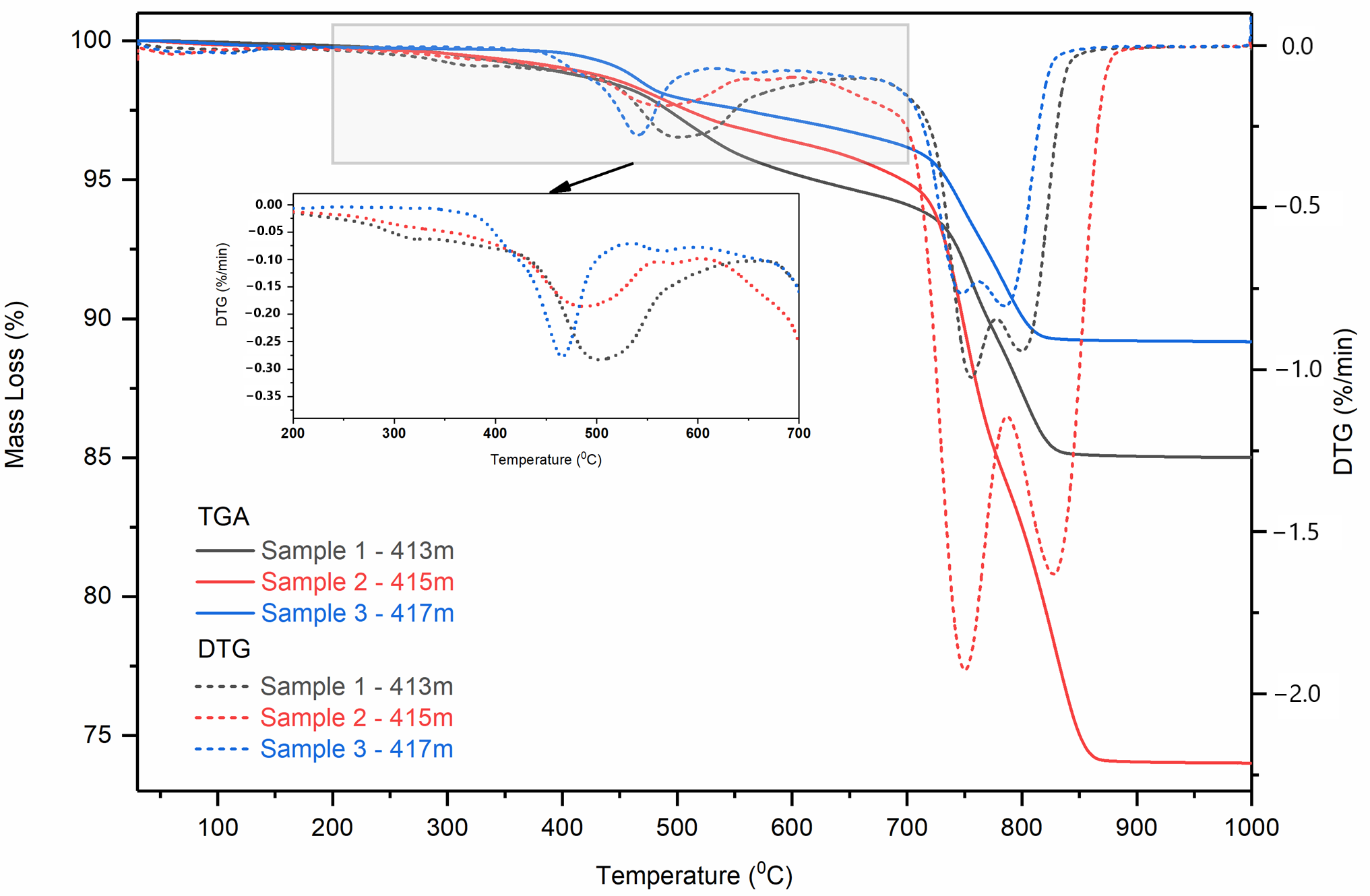
2.3. Thermal Analysis of the Initial Oil-Saturated Samples
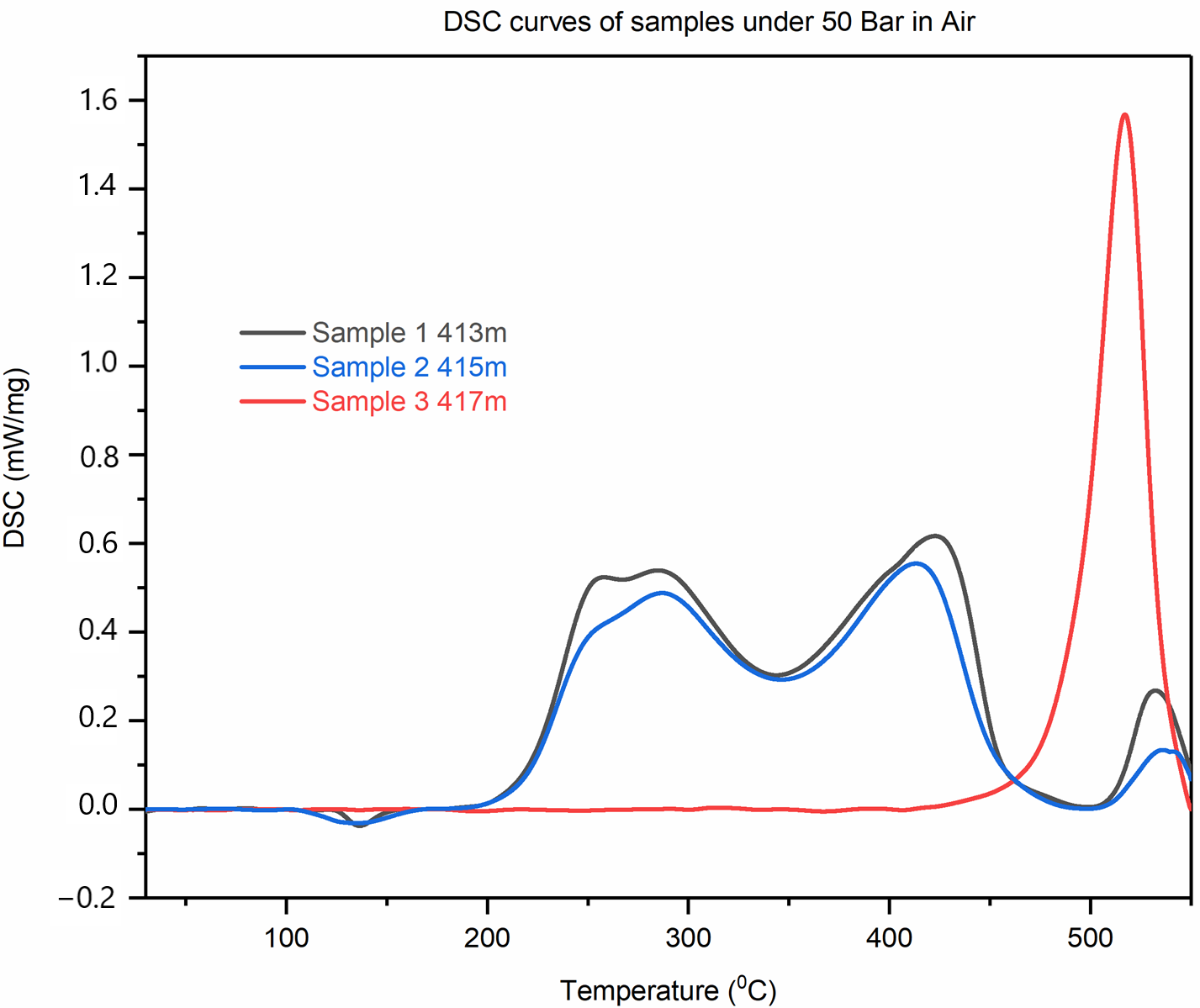
Differential Scanning Calorimetric Analysis of the Initial Oil-Saturated Samples
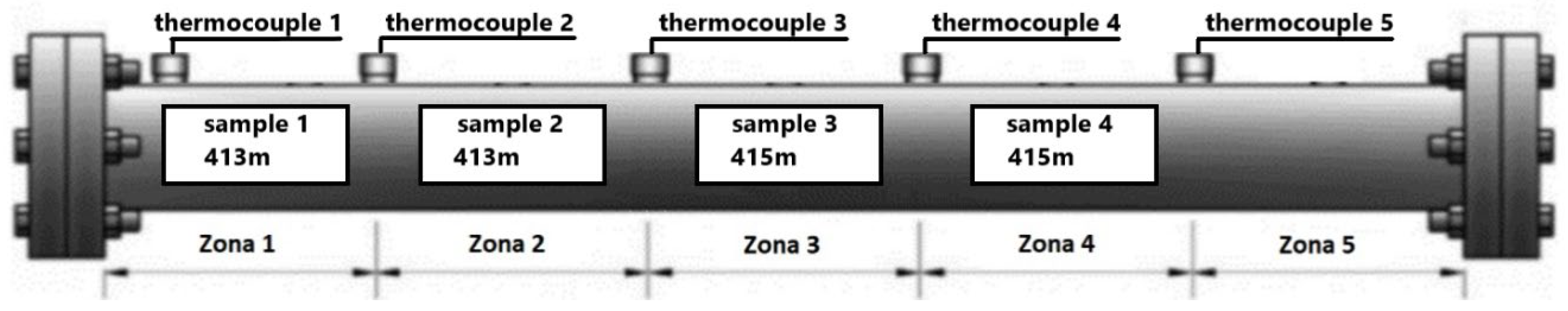
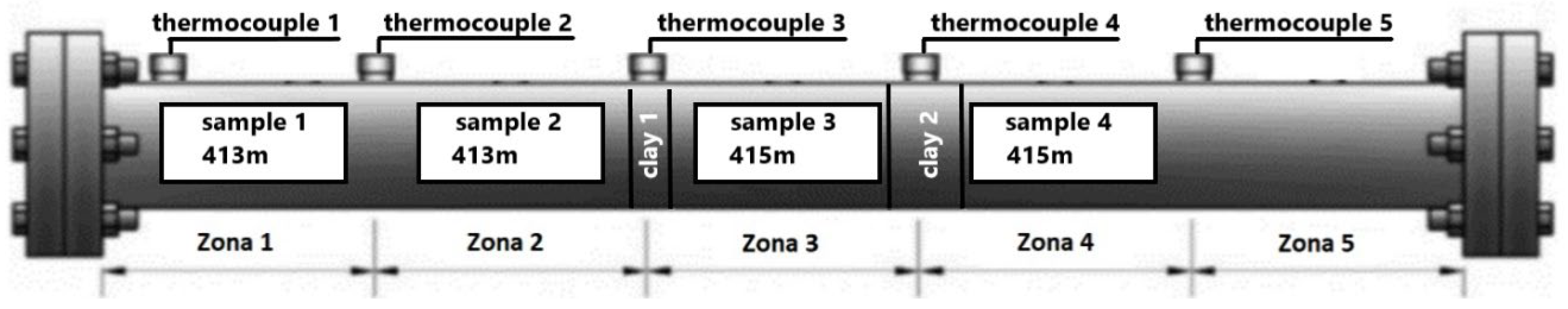
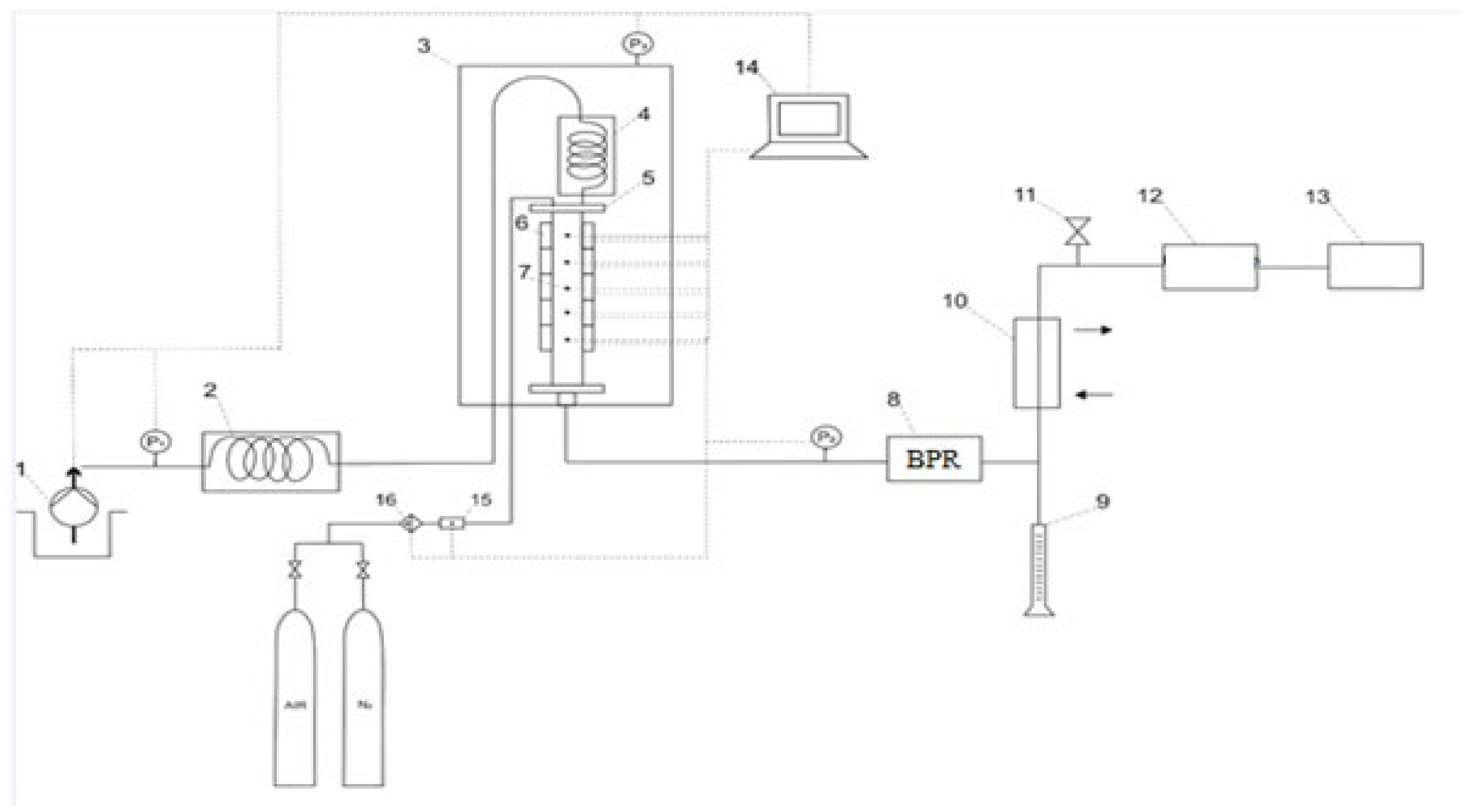
2.4. Combustion Tube Experiments
3. Results and Discussion
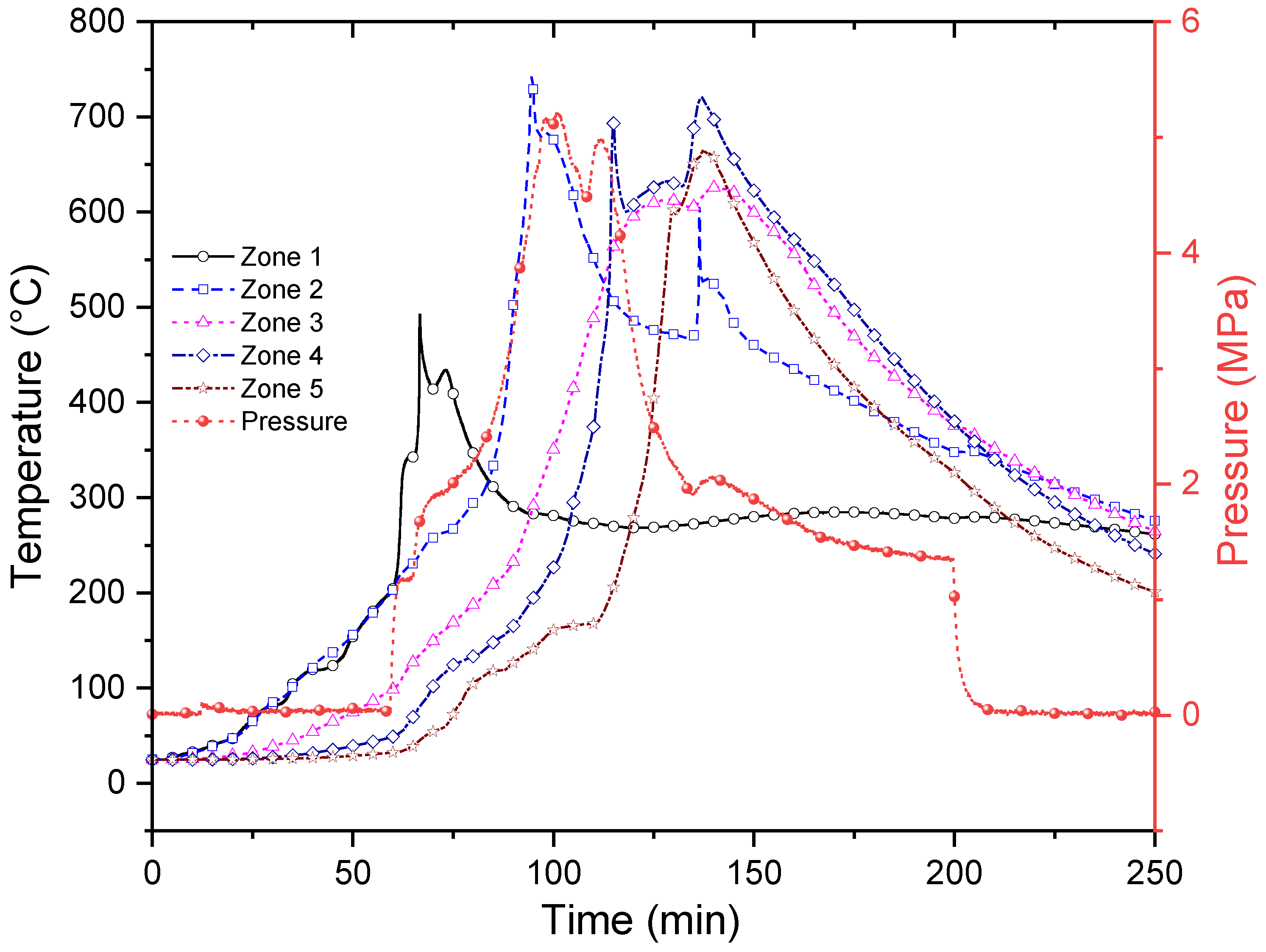
3.1. In Situ Combustion Test for Standard Core Model (ISC-1)
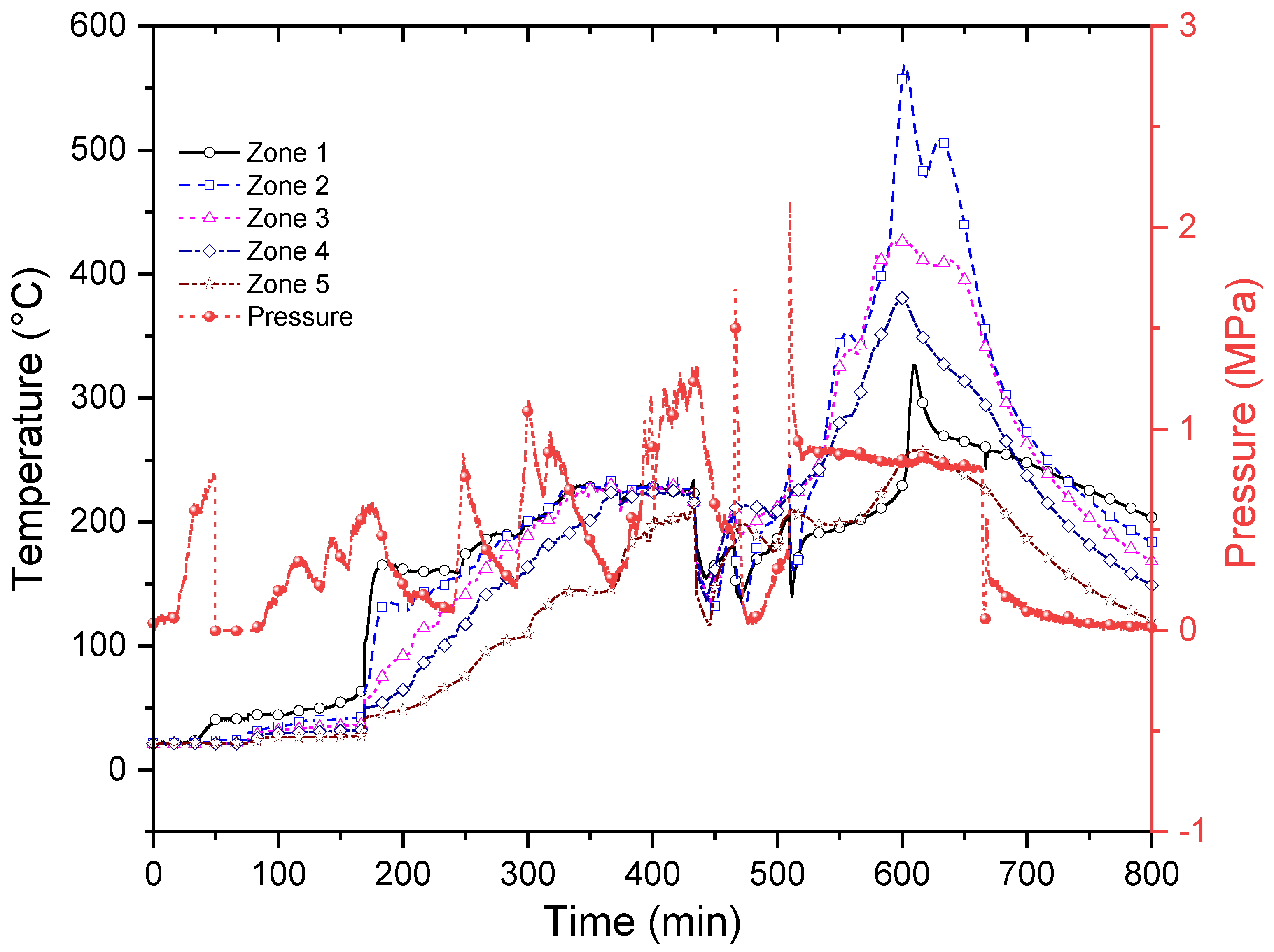
3.2. In Situ Combustion Test for Core Model with Clay Interlayers (ISC-2)
3.3. In Situ Combustion Test for Core Model with Clay Interlayers in the Presence of Hydrothermal Conditions (ISC-3)
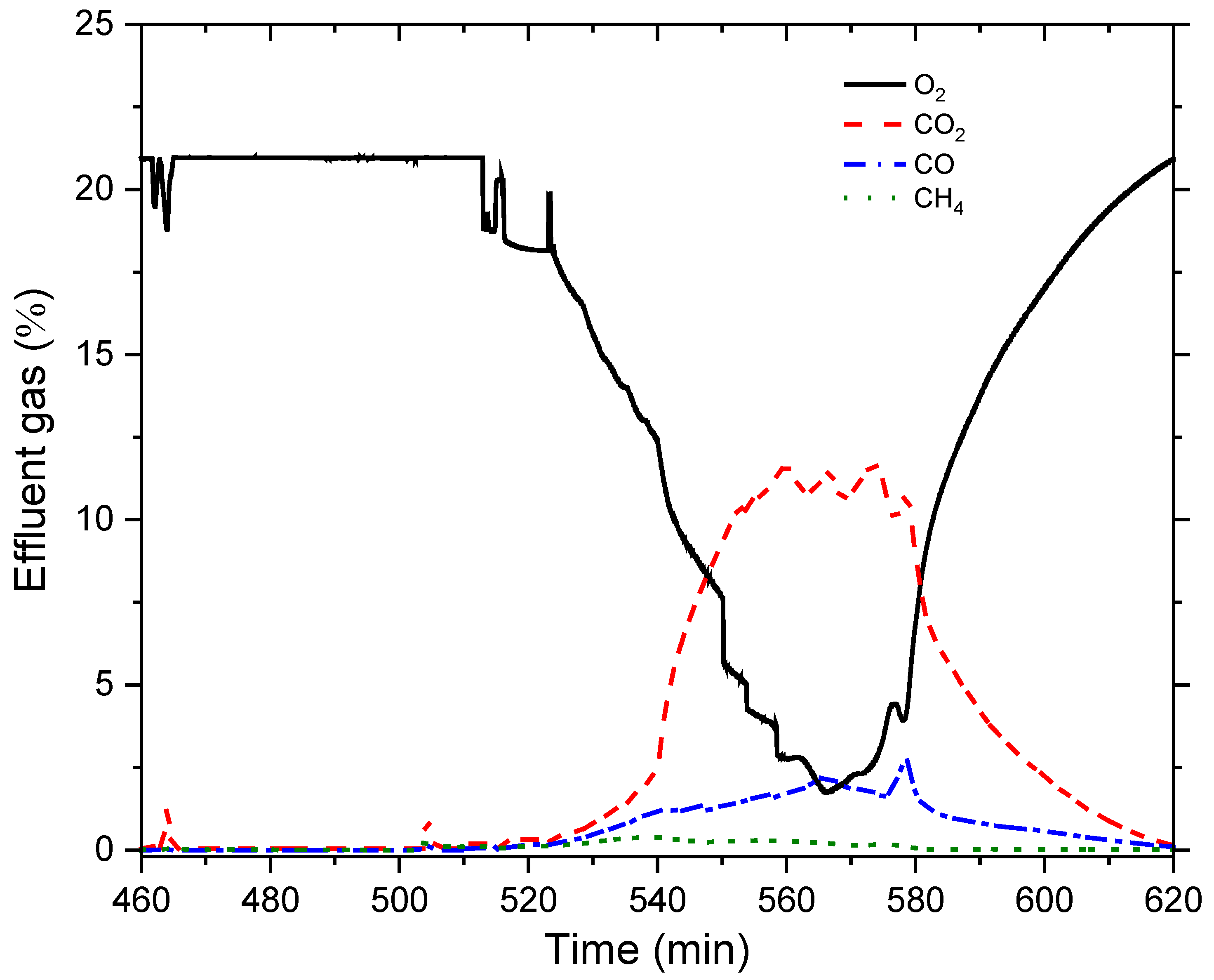
Analysis of Combustion Tube Data
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial Sciences in Unconventional Petroleum Production: From Fundamentals to Applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef] [PubMed]
- Caineng, Z.; Guangya, Z.; Shizhen, T.; Suyun, H.; Xiaodi, L.; Jianzhong, L.; Dazhong, D.; Rukai, Z.; Xuanjun, Y.; Lianhua, H. Geological Features, Major Discoveries and Unconventional Petroleum Geology in the Global Petroleum Exploration. Pet. Explor. Dev. 2010, 37, 129–145. [Google Scholar] [CrossRef]
- Zou, C. Unconventional Petroleum Geology; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 0128122358. [Google Scholar]
- Ganat, T.A.-A.O. Unconventional Petroleum Reservoirs. In Fundamentals of Reservoir Rock Properties; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–129. [Google Scholar]
- Mukherjee, S.; Kumar, A.; Zaworotko, M.J. Metal-Organic Framework Based Carbon Capture and Purification Technologies for Clean Environment. In Metal-Organic Frameworks (MOFs) for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–61. [Google Scholar]
- Mukherjee, S.; Kumar, A.; Zaworotko, M.J. Department of Chemical Sciences; Bernal Institute, University of Limerick: Limerick, Ireland, 2019. [Google Scholar]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010; ISBN 0080961630. [Google Scholar]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery; Henry, L., Ed.; Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998; Volume 6. [Google Scholar]
- Mokheimer, E.M.A.; Hamdy, M.; Abubakar, Z.; Shakeel, M.R.; Habib, M.A.; Mahmoud, M. A Comprehensive Review of Thermal Enhanced Oil Recovery: Techniques Evaluation. J. Energy Resour. Technol. 2019, 141, 30801. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Ignashev, N.E.; Krapivnitskaya, T.O.; Peskov, N.Y.; Glyavin, M.Y.; Bulanova, S.A.; Slavkina, O.V.; Schekoldin, K.A. Microwave Radiation Impact on Heavy Oil Upgrading from Carbonate Deposits in the Presence of Nano-Sized Magnetite. Processes 2021, 9, 2021. [Google Scholar] [CrossRef]
- Sitnov, S.; Mukhamatdinov, I.; Aliev, F.; Khelkhal, M.A.; Slavkina, O.; Bugaev, K. Heavy Oil Aquathermolysis in the Presence of Rock-Forming Minerals and Iron Oxide (II, III) Nanoparticles. Pet. Sci. Technol. 2020, 38, 574–579. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2021, 35, 374–385. [Google Scholar] [CrossRef]
- Santos, R.G.; Loh, W.; Bannwart, A.C.; Trevisan, O.V. An Overview of Heavy Oil Properties and Its Recovery and Transportation Methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Mahinpey, N.; Ambalae, A.; Asghari, K. In Situ Combustion in Enhanced Oil Recovery (EOR): A Review. Chem. Eng. Commun. 2007, 194, 995–1021. [Google Scholar] [CrossRef]
- Sharma, J.; Dean, J.; Aljaberi, F.; Altememee, N. In-Situ Combustion in Bellevue Field in Louisiana–History, Current State and Future Strategies. Fuel 2021, 284, 118992. [Google Scholar] [CrossRef]
- Carcoana, A.N.; Machedon, V.C.; Pantazi, I.G.; Petcovici, V.C. In Situ Combustion-an Effective Method to Enhance Oil Recovery in Romania. Proc. World Pet. Congr. 1983. [Google Scholar]
- Khelkhal, M.A.; Eskin, A.A.; Nurgaliev, D.K.; Vakhin, A.V. Thermal Study on Stabilizing the Combustion Front via Bimetallic Mn@ Cu Tallates during Heavy Oil Oxidation. Energy Fuels 2019, 34, 5121–5127. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.; Tajik, A.; Lapuk, S.E.; Rezaeisadat, M.; Eskin, A.A.; Rodionov, N.O.; Vakhin, A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.; Vakhin, A.V. Kinetic Study on Heavy Oil Oxidation by Copper Tallates. Energy Fuels 2019, 33, 12690–12695. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Sitnov, S.A.; Vakhin, A.V. Impact of Iron Tallate on the Kinetic Behavior of the Oxidation Process of Heavy Oils. Energy Fuels 2019, 33, 7678–7683. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Mukhamatdinov, I.I.; Feoktistov, D.A.; Vakhin, A.V. Comparative Kinetic Study on Heavy Oil Oxidation in the Presence of Nickel Tallate and Cobalt Tallate. Energy Fuels 2019, 33, 9107–9113. [Google Scholar] [CrossRef]
- Galukhin, A.; Khelkhal, M.A.; Gerasimov, A.; Biktagirov, T.; Gafurov, M.; Rodionov, A.; Orlinskii, S. Mn-Catalyzed Oxidation of Heavy Oil in Porous Media: Kinetics and Some Aspects of the Mechanism. Energy Fuels 2016, 30, 7731–7737. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Sharifullin, A.V.; Vakhin, A.V. Differential Scanning Calorimetric Study of Heavy Oil Catalytic Oxidation in the Presence of Manganese Tallates. Pet. Sci. Technol. 2019, 37, 1194–1200. [Google Scholar] [CrossRef]
- Galukhin, A.; Nosov, R.; Eskin, A.; Khelkhal, M.; Osin, Y. Manganese Oxide Nanoparticles Immobilized on Silica Nanospheres as a Highly Efficient Catalyst for Heavy Oil Oxidation. Ind. Eng. Chem. Res. 2019, 58, 8990–8995. [Google Scholar] [CrossRef]
- Galukhin, A.V.; Khelkhal, M.A.; Eskin, A.V.; Osin, Y.N. Catalytic Combustion of Heavy Oil in the Presence of Manganese-Based Submicroparticles in a Quartz Porous Medium. Energy Fuels 2017, 31, 11253–11257. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Yuan, C.; Bolotov, A.V.; Minkhanov, I.F.; Mehrabi-Kalajahi, S.; Saifullin, E.R.; Marvanov, M.M.; Baygildin, E.R.; Sabiryanov, R.M.; Rojas, A. Effect of Copper Stearate as Catalysts on the Performance of In-Situ Combustion Process for Heavy Oil Recovery and Upgrading. J. Pet. Sci. Eng. 2021, 207, 109125. [Google Scholar] [CrossRef]
- Yuan, C.; Emelianov, D.A.; Varfolomeev, M.A.; Rodionov, N.O.; Suwaid, M.A.; Vakhitov, I.R. Mechanistic and Kinetic Insight into Catalytic Oxidation Process of Heavy Oil in In-Situ Combustion Process Using Copper (Ⅱ) Stearate as Oil Soluble Catalyst. Fuel 2021, 284, 118981. [Google Scholar] [CrossRef]
- Amanam, U.U.; Kovscek, A.R. Analysis of the Effects of Copper Nanoparticles on In-Situ Combustion of Extra Heavy-Crude Oil. J. Pet. Sci. Eng. 2017, 152, 406–415. [Google Scholar] [CrossRef]
- Li, Y.-B.; Gao, H.; Pu, W.-F.; Li, L.; Chen, Y.; Bai, B. Study of the Catalytic Effect of Copper Oxide on the Low-Temperature Oxidation of Tahe Ultra-Heavy Oil. J. Therm. Anal. Calorim. 2019, 135, 3353–3362. [Google Scholar] [CrossRef]
- Ariskina, K.A.; Yuan, C.; Abaas, M.; Emelianov, D.A.; Rodionov, N.; Varfolomeev, M.A. Catalytic Effect of Clay Rocks as Natural Catalysts on the Combustion of Heavy Oil. Appl. Clay Sci. 2020, 193, 105662. [Google Scholar] [CrossRef]
- Kök, M.V.; Varfolomeev, M.A.; Nurgaliev, D.K. TGA and DSC Investigation of Different Clay Mineral Effects on the Combustion Behavior and Kinetics of Crude Oil from Kazan Region, Russia. J. Pet. Sci. Eng. 2021, 200, 108364. [Google Scholar] [CrossRef]
- Minkhanov, I.F.; Bolotov, A.V.; Sagirov, R.N.; Varfolomeev, M.A.; Anikin, O.V.; Tazeev, A.R.; Derevyanko, V.K. The Influence of the Content of Clay Minerals on the Efficiency of Steam Treatment Injection for the Bitumen Oils Recovery. SOCAR Proc. 2021. [Google Scholar] [CrossRef]
- Vossoughi, S.; Willhite, G.P.; Kritikos, W.P.; Guvenir, I.M.; El Shoubary, Y. Automation of an In-Situ Combustion Tube and Study of the Effect of Clay on the in-Situ Combustion Process. Soc. Pet. Eng. J. 1982, 22, 493–502. [Google Scholar] [CrossRef]
- Li, Y.-B.; Luo, C.; Lin, X.; Li, K.; Xiao, Z.-R.; Wang, Z.-Q.; Pu, W.-F. Characteristics and Properties of Coke Formed by Low-Temperature Oxidation and Thermal Pyrolysis during in Situ Combustion. Ind. Eng. Chem. Res. 2020, 59, 2171–2180. [Google Scholar]
- Ariskina, K.A.; Ding, Z.; Abaas, M.; Yuan, C.; Emelianov, D.A.; Chen, Q.; Varfolomeev, M.A. Influence of Carbonate Minerals on Heavy Oil Oxidation Behavior and Kinetics by TG-FTIR. Energies 2021, 14, 8136. [Google Scholar] [CrossRef]
- Kozlowski, M.L.; Punase, A.; Nasr-El-Din, H.A.; Hascakir, B. The Catalytic Effect of Clay on In-Situ Combustion Performance. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Quito, Ecuador, 18–20 November 2015; OnePetro: Houston, TX, USA, 2015. [Google Scholar]
- Galeev, R.I.; Sakharov, B.V.; Khasanova, N.M.; Volkov, V.Y.; Fazlyyyakhmatov, M.G.; Shamanov, I.N.; Emelianov, D.A.; Kozlova, E.V.; Petrashov, O.V.; Varfolomeev, M.A. Novel Low-Field NMR Method for Characterization Content and SARA Composition of Bitumen in Rocks. J. Pet. Sci. Eng. 2022, 214, 110486. [Google Scholar] [CrossRef]
- Pituganova, A.; Nassan, T.; Amro, M.; Minkhanov, I.; Varfolomeev, M.; Bolotov, A. Experimental and Numerical Analysis of Thermal EOR Recovery Schemes for Extra-Heavy Oil of the Oykino-Altuninsky Uplift of the Romashkinskoye Oilfield. In Proceedings of the International Petroleum Technology Conference, Riyadh, Saudi Arabia, 21–23 February 2022; OnePetro: Houston, TX, USA, 2022. [Google Scholar]
- Ushakova, A.S.; Zatsepin, V.; Khelkhal, M.A.; Sitnov, S.A.; Vakhin, A.V. In Situ Combustion of Heavy, Medium, and Light Crude Oils: Low-Temperature Oxidation in Terms of a Chain Reaction Approach. Energy Fuels 2022, 36, 7710–7721. [Google Scholar] [CrossRef]
- Minkhanov, I.F.; Bolotov, A.V.; Al-Muntaser, A.A.; Mukhamatdinov, I.I.; Vakhin, A.V.; Varfolomeev, M.A.; Slavkina, O.V.; Shchekoldin, K.A.; Darishchev, V.I. Experimental Study on the Improving the Efficiency of Oil Displacement by Co-Using of the Steam-Solvent Catalyst (Russian). Neft. Khozyaystvo-Oil Ind. 2021, 2021, 54–57. [Google Scholar] [CrossRef]
- Minkhanov, I.F.; Marvanov, M.M.; Bolotov, A.V.; Varfolomeev, M.A.; Khairtdinov, R.K. Improvement of Heavy Oil Displacement Efficiency by Using Aromatic Hydrocarbon Solvent. Int. Multidiscip. Sci. GeoConference SGEM 2020, 20, 711–718. [Google Scholar]
- Speight, J.G. Oil Sand Production Processes; Gulf Professional Publishing: Houston, TX, USA, 2012; ISBN 0124045529. [Google Scholar]







| Composition (%) | Sample | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Quartz | 17 | 50 | 64 |
| Microcline | 14 | 12 | 13 |
| Dolomite | 55 | 28 | 10 |
| Calcite | 0 | 2 | 0 |
| Pyrite | 5 | <1 | 1 |
| Kaolinite | 3 | 3 | 4 |
| Mica | 6 | 5 | 8 |
| Sample | Initial Oil Saturation, % |
|---|---|
| Three-dimensional model | 4.12 |
| 1 | 4.4 |
| 2 | 3.41 |
| 3 (clay) | 0.4 (low organic content) |
| Sample | 1 | 2 | 3 |
|---|---|---|---|
| Total organic content (TOC) | 5.2% | 3.2% | 1.7% |
| Input Parameters | ISC-1 | ISC-2 | ISC-3 |
|---|---|---|---|
| Porosity, % | 7.2 | 6.8 | 6.8 |
| Permeability, mD | 90 | 35 | 41 |
| Viscosity, mPa*s (20 °C) | 20,306 | ||
| Reservoir temperature | 25 °C | ||
| Model | Mode | Description of the Experiment |
|---|---|---|
| 1 | ISC-1 | Uniform distribution of clay minerals (more than 8%) |
| 2 | ISC-2 | Clay layers of 1 cm before zone 3 and 2 cm after it (Figure 5) |
| 3 | Steam Injection + ISC (ISC-3) | Clay layers of 1 cm before zone 3 and 2 cm after it (Figure 5) |
| The Content of Organic Matter in the Model, g | Weight of Extracted Oil, g | Displacement Coefficient, % |
|---|---|---|
| 49.4 | 39.8 | 80.5 |
| Sample | SARA Analysis (%) | |||
|---|---|---|---|---|
| Saturates | Aromatics | Resins | Asphaltenes | |
| Original Oil | 34.89 | 35.53 | 13.51 | 16.07 |
| Produced Oil | 50.79 | 26.92 | 11.53 | 10.76 |
| Zone | Oil Saturation, % | ||
|---|---|---|---|
| Before Combustion | After Combustion | ||
| Zone 1 | № 1 | 4.19 | 1.61 |
| Rock | |||
| Zone 2 | № 1 | 4.20 | 1.85 |
| Rock | |||
| Clay 1 | 0.40 | 3.55 | |
| Zone 3 | № 2 | 3.94 | 5.03 |
| Rock | |||
| Clay 2 | 0.40 | 6.02 | |
| Zone 4 | № 2 | 3.92 | 6.51 |
| Rock | |||
| Zone | Oil Saturation, % | ||
|---|---|---|---|
| Before Hydrothermal Combustion | After Hydrothermal Combustion | ||
| Zone 1 | № 1 | 4.19 | 1.47 |
| Rock | |||
| Zone 2 | № 1 | 4.20 | 1.73 |
| Rock | |||
| Clay 1 | 0.40 | 1.99 | |
| Zone 3 | № 2 | 3.92 | 4.56 |
| Rock | |||
| Clay 2 | 0.40 | 5.24 | |
| Zone 4 | № 2 | 3.93 | 5.02 |
| Rock | |||
| Parameters | Elements | Symbol | ISC-1 | ISC-2 | ISC-3 |
|---|---|---|---|---|---|
| Composition of air, mol % | Nitrogen Oxygen | N2i O2i CH4 O2 | 79.04 20.96 1.76 4.6 | 79.04 20.96 0.15 2.96 | 79.04 20.96 0.22 7.43 |
| Average gas compositions of produced gases, mol % | Methane Oxygen Carbon dioxide Carbon monoxide Nitrogen (Calculated) | CO2 CO N2 | 17.76 4.21 71.67 | 9.53 0.44 86.92 | 7.76 1.44 83.15 |
| Normalization of produced gases, mol % | Oxygen Carbon dioxide Carbon monoxide Nitrogen | O2 CO2 CO N2 | 4.68 18.07 4.28 72.95 | 2.96 9.54 0.44 87.05 | 7.44 7.77 1.44 83.33 |
| Produced (CO2, CO, and N2) and unreacted (O2) gases | Unreacted Oxygen Carbon dioxide Carbon monoxide Nitrogen | O2 CO2 CO N2 | 0.273 1.056 0.25 4.18 | 0.142 0.45 0.021 4.18 | 0.37 0.39 0.072 4.18 |
| Injected gas volume (scf) | Vi-lab | Vi | 5.297 | 5.297 | 5.297 |
| Burned sand volume (ft3) | Vburned-sand-lab | vi-t | 0.0142 | 0.0173 | 0.0180 |
| Parameters | Symbol | ISC-1 | ISC-2 | ISC-3 |
|---|---|---|---|---|
| O2/Fuel, scf/lbm m3/kg | O2/fuel | 1.85 | 3.37 | 3.85 |
| N (H/C) ratio | N | 0.9994 | 4.148 | 2.669 |
| Air/Fuel, scf/lbm m3/kg | Air/fuel | 8.87 | 16.11 | 18.40 |
| Oxygen utilization | Y | 0.75 | 0.871 | 0.66 |
| Ar, scf/ft3 m3/m3 | Ar | 272.30 | 551.71 | 378.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minkhanov, I.F.; Bolotov, A.V.; Tazeev, A.R.; Chalin, V.V.; Kacou, A.F.D.; Galeev, R.I.; Sagirov, R.N.; Al-Muntaser, A.A.; Emelianov, D.A.; Khelkhal, M.A.; et al. The Influence of Reservoir Clay Composition on Heavy Oil In Situ Combustion. Processes 2022, 10, 2308. https://doi.org/10.3390/pr10112308
Minkhanov IF, Bolotov AV, Tazeev AR, Chalin VV, Kacou AFD, Galeev RI, Sagirov RN, Al-Muntaser AA, Emelianov DA, Khelkhal MA, et al. The Influence of Reservoir Clay Composition on Heavy Oil In Situ Combustion. Processes. 2022; 10(11):2308. https://doi.org/10.3390/pr10112308
Chicago/Turabian StyleMinkhanov, Ilgiz F., Alexander V. Bolotov, Aidar R. Tazeev, Vladislav V. Chalin, Anini Franck D. Kacou, Ranel I. Galeev, Rustam N. Sagirov, Ameen A. Al-Muntaser, Dmitrii A. Emelianov, Mohammed Amine Khelkhal, and et al. 2022. "The Influence of Reservoir Clay Composition on Heavy Oil In Situ Combustion" Processes 10, no. 11: 2308. https://doi.org/10.3390/pr10112308
APA StyleMinkhanov, I. F., Bolotov, A. V., Tazeev, A. R., Chalin, V. V., Kacou, A. F. D., Galeev, R. I., Sagirov, R. N., Al-Muntaser, A. A., Emelianov, D. A., Khelkhal, M. A., & Varfolomeev, M. A. (2022). The Influence of Reservoir Clay Composition on Heavy Oil In Situ Combustion. Processes, 10(11), 2308. https://doi.org/10.3390/pr10112308









