Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review
Abstract
:1. Introduction
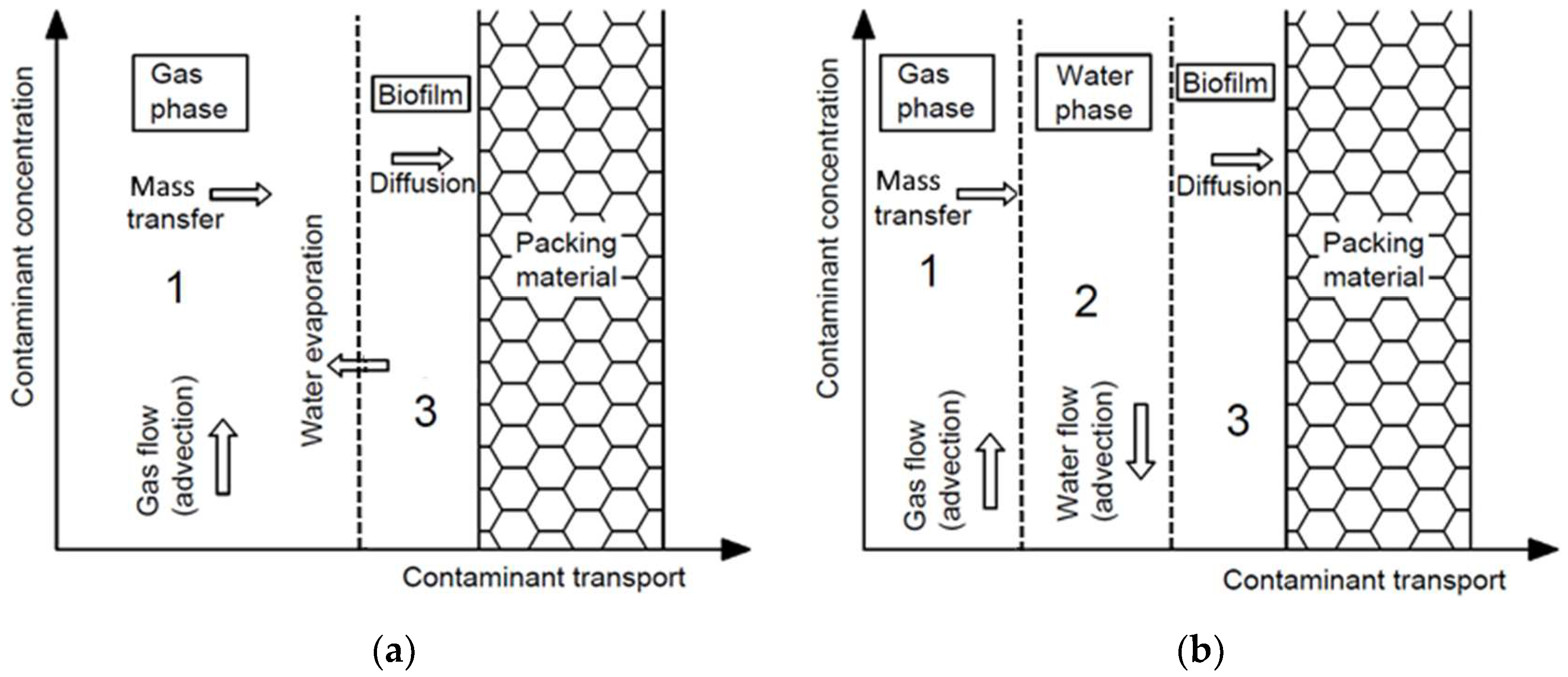
2. Comparison of Biofiltration Processes in Biofilters and Biotrickling Filters
3. Configurations of Biofilter Irrigation Systems
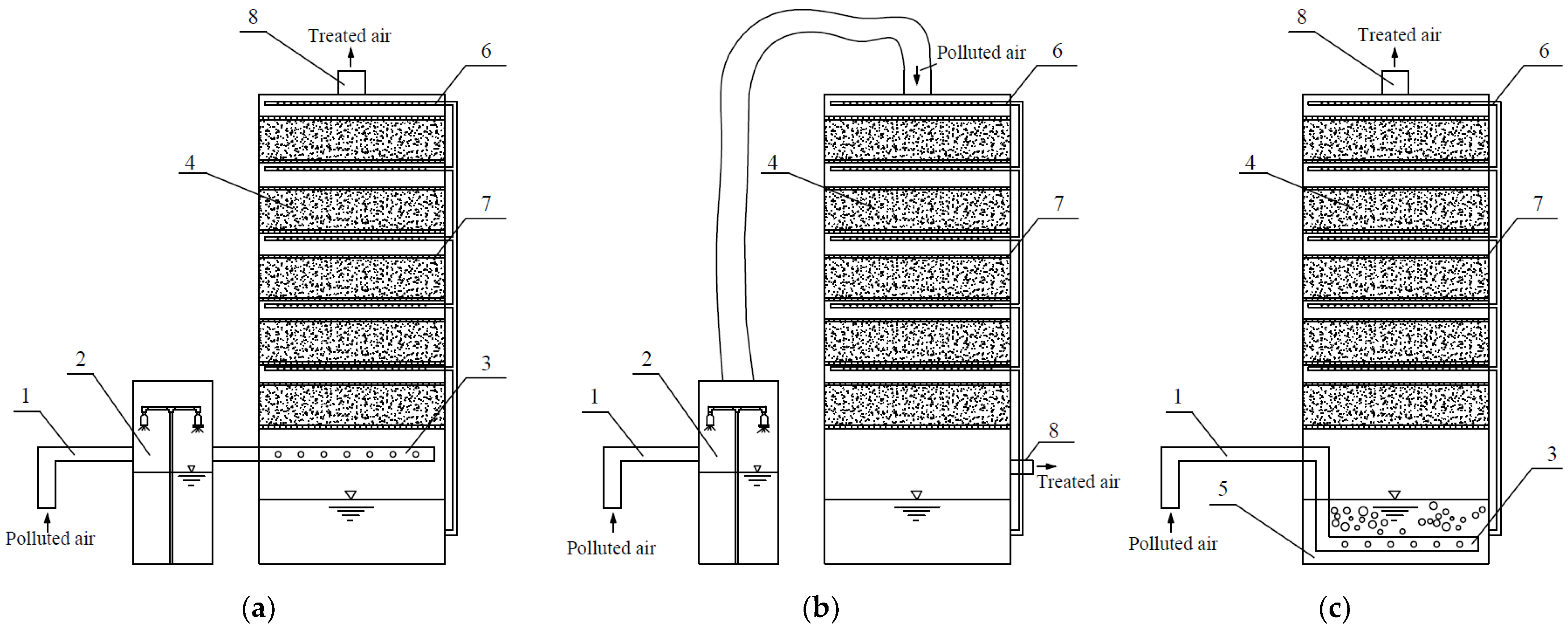
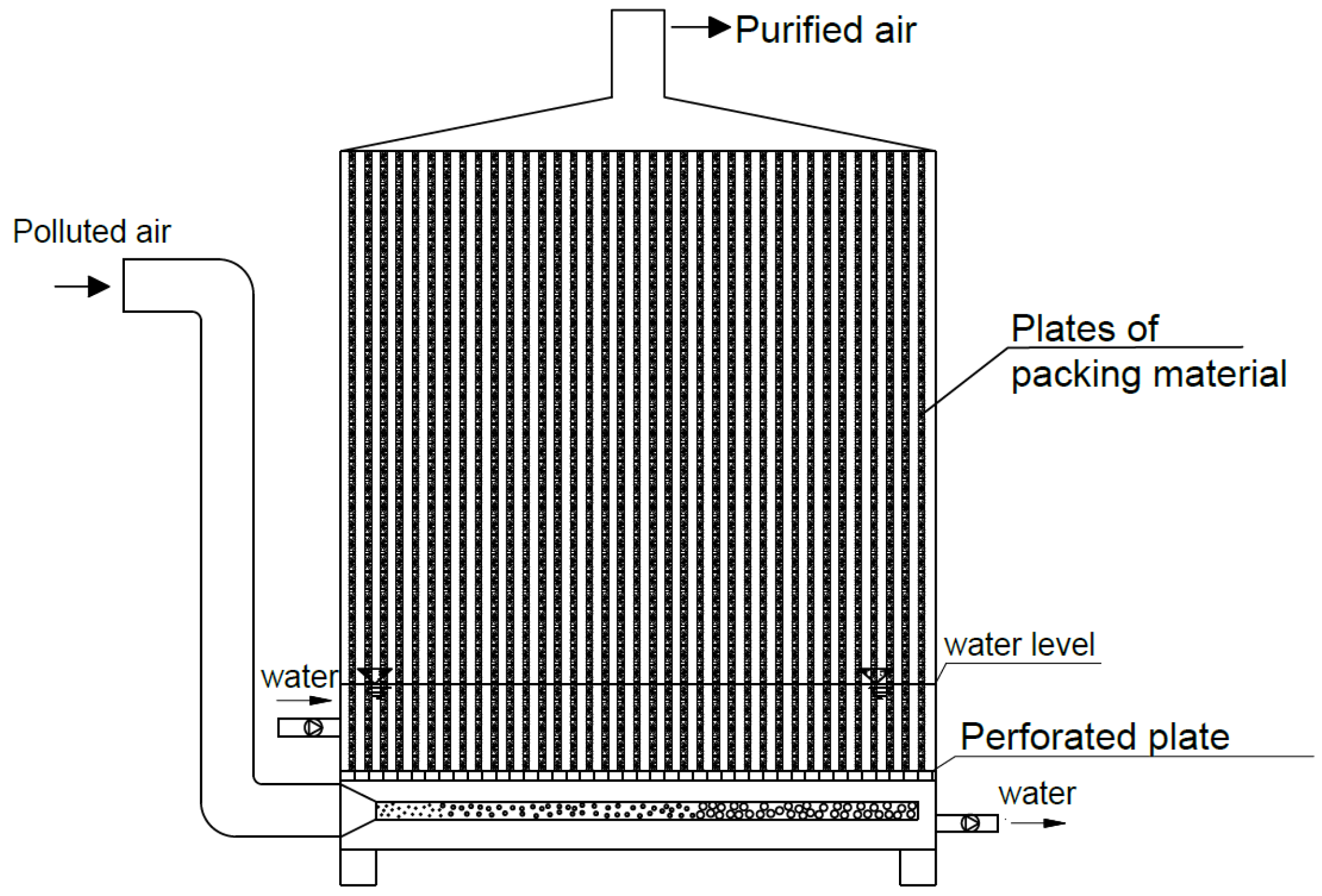
3.1. Humidification of Inlet Gas and Packing Material by Spraying
- Open-type biofilters;
- Closed-type biofilters.
3.2. Irrigation of Biofilter Packing Material by Immersing It in Liquid
4. Biotrickling Filter Irrigation Systems
5. Influence of Packing Material Moisture Content on Biofiltration Efficiency
5.1. Packing Materials
5.2. Influence of Packing Material Moisture Content on Biofiltration Efficiency
5.2.1. Biofilters
5.2.2. Biotrickling Filters
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iliuta, I.; Iliuta, M.C.; Larachi, F. Hydrodynamics Modeling of Bioclogging in Waste Gas Treating Trickle-Bed Bioreactors. Ind. Eng. Chem. Res. 2005, 44, 5044–5052. [Google Scholar] [CrossRef]
- Auria, R.; Aycaguer, A.C.; Devinny, J.S. Influence of Water Content on Degradation Rates for Ethanol in Biofiltration. J. Air Waste Manag. Assoc. 1998, 48, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybarczyk, P.; Szulczyński, B.; Gębicki, J.; Hupka, J. Treatment of malodorous air in biotrickling filters: A review. Biochem. Eng. J. 2019, 141, 146–162. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Zagorskis, A. Investigation into the air treatment efficiency of biofilters of different structures. J. Environ. Eng. Landsc. Manag. 2010, 18, 23–31. [Google Scholar] [CrossRef]
- Pineda, P.A.L.; Demeestere, K.; Toledo, M.; Van Langenhove, H.; Walgraeve, C. Enhanced removal of hydrophobic volatile organic compounds in biofilters and biotrickling filters: A review on the use of surfactants and the addition of hydrophilic compounds. Chemosphere 2021, 279, 130757. [Google Scholar] [CrossRef]
- Deshusses, M.A.; Johnson, C.T. Development and Validation of a Simple Protocol To Rapidly Determine the Performance of Biofilters for VOC Treatment. Environ. Sci. Technol. 2000, 34, 461–467. [Google Scholar] [CrossRef]
- Canet, X.; Gilles, F.; Su, B.L.; de Weireld, G.; Frère, M. Adsorption of Alkanes and Aromatic Compounds on Various Faujasites in the Henry Domain. 1. Compensating Cation Effect on Zeolites Y. J. Chem. Eng. Data 2007, 52, 2117–2126. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, D.; Sorial, G.A. Performance of Trickle-Bed Air Biofilter: A Comparative Study of a Hydrophilic and a Hydrophobic Voc. Water Air Soil Pollut. Focus 2006, 6, 57–69. [Google Scholar] [CrossRef]
- Malhautier, L.; Khammar, N.; Bayle, S.; Fanlo, J.L. Biofiltration of volatile organic compounds. Appl. Microbiol. Biotechnol. 2005, 68, 16–22. [Google Scholar] [CrossRef]
- Chan, W.C.; Chang, L.Y. Effects of Temperature and Inlet Concentration on Acetone Biofiltration in a Composite Bead Biofilter. J. Polym. Environ. 2006, 14, 1–8. [Google Scholar] [CrossRef]
- Khanongnuch, R.; Abubackar, H.N.; Keskin, T.; Gungormusler, M.; Duman, G.; Aggarwal, A.; Behera, S.K.; Li, L.; Bayar, B.; Rene, E.R. Bioprocesses for resource recovery from waste gases: Current trends and industrial applications. Renew. Sustain. Energy Rev. 2022, 156, 111926. [Google Scholar] [CrossRef]
- Han, M.F.; Wang, C.; Yang, N.Y.; Hu, X.R.; Wang, Y.C.; Duan, E.H.; Ren, H.W.; Hsi, H.C.; Deng, J.G. Performance enhancement of a biofilter with pH buffering and filter bed supporting material in removal of chlorobenzene. Chemosphere 2020, 251, 126358. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, M.; Jin, B.; Yang, J.; Li, S. Performance evaluation and microbial community analysis of the biofilter for removing grease and volatile organic compounds in the kitchen exhaust fume. Bioresour. Technol. 2021, 319, 124132. [Google Scholar] [CrossRef]
- Lee, S.; Li, C.; Heber, A.J.; Ni, J.; Huang, H. Biofiltration of a mixture of ethylene, ammonia, n-butanol, and acetone gases. Bioresour. Technol. 2013, 127, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Bruneel, J.; Walgraeve, C.; Mukurarinda, J.; Boon, N.; Van Langenhove, H. Biofiltration of hexane, acetone and dimethyl sulphide using wood, compost and silicone foam. J. Chem. Technol. Biotechnol. 2018, 93, 2234–2243. [Google Scholar] [CrossRef]
- Dorado, A.D.; Lafuente, J.; Gabriel, D.; Gamisans, X. The role of water in the performance of biofilters: Parameterization of pressure drop and sorption capacities for common packing materials. J. Hazard. Mater. 2010, 180, 693–702. [Google Scholar] [CrossRef]
- Sun, Y.; Quan, X.; Chen, J.; Yang, F.; Xue, D.; Liu, Y.; Yang, Z. Toluene vapour degradation and microbial community in biofilter at various moisture content. Process Biochem. 2002, 38, 109–113. [Google Scholar] [CrossRef]
- Rene, E.R.; Sergienko, N.; Goswami, T.; López, M.E.; Kumar, G.; Saratale, G.D.; Venkatachalam, P.; Pakshirajan, K.; Swaminathan, T. Effects of concentration and gas flow rate on the removal of gas-phase toluene and xylene mixture in a compost biofilter. Bioresour. Technol. 2018, 248, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Carvalho, G.; Yuan, Z.; Bond, P.; Jiang, G. Biotrickling filter for the removal of volatile sulfur compounds from sewers: A review. Chemosphere 2021, 277, 130333. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yan, H.; Quan, Y.; Zhao, H.; Jiang, N.; Yin, C. Recent progress and perspectives in biotrickling filters for VOCs and odorous gases treatment. J. Environ. Manag. 2018, 222, 409–419. [Google Scholar] [CrossRef]
- Elmrini, H.; Bredin, N.; Shareefdeen, Z.; Heitz, M. Biofiltration of xylene emissions: Bioreactor response to variations in the pollutant inlet concentration and gas flow rate. Chem. Eng. J. 2004, 100, 149–158. [Google Scholar] [CrossRef]
- Lee, S.H.; Kurade, M.B.; Jeon, B.H.; Kim, J.; Zheng, Y.; Salama, E.S. Water condition in biotrickling filtration for the efficient removal of gaseous contaminants. Crit. Rev. Biotechnol. 2021, 41, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Zhu, X.; Suidan, M.T.; Kim, B.R.; Kim, B.J. Mathematical Model of Biofiltration of VOCs: Effect of Nitrate Concentration and Backwashing. J. Environ. Eng. 2001, 127, 655–664. [Google Scholar] [CrossRef]
- López, M.E.; Boger, Z.; Rene, E.R.; Veiga, M.C.; Kennes, C. Transient-state studies and neural modeling of the removal of a gas-phase pollutant mixture in a biotrickling filter. J. Hazard. Mater. 2014, 269, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and solutions for biofiltration of hydrophobic volatile organic compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Kalantar, M.; Zamir, S.M.; Ferdowsi, M.; Shojaosadati, S.A. Removal of toluene in a biotrickling filter in the presence of methanol vapors: Experimental study, mathematical modeling, and kinetic parameters optimization. J. Environ. Chem. Eng. 2021, 9, 104617. [Google Scholar] [CrossRef]
- Barbusiński, K.; Urbaniec, K.; Kasperczyk, D.; Thomas, M. Biofilters versus bioscrubbers and biotrickling filters: State-of-the-art biological air treatment. In From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment; Soreanu, G., Dumont, É., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–51. [Google Scholar]
- Merouani, E.F.O.; Khabiri, B.; Ferdowsi, M.; Benyoussef, E.H.; Malhautier, L.; Buelna, G.; Jones, J.P.; Heitz, M. Biofiltration of methane in presence of ethylbenzene or xylene. Atmos. Pollut. Res. 2022, 13, 101271. [Google Scholar] [CrossRef]
- Van Lith, C.; Leson, G.; Michelsen, R. Evaluating Design Options for Biofilters. J. Air Waste Manag. Assoc. 1997, 47, 37–48. [Google Scholar] [CrossRef]
- Xue, S.; Chen, W.; Deng, M.; Luo, H.; Huang, W.; Han, Y.; Li, L. Effects of moisture content on the performance of a two-stage thermophilic biofilter and choice of irrigation rate. Process Saf. Environ. Prot. 2018, 113, 164–173. [Google Scholar] [CrossRef]
- Bagherpour, M.B.; Nikazar, M.; Welander, U.; Bonakdarpour, B.; Sanati, M. Effects of irrigation and water content of packings on alpha-pinene vapours biofiltration performance. Biochem. Eng. J. 2005, 24, 185–193. [Google Scholar] [CrossRef]
- Bruneel, J.; Huepe Follert, J.L.; Laforce, B.; Vincze, L.; Van Langenhove, H.; Walgraeve, C. Dynamic performance of a fungal biofilter packed with perlite for the abatement of hexane polluted gas streams using SIFT-MS and packing characterization with advanced X-ray spectroscopy. Chemosphere 2020, 253, 126684. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Guo, L.; Veiga, M.C.; Kennes, C. Fungal biofiltration of α-pinene: Effects of temperature, relative humidity, and transient loads. Biotechnol. Bioeng. 2007, 96, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Cruz-García, B.; Geronimo-Meza, A.S.; Martínez-Lievana, C.; Arriaga, S.; Huante-González, Y.; Aizpuru, A. Biofiltration of high concentrations of methanol vapors: Removal performance, carbon balance and microbial and fly populations. J. Chem. Technol. Biotechnol. 2019, 94, 1925–1936. [Google Scholar] [CrossRef]
- Ghasemi, R.; Golbabaei, F.; Rezaei, S.; Pourmand, M.R.; Nabizadeh, R.; Jafari, M.J.; Masoorian, E. A comparison of biofiltration performance based on bacteria and fungi for treating toluene vapors from airflow. AMB Express 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Saha, P.D.; Baskaran, D.; Rajamanickam, R. Microbial biofilter for toluene removal: Performance evaluation, transient operation and theoretical prediction of elimination capacity. Sustain. Environ. Res. 2018, 28, 121–127. [Google Scholar] [CrossRef]
- Yang, K.; Li, L.; Ding, W.; Liu, J.; Xue, S. A full-scale thermophilic biofilter in the treatment of sludge drying exhaust: Performance, microbial characteristics and bioaerosol emission. J. Chem. Technol. Biotechnol. 2018, 93, 2216–2225. [Google Scholar] [CrossRef]
- Saravanan, V.; Rajamohan, N. Treatment of xylene polluted air using press mud-based biofilter. J. Hazard. Mater. 2009, 162, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chai, F.; Liang, C.; Wang, Y.; Zhang, X.; Yang, K.; Xiao, B. Comparison and application of biofilter and suspended bioreactor in removing gaseous o-xylene. Environ. Res. 2020, 188, 109853. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.X. Removal of xylene from off-gas using a bioreactor containing bacteria and fungi. Int. Biodeterior. Biodegrad. 2006, 58, 60–64. [Google Scholar] [CrossRef]
- Mansoori, A.M.; Ando, N.; Higuchi, T. Influence of phosphorus and trace metals in biofilters treating gaseous VOCs using a novel irrigation system. J. Air Waste Manag. Assoc. 2019, 69, 1348–1360. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Januševičius, T.; Zagorskis, A.; Baltrėnaitė-Gedienė, E. Removal of ammonia by biofilters with straight and wavy lamellar plates. Int. J. Environ. Sci. Technol. 2021, 18, 1181–1190. [Google Scholar] [CrossRef]
- Lu, L.; Dong, D.; Yeung, M.; Sun, Z.; Xi, J. Sustaining low pressure drop and homogeneous flow by adopting a fluidized bed biofilter treating gaseous toluene. Chemosphere 2022, 291, 132951. [Google Scholar] [CrossRef] [PubMed]
- Marycz, M.; Brillowska-Dąbrowska, A.; Muñoz, R.; Gębicki, J. A state of the art review on the use of fungi in biofiltration to remove volatile hydrophobic pollutants. Rev. Environ. Sci. Bio/Technol. 2022, 21, 225–246. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Cheng, Y.; Huang, J.; Yang, H.; Zeng, G.; Lu, L.; He, S. Enhanced removal of ethylbenzene from gas streams in biotrickling filters by Tween-20 and Zn(II). J. Environ. Sci. 2014, 26, 2500–2507. [Google Scholar] [CrossRef]
- Yang, C.; Yu, G.; Zeng, G.; Yang, H.; Chen, F.; Jin, C. Performance of biotrickling filters packed with structured or cubic polyurethane sponges for VOC removal. J. Environ. Sci. 2011, 23, 1325–1333. [Google Scholar] [CrossRef]
- San-Valero, P.; Penya-roja, J.; Alvarez-Hornos, F.J.; Marzal, P.; Gabaldón, C. Dynamic Mathematical Modelling of the Removal of Hydrophilic VOCs by Biotrickling Filters. Int. J. Environ. Res. Public Health 2015, 12, 746–766. [Google Scholar] [CrossRef] [Green Version]
- Khoramfar, S.; Jones, K.D.; Boswell, J.; Ghobadi, J.; Paca, J. Evaluation of a sequential biotrickling-biofiltration unit for removal of VOCs from the headspace of crude oil storage tanks. J. Chem. Technol. Biotechnol. 2018, 93, 1778–1789. [Google Scholar] [CrossRef]
- Song, J.; Kinney, K.A. Effect of vapor-phase bioreactor operation on biomass accumulation, distribution, and activity: Linking biofilm properties to bioreactor performance. Biotechnol. Bioeng. 2000, 68, 508–516. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Xu, J.; Li, J.; Mao, Z. Process Performance of a Biotrickling Filter Using a Flow-Directional-Switching Method. CLEAN-Soil Air Water 2013, 41, 522–527. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Liu, H.; Yang, C.; Wu, S.; Du, C.; Nie, L.; Zhong, Y. Effect of presence of hydrophilic volatile organic compounds on removal of hydrophobic n-hexane in biotrickling filters. Chemosphere 2020, 252, 126490. [Google Scholar] [CrossRef]
- Zagorskis, A.; Januševičius, T.; Danila, V. Removal of Acetone Vapor from Air Using a Biotrickling Filter Packed with Polymeric Bioballs. Processes 2021, 10, 57. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Xing, H.; Li, J. Effect of liquid supply on the performance of a fungal bio-trickling filter treating hydrophobic VOC. Biochem. Eng. J. 2020, 161, 107658. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Liu, J.; Cao, J.; Sheng, D.; Cai, T. Evaluation of a pilot-scale bio-trickling filter as a VOCs control technology for the chemical fibre wastewater treatment plant. J. Environ. Manag. 2019, 246, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yin, Z.; Quan, Y.; Fang, Y.; Yin, C. Removal of methyl acrylate by ceramic-packed biotrickling filter and their response to bacterial community. Bioresour. Technol. 2016, 209, 237–245. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lu, B.H.; Zhou, X.X.; Li, W. Evaluation of o-xylene and other volatile organic compounds removal using a xylene-acclimated biotrickling filter. Environ. Technol. 2013, 34, 2691–2699. [Google Scholar] [CrossRef]
- Xue, N.; Wang, Q.; Wang, J.; Wang, J.; Sun, X. Odorous composting gas abatement and microbial community diversity in a biotrickling filter. Int. Biodeterior. Biodegrad. 2013, 82, 73–80. [Google Scholar] [CrossRef]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for treatment of VOCs and odours—A review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef]
- Xu, P.; Wei, Y.; Cheng, N.; Li, S.; Li, W.; Guo, T.; Wang, X. Evaluation on the removal performance of dichloromethane and toluene from waste gases using an airlift packing reactor. J. Hazard. Mater. 2019, 366, 105–113. [Google Scholar] [CrossRef]
- Wongbunmak, A.; Panthongkham, Y.; Suphantharika, M.; Pongtharangkul, T. A fixed-film bioscrubber of Microbacterium esteraromaticum SBS1-7 for toluene/styrene biodegradation. J. Hazard. Mater. 2021, 418, 126287. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J.; Deshusses, M.A.; Schroeder, E.D. Literature review of air pollution control biofilters and biotrickling filters for odor and volatile organic compound removal. Environ. Prog. 2005, 24, 254–267. [Google Scholar] [CrossRef]
- Huan, C.; Lyu, Q.; Tong, X.; Li, H.; Zeng, Y.; Liu, Y.; Jiang, X.; Ji, G.; Xu, L.; Yan, Z. Analyses of deodorization performance of mixotrophic biotrickling filter reactor using different industrial and agricultural wastes as packing material. J. Hazard. Mater. 2021, 420, 126608. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Wu, H.; Guo, C.; Han, Y.; Yin, C. Enhancement of TCE removal by a static magnetic field in a fungal biotrickling filter. Bioresour. Technol. 2018, 259, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Rajamanickam, R.; Baskaran, D. Biodegradation of gaseous toluene with mixed microbial consortium in a biofilter: Steady state and transient operation. Bioprocess Biosyst. Eng. 2017, 40, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Giri, B.S.; Kim, K.H.; Singh, R.P.; Rene, E.R.; López, M.E.; Rai, B.N.; Singh, H.; Prasad, D.; Singh, R.S. Performance of a biofilter with compost and activated carbon based packing material for gas-phase toluene removal under extremely high loading rates. Bioresour. Technol. 2019, 285, 121317. [Google Scholar] [CrossRef]
- Baskaran, D.; Rajamanickam, R.; Vaidyalingam, B. Effect of concentration and gas flow rate on the removal of gas-phase trichloroethylene in a novel packed biofilter. Bioresour. Technol. Rep. 2020, 9, 100387. [Google Scholar] [CrossRef]
- Liu, J.; Yue, P.; Huang, L.; Zhao, M.; Kang, X.; Liu, X. Styrene removal with an acidic biofilter with four packing materials: Performance and fungal bioaerosol emissions. Environ. Res. 2020, 191, 110154. [Google Scholar] [CrossRef]
- Zou, J.; Xu, G.; Pan, K.; Zhou, W.; Dai, Y.; Wang, X.; Zhang, D.; Hu, Y.C.; Ma, M. Nitrogen removal and biofilm structure affected by COD/NH4+–N in a biofilter with porous sludge-ceramsite. Sep. Purif. Technol. 2012, 94, 9–15. [Google Scholar] [CrossRef]
- Sun, M.T.; Zhao, Y.Z.; Yang, Z.M.; Shi, X.S.; Wang, L.; Dai, M.; Wang, F.; Guo, R.B. Methane Elimination Using Biofiltration Packed With Fly Ash Ceramsite as Support Material. Front. Bioeng. Biotechnol. 2020, 8, 351. [Google Scholar] [CrossRef] [Green Version]
- Paca, J.; Koutsky, B.; Maryska, M.; Halecky, M. Styrene degradation along the bed height of perlite biofilter. J. Chem. Technol. Biotechnol. 2001, 76, 873–878. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.; Chai, F.; Ma, J.; Li, L. Biofilters for the co-treatment of volatile organic compounds and odors in a domestic waste landfill site. J. Clean. Prod. 2020, 277, 124012. [Google Scholar] [CrossRef]
- Sun, Z.; Ding, C.; Xi, J.; Lu, L.; Yang, B. Enhancing biofilm formation in biofilters for benzene, toluene, ethylbenzene, and xylene removal by modifying the packing material surface. Bioresour. Technol. 2020, 296, 122335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hu, L.; Dai, L.; Wang, Z.; He, J.; Wang, Z.; Chen, J.; Hrynsphan, D.; Tatsiana, S. Bamboo charcoal powder-based polyurethane as packing material in biotrickling filter for simultaneous removal of n-hexane and dichloromethane. Bioresour. Technol. 2022, 345, 126427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Xing, H.; Li, J. Performance and fungal diversity of bio-trickling filters packed with composite media of polydimethylsiloxane and foam ceramics for hydrophobic VOC removal. Chemosphere 2020, 256, 127093. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Baltrėnaitė, E.; Spudulis, E. Biochar from Pine and Birch Morphology and Pore Structure Change by Treatment in Biofilter. Water Air Soil Pollut. 2015, 226, 69. [Google Scholar] [CrossRef]
- Liu, S.H.; Lin, H.H.; Lai, C.Y.; Lin, C.W.; Chang, S.H.; Yau, J.T. Microbial community in a pilot-scale biotrickling filter with cell-immobilized biochar beads and its performance in treating toluene-contaminated waste gases. Int. Biodeterior. Biodegrad. 2019, 144, 104743. [Google Scholar] [CrossRef]
- Dolphen, R.; Treesubsuntorn, C.; Santawee, N.; Setsungnoen, A.; Thiravetyan, P. Modified coir pith with glucose syrup as a supporter in non-external nutrient supplied biofilter for benzene removal by Bacillus megaterium. Environ. Technol. 2020, 41, 3607–3618. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, K.; Arancibia, A.; Caceres, M.; Aroca, G. Release of formaldehyde during the biofiltration of methanol vapors in a peat biofilter inoculated with Pichia pastoris GS115. Electron. J. Biotechnol. 2019, 40, 10–16. [Google Scholar] [CrossRef]
- Saravanan, V.; Ashokkumar, S.; Rajamohan, N.; Joo, S.W.; Vasseghian, Y.; Rajasimman, M. A mixed agro-waste based biofilter for the removal of methyl ethyl ketone: Kinetics and modeling. Process Saf. Environ. Prot. 2022, 162, 83–96. [Google Scholar] [CrossRef]
- Li, K.; Zhou, J.; Wang, L.; Mao, Z.; Xu, R. The styrene purification performance of biotrickling filter with toluene-styrene acclimatization under acidic conditions. J. Air Waste Manag. Assoc. 2019, 69, 944–955. [Google Scholar] [CrossRef]
- Portune, K.J.; Pérez, M.C.; Álvarez-Hornos, J.; Gabaldón, C. Contribution of bacterial biodiversity on the operational performance of a styrene biotrickling filter. Chemosphere 2020, 247, 125800. [Google Scholar] [CrossRef]
- You, J.; Chen, J.; Sun, Y.; Fang, J.; Cheng, Z.; Ye, J.; Chen, D. Treatment of mixed waste-gas containing H2S, dichloromethane and tetrahydrofuran by a multi-layer biotrickling filter. J. Clean. Prod. 2021, 319, 128630. [Google Scholar] [CrossRef]
- Kim, S.; Deshusses, M.A. Determination of mass transfer coefficients for packing materials used in biofilters and biotrickling filters for air pollution control. 1. Experimental results. Chem. Eng. Sci. 2008, 63, 841–855. [Google Scholar] [CrossRef]
- Shareefdeen, Z.; Herner, B.; Webb, D.; Wilson, S. Biofiltration eliminates nuisance chemical odors from industrial air streams. J. Ind. Microbiol. Biotechnol. 2003, 30, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.C.; Álvarez-Hornos, F.J.; Portune, K.; Gabaldón, C. Abatement of styrene waste gas emission by biofilter and biotrickling filter: Comparison of packing materials and inoculation procedures. Appl. Microbiol. Biotechnol. 2015, 99, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caicedo, F.; Estrada, J.M.; Silva, J.P.; Muñoz, R.; Lebrero, R. Effect of packing material configuration and liquid recirculation rate on the performance of a biotrickling filter treating VOCs. J. Chem. Technol. Biotechnol. 2018, 93, 2299–2306. [Google Scholar] [CrossRef]
- Álvarez-Hornos, F.J.; Gabaldón, C.; Martínez-Soria, V.; Martín, M.; Marzal, P.; Penya-roja, J.M. Biofiltration of ethylbenzene vapours: Influence of the packing material. Bioresour. Technol. 2008, 99, 269–276. [Google Scholar] [CrossRef]
- Yang, L.; Kent, A.D.; Wang, X.; Funk, T.L.; Gates, R.S.; Zhang, Y. Moisture effects on gas-phase biofilter ammonia removal efficiency, nitrous oxide generation, and microbial communities. J. Hazard. Mater. 2014, 271, 292–301. [Google Scholar] [CrossRef]
- Vergara-Fernández, A.; Salgado-Ísmodes, V.; Pino, M.; Hernández, S.; Revah, S. Temperature and moisture effect on spore emission in the fungal biofiltration of hydrophobic VOCs. J. Environ. Sci. Health Part A 2012, 47, 605–613. [Google Scholar] [CrossRef]
- Rene, E.R.; Veiga, M.C.; Kennes, C. Performance of a biofilter for the removal of high concentrations of styrene under steady and non-steady state conditions. J. Hazard. Mater. 2009, 168, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.A.; Padoley, K.V.; Mukherji, S.S.; Mudliar, S.N.; Vaidya, A.N.; Rajvaidya, A.S.; Subbarao, T.V. Biotreatment of waste gas containing pyridine in a biofilter. Bioresour. Technol. 2007, 98, 2258–2267. [Google Scholar] [CrossRef]
- Rezaei, M.; Moussavi, G.; Naddafi, K.; Johnson, M.S. Enhanced biodegradation of styrene vapors in the biotrickling filter inoculated with biosurfactant-generating bacteria under H2O2 stimulation. Sci. Total Environ. 2020, 704, 135325. [Google Scholar] [CrossRef] [PubMed]
- Deshusses, M.A.; Gabriel, D. Biotrickling Filter Technology. In Biotechnology for Odor and Air Pollution Control; Shareefdeen, Z., Singh, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 147–168. [Google Scholar]
- Lu, C.; Lin, M.R.; Chu, C. Effects of pH, moisture, and flow pattern on trickle-bed air biofilter performance for BTEX removal. Adv. Environ. Res. 2002, 6, 99–106. [Google Scholar] [CrossRef]
- Jia, T.; Sun, S.; Chen, K.; Zhang, L.; Peng, Y. Simultaneous methanethiol and dimethyl sulfide removal in a single-stage biotrickling filter packed with polyurethane foam: Performance, parameters and microbial community analysis. Chemosphere 2020, 244, 125460. [Google Scholar] [CrossRef]
- San-Valero, P.; Penya-Roja, J.M.; Álvarez-Hornos, F.J.; Gabaldón, C. Modelling mass transfer properties in a biotrickling filter for the removal of isopropanol. Chem. Eng. Sci. 2014, 108, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.C.; Álvarez-Hornos, F.J.; Engesser, K.H.; Dobslaw, D.; Gabaldón, C. Removal of 2-butoxyethanol gaseous emissions by biotrickling filtration packed with polyurethane foam. N. Biotechnol. 2016, 33, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, G.; Wang, S.; Yang, C.; Chen, H.; Zhu, Y.; Yu, L.; Li, J.; Kazemian, H. Performance promotion and its mechanism for n-hexane removal in a lab-scale biotrickling filter with reticular polyurethane sponge under intermittent spraying mode. Process Saf. Environ. Prot. 2021, 152, 654–662. [Google Scholar] [CrossRef]
- Alinejad, A.; Zamir, S.M.; Shojaosadati, S.A. Different strategies for transient-state operation of a biotrickling filter treating toluene vapor. Appl. Microbiol. Biotechnol. 2017, 101, 3451–3462. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, B.; Wang, L.; Ding, C.; Li, Z. Toluene-styrene secondary acclimation improved the styrene removal ability of biotrickling filter. Chem. Speciat. Bioavailab. 2017, 29, 54–59. [Google Scholar] [CrossRef] [Green Version]
- San-Valero, P.; Penya-Roja, J.M.; Sempere, F.; Gabaldón, C. Biotrickling filtration of isopropanol under intermittent loading conditions. Bioprocess Biosyst. Eng. 2013, 36, 975–984. [Google Scholar] [CrossRef] [Green Version]


| Packing Material; Inoculation | Bioreactor | Pollutant | Humidity Control | EBRT | ILR or IC | EC or RE | Packing Material Properties | References |
|---|---|---|---|---|---|---|---|---|
| Coir pith glucose syrup beads + sterile distilled water + Bacillus megaterium | BF | Benzene | External nutrients were not added | 60 s | ILR (max): 22.15 g/(m3 h) | EC (max): 18.82 g/(m3 h) | Air filled porosity: 0.4 m3/m3; Moisture content of PM: 53% (day 0); 6.8% (day 215) | [77] |
| Mixture of bamboo-clay and turkey litter compost (3:2, w/w); microbial consortium of compost with toluene degraded consortia | BF | TCE | Inlet gas humidification (RH = 70%), periodical spraying of packing material with moisture and nutrients | 1.4–2.0 min | 8–67 g/(m3 h) | 3–38 g/(m3 h) | Porosity of bamboo: 75%; Moisture content of bamboo: 65%; Porosity of clay: 64%; Moisture content of clay: 10% | [66] |
| Mixture of compost and ceramic beads (3:2, v/v) | BF | Toluene | Inlet gas humidification (RH = 45%), periodical spraying of packing material with nutrient solution | 1.2–2.0 min | 0.052–3.810 g/m3 | EC (max): 93 g/(m3 h) at ILR of 114 g/(m3 h) | SSA of ceramic beads: 500 m2/m3; Water retention of ceramic beads: 0.3 w/w. Porosity of compost: 50%; Moisture content of compost: 42% | [64] |
| Mixture of compost and GAC prepared in the form of spherical beads; activated sludge | BF | Toluene | Inlet gas humidification; periodical spraying of packing material with water (100 ml/day) | 19–42 s | 160–8750 g/(m3 h) | RE: 70–96% EC (max): 6665 g/(m3 h) at ILR of 8750 g/(m3 h) | Porosity of PM: 68%; Moisture content of PM: 55% (under wet conditions) | [65] |
| Mixture of vermi-compost and wood charcoal (2:1, v/v); Pseudomonas putida PTCC 1694 | BF | Toluene | Inlet gas humidification; spraying packing material with nutrient solution every 3 days | 21 s | 21.27 ± 3.3 g/(m3 h) | 16.23 ± 3.37 g/(m3 h) | Porosity of compost: 55%; moisture content of compost: 44–53%; porosity of wood charcoal particles: 50%; moisture content of wood charcoal particles after 24 h immersion in water: 78% | [35] |
| Mixture of 60% peat and 40% perlite (v/v); Pichia pastoris GS115 | BF | Methanol | Inlet gas humidification | 60–120 s | 81.12–672.12 g/(m3 h) | 80.28–322.74 g/(m3 h) | Moisture content of PM: 63%; Porosity of PM: 66.8% | [78] |
| Perlite; activated sludge | BF | Methanol | Inlet gas saturation in a liquid methanol; daily mineral medium spraying | 60–160 s | 98.8 ± 4.8–341.5 ± 47.5 g/(m3 h) | EC (max): 343.8 g/(m3 h) | Moisture content of PM: 50–73%; moisture level was higher in the lower BF module due to effect of gravity | [34] |
| PU foam cubes; Brevibacillus (58.8%) | BF | Total VOCs | Spraying of packing material with nutrient solution | 1.5 min | 0.5–200 mg/m3 | >85% | Size of 1 cube: 1.0 cm3; Porosity: 95% | [71] |
| Pressmud and cornstack (80%/20%); sludge | BF | MEK | Inlet gas humidification, spraying packing material with nutrient solution | 0.7–2.81 min | 4.16–100.08 g/(m3 h) | RE: 58–97% | MEK removal decreased, when the cornstack ratio increased | [79] |
| Cell-immobilized bamboo-biochar beads; mixed culture from activated sludge | BTF | Toluene | Trickling liquid rate: 2 L/min | 99 s | 0.14–99.1 g/(m3 h) | EC (max): 34.9 g/(m3 h) at ILR of 46.2 g/(m3 h) | Water content: 82 ± 1.14%; | [76] |
| Ceramic Raschig rings; activated sludge | BTF | Styrene | Continuous liquid trickling | 34–136 s | 12.3–159.8 g/(m3 h) | EC (max): 126 g/(m3 h) at ILR of 160 g/(m3 h) | SSA: 526 m2/m3, Porosity: 68.2% | [80] |
| Ceramsite; Fusarium oxysporum | BTF | Toluene | Intermittent nutrient solution spraying | 37–73 s | 20–100 g/(m3 h) | EC (max): 79.9 g/(m3 h) | A diameter of ceramsite: 4–5 mm; porosity 46.3%; SSA: 1.08 m2/g | [53] |
| HDPE pall rings; Pandoraea pnomenusa DSM 16536, Ralstonia eutropha PTCC1615 | BTF | Toluene; methanol | Trickling liquid rate: 20 mL/min | 60 s | 18–36 g/(m3 h) (toluene); 0–226 g/(m3 h) (methanol) | RE: 30–99% (toluene); EC (average): 220 g/(m3 h) for an ILR of 226 g/(m3 h) (methanol) | SSA: 480 m2/m3; Porosity of pall rings: 90%; Porosity of PM: 68% | [26] |
| PDMS and foam ceramic composite; Cladophialophora fungus | BTF | Toluene | Intermittent nutrient solution spraying | 10–57 s | 20–265 g/(m3 h) | EC (max): 264.4 g/(m3 h) | Porosity of PM: 73.0% | [74] |
| Plastic biological ball filters (bioballs); Pseudomonas putida, Rhodococcus aerolatus, and Aquaspirillum anulus | BTF | Acetone | Trickling liquid rate: 0.1 L/min | 6.3–12.5 s | 51.8–230.4 g/(m3 h) | EC (max): 207.8 g/(m3 h) at ILR of 230.4 g/(m3 h) | SSA: 620 m2/m3. Porosity of PM: 75% | [52] |
| Polypropylene rings; activated sludge | BTF | Styrene | Intermittent nutrient solution spraying (2.5–3 L per min for 15 min every 2 h) | 30–60 s | 13–41 g/(m3 h) | 11.8–31.8 g/(m3 h) | Nominal diameter: 25 mm, SSA: 207 m2/m3, Porosity: 92% | [81] |
| PU pall rings; H2S degraders, Pseudomonas oleovorans DT4, Methylobacterium rhodesianum H13 | BTF | H2S, THF, and DCM | Continuous nutrient solution trickling | 20–50 s | 200, 100, and 100 mg/m3 for H2S, THF, and DCM, respectively | EC (max): 52.5 g/(m3 h) (H2S), 26.7 g/(m3 h) (THF), 17.2 g/(m3 h) (DCM) | SSA: 350 m2/m3; Porosity: 91.3% | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danila, V.; Zagorskis, A.; Januševičius, T. Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review. Processes 2022, 10, 1304. https://doi.org/10.3390/pr10071304
Danila V, Zagorskis A, Januševičius T. Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review. Processes. 2022; 10(7):1304. https://doi.org/10.3390/pr10071304
Chicago/Turabian StyleDanila, Vaidotas, Alvydas Zagorskis, and Tomas Januševičius. 2022. "Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review" Processes 10, no. 7: 1304. https://doi.org/10.3390/pr10071304






