LCA-Based Carbon Footprint Accounting of Mixed Rare Earth Oxides Production from Ionic Rare Earths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbon Footprint Accounting
2.2. Life Cycle Assessment Approach
2.3. System Boundary and Process Unit
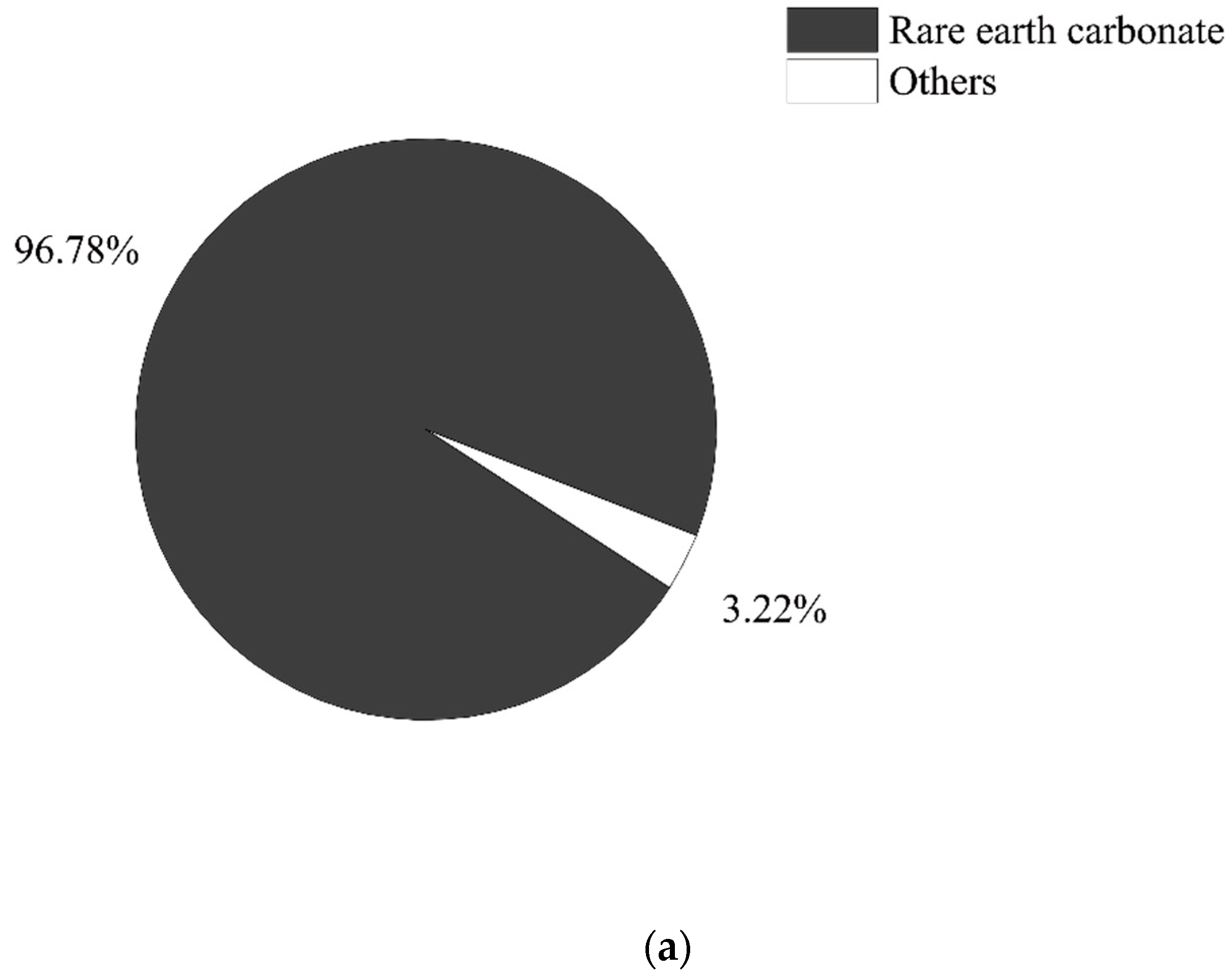
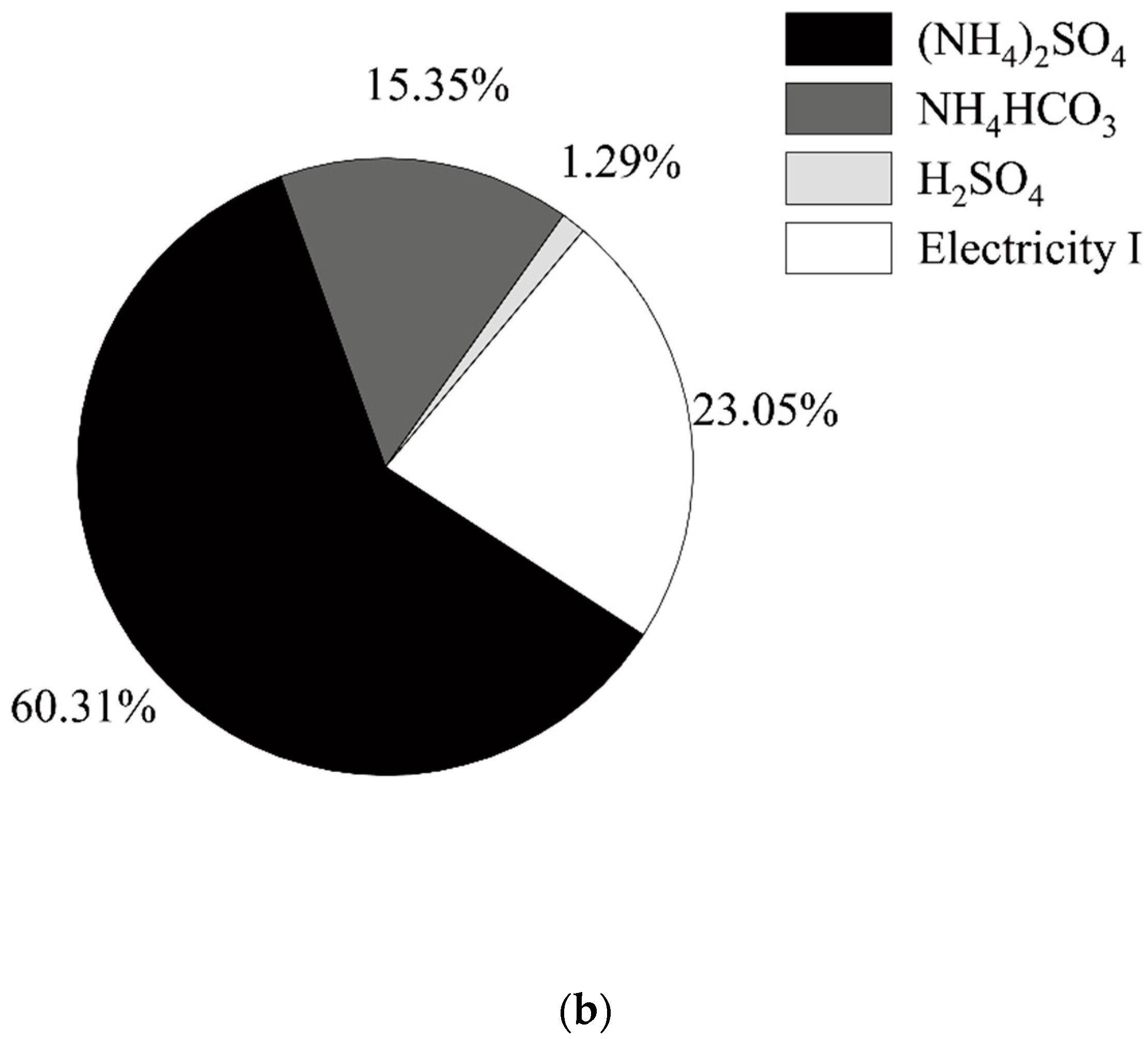
2.4. Data Sources and Assumptions
3. Results
3.1. Carbon Footprint Analysis of Rare Earth Development
3.2. Carbon Footprint Analysis under the Total Mining Control Target from 2012 to 2021
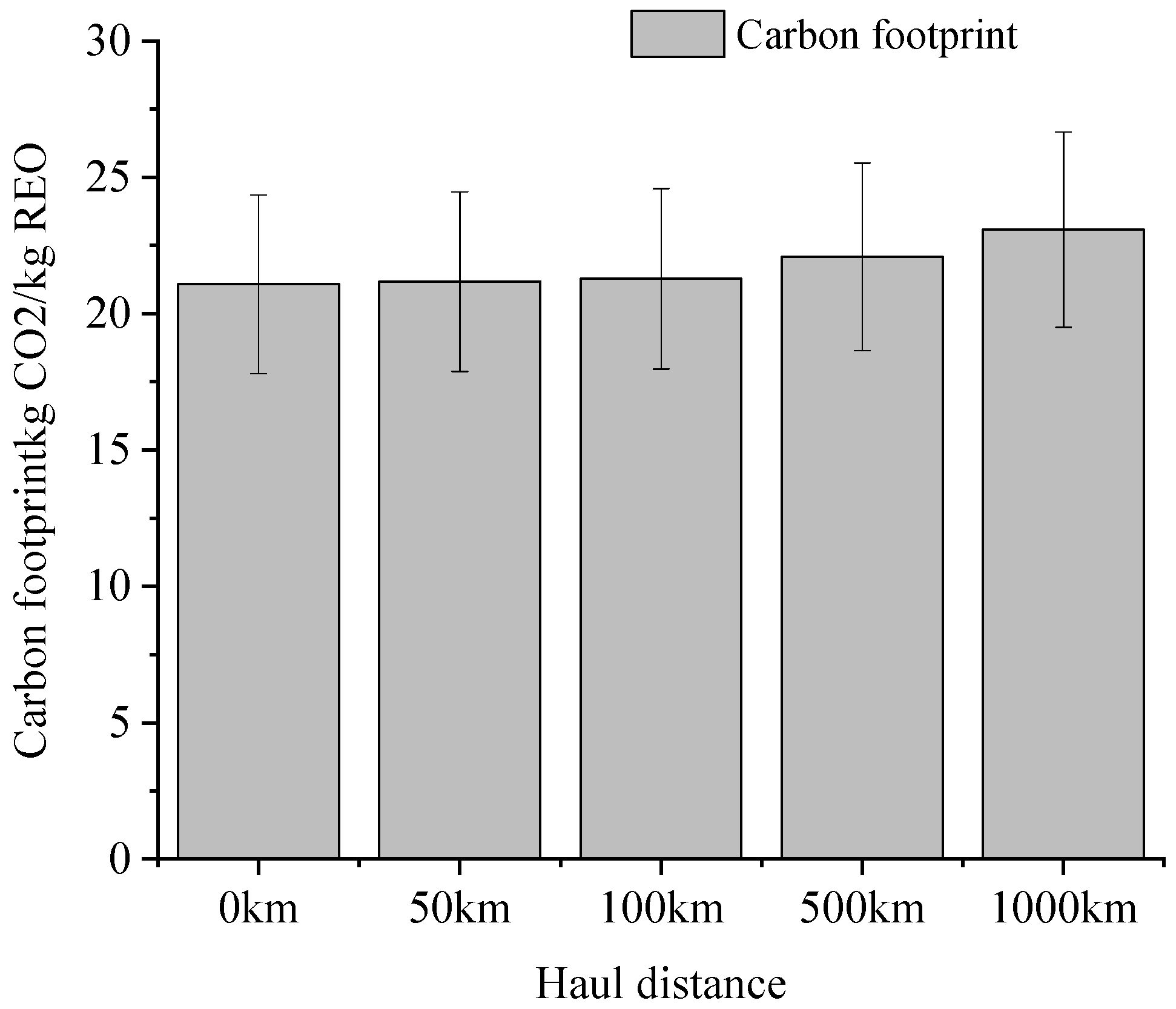
3.3. Effect of Material Transport Distance on Carbon Footprint of Ionic Rare Earth Development
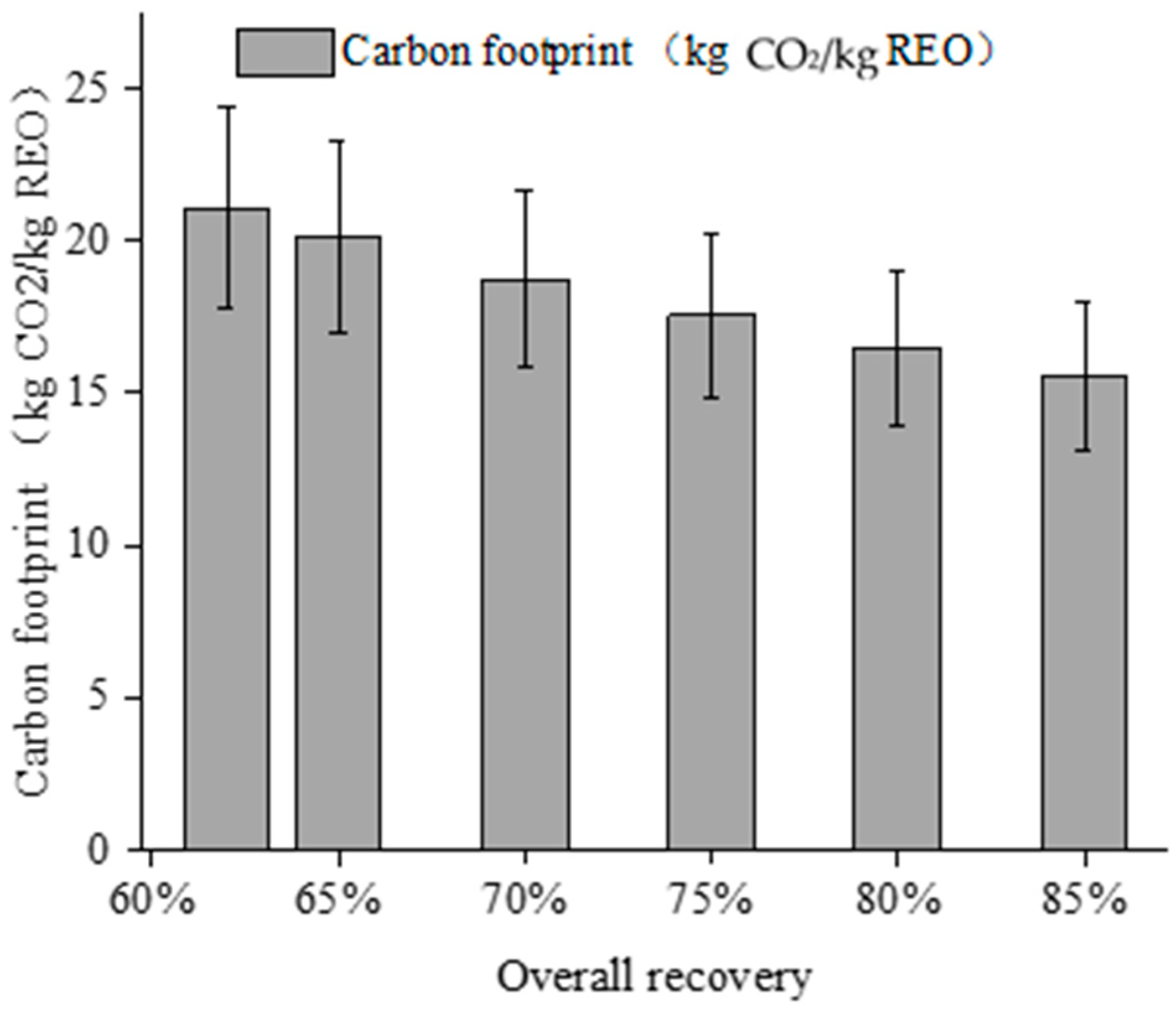
3.4. Effect of Product Recovery on the Carbon Footprint of Ionic Rare Earth Development
3.5. Impact of Technological Change on Carbon Footprint
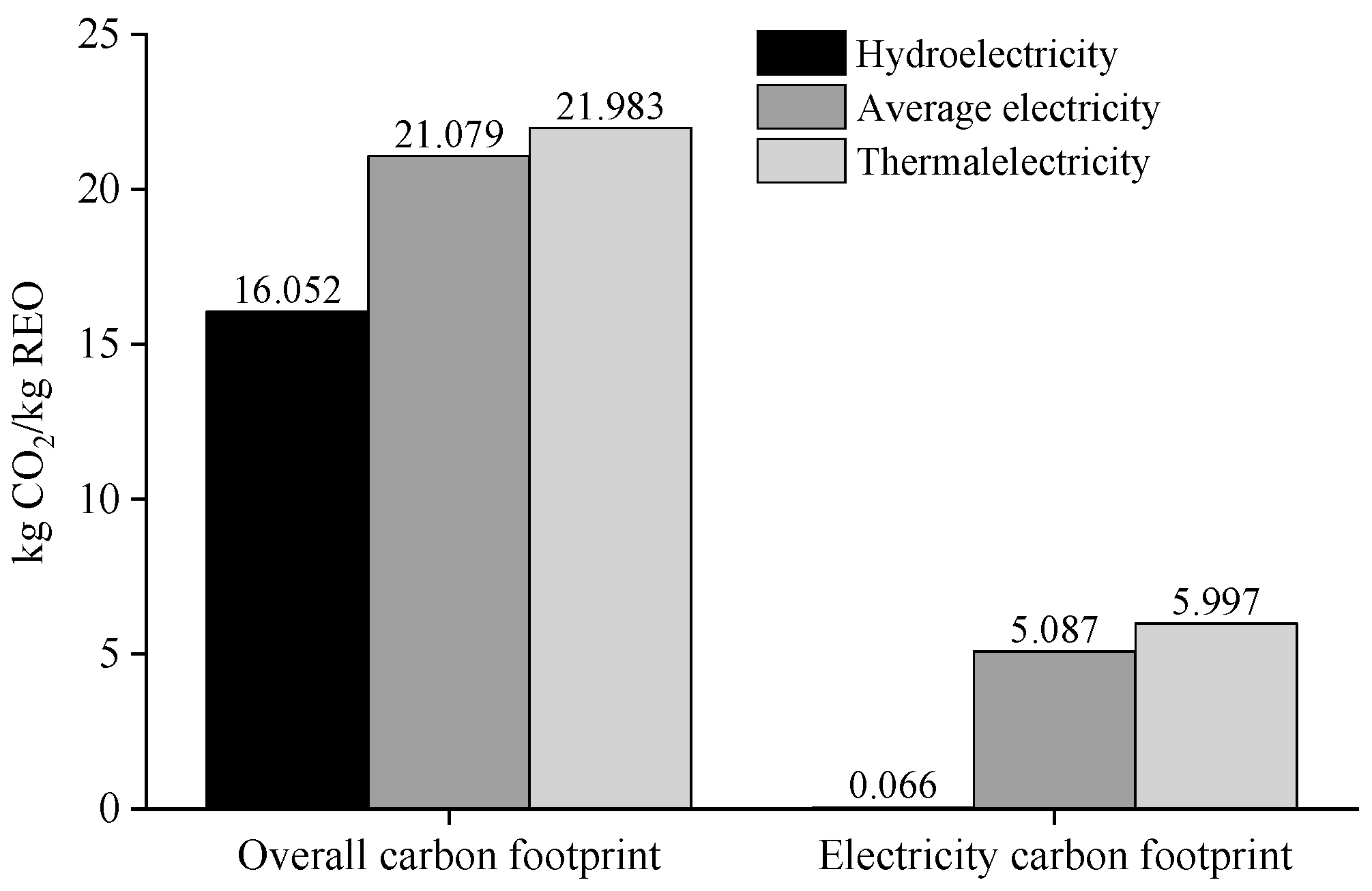
3.6. Impact of Clean Energy Use on Carbon Footprint
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating rare earth element availability: A case with revolutionary demand from clean technologies. Environ. Sci. Technol. 2012, 46, 3406–3414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ning, Y.K.; Cao, F.; Zhang, Y. Current situation of worldwide rare earth resources. Multipurp. Util. Miner. Resour. 2018, 4, 7–12. [Google Scholar]
- USGS (U.S. Geological Survey). Mineral Commodity Summaries (1996–2021) [R/OL]. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-rare-earths.pdf (accessed on 20 April 2022).
- Zheng, G.D.; Wang, K.; Chen, Q.S.; Zhang, Y.F. The change of world rare earth industrial structure and the problems faced by China’s rare earth industry. Acta Geosci. Sin. 2021, 42, 265–272. [Google Scholar]
- Lee, J.C.K.; Wen, Z.G. Pathways for greening the supply of rare earth elements in China. Nat. Sustain. 2018, 1, 598–605. [Google Scholar] [CrossRef]
- Wang, Z. Research on the development of China’s rare earth resources and environment protection. J. Zhejiang Univ. Tech. Soc. Sci. 2015, 14, 13–17. [Google Scholar]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Tian, S.; Li, K.; Møller, P.; Ying, S.C.; Wang, L.; Li, Z.; Roursgaard, M.; Liang, T. Assessment of reactive oxygen species production and genotoxicity of rare earth mining dust: Implications for public health and mining management. Sci. Total Environ. 2020, 740, 139759. [Google Scholar] [CrossRef]
- Ma, G.; Wang, X.; Yu, F.; Wu, Q. Evaluation of environmental costs of rare earth exploitation and analysis of their spatial difference characteristics in China. Res. Environ. Sci. 2017, 30, 817–824. [Google Scholar]
- Xu, F.; Li, H.; Wang, X. Progress in environmental problems and cost assessment of Ion-adsorption rare earth mining. Chin. Rare Earths 2020, 41, 119–127. [Google Scholar]
- Marx, J.; Schreiber, A.; Zapp, P.; Walachowicz, F. Comparative life cycle assessment of NdFeB permanent magnet production from different rare earth deposits. ACS Sustain. Chem. Eng. 2018, 6, 5858–5867. [Google Scholar] [CrossRef]
- Hu, A.H.; Kuo, C.H.; Huang, L.H.; Su, C.C. Carbon footprint assessment of recycling technologies for rare earth elements: A case study of recycling yttrium and europium from phosphor. Waste Manag. 2017, 60, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Li, C.; Yang, P.; Lin, J. Recent progress in rare earth micro/nanocrystals: Soft chemical synthesis, luminescent properties, and biomedical applications. Chem. Rev. 2014, 114, 2343–2389. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qin, L.; Wang, G.S.; Luo, S.H.; Long, P.; Peng, C. Status of research on ion adsorption type rare earth leaching mechanism. Chin. J. Rare Earths 2021, 39, 543–554. [Google Scholar]
- Lohmann, P.M.; Gsottbauser, E.; Doherty, A.; Kontoleon, A. Do carbon footprint labels promote climatarian diets? Evidence from a large-scale field experiment. J. Environ. Econ. Manag. 2022, 114, 102693. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, J.; Dong, H. Analysis of carbon footprint and reduction approach of magnesia production in China. J. Clean. Prod. 2022, 334, 130194. [Google Scholar] [CrossRef]
- Bailey, G.; Joyce, P.J.; Schrijvers, D.; Schulze, R. Review and new life cycle assessment for rare earth production from bastnsite, ion adsorption clays and lateritic monazite. Resour. Conserv. Recycl. 2020, 155, 104675. [Google Scholar] [CrossRef]
- Vahidi, E.; Navarro, J.; Zhao, F. An initial life cycle assessment of rare earth oxides production from ion-adsorption clays. Resour. Conserv. Recycl. 2016, 113, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Wen, Z. Rare earths from mines to metals: Comparing environmental impacts from China’s main production pathways. J. Ind. Ecol. 2017, 21, 1277–1290. [Google Scholar] [CrossRef]
- Schulze, R.; Abbasalizadeh, A.; Bulach, W.; Schebek, L.; Buchert, M. An ex-ante LCA study of rare earth extraction from NdFeB magnet scrap using molten salt electrolysis. J. Sustain. Met. 2018, 4, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Wiedmann, T.; Barrett, J. A Review of the ecological footprint indicator-perceptions and methods. Sustainability 2010, 2, 1645–1693. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Su, J.; Xi, B.; Yu, Y.; Ji, D.; Sun, Y.; Cui, C.; Zhu, J. Life cycle assessment of lithium-ion batteries for greenhouse gas emissions. Resour. Conserv. Recycl. 2016, 117, 285–293. [Google Scholar] [CrossRef]
- Vahidi, E.; Fu, Z. Environmental life cycle assessment on the separation of rare earth oxides through solvent extraction. J. Environ. Manag. 2017, 203, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Pryshlakivsky, J.; Searcy, C. Life cycle assessment as a decision-making tool: Practitioner and managerial considerations. J. Clean. Prod. 2021, 309, 127344. [Google Scholar] [CrossRef]
- Pryshlakivsky, J.; Searcy, C. Fifteen years of ISO 14040: A review. J. Clean. Prod. 2013, 57, 115–123. [Google Scholar] [CrossRef]
- Guo, Z.; Jin, J.; Zhao, K. Current status of ion adsorption-based rare earth mining process and theory research. Rare Earths 2018, 39, 132–141. [Google Scholar]
- Li, C. Application of new in-situ leaching process in ionic rare earth ore. Nonferrous Met. Sci. Eng. 2011, 2, 63–67. [Google Scholar]
- Yi., L.; Zheng, M. Assessment of the effect of the total rare earth mining control policy in China. Nonferrous Met. Sci. Eng. 2021, 12, 120–126. [Google Scholar]
- Wang, G.; Xie, F.; Luo, S.; Peng, C.; Qin, L.; Hong, B.; Yao, K. Ionic rare earth leaching and ammonia-nitrogen residual pattern under empirical injection of in-situ leaching ore. Chin. J. Nonferrous Met. 2020, 30, 1454–1465. [Google Scholar]



| List Inventory | Unit | Quantity | Specifications | |
|---|---|---|---|---|
| Inputs | Ammonium sulfate (NH4)2 SO4 | kg | 6.00 | Technical pure |
| Ammonium bicarbonate NH4 HCO3 | kg | 4.00 | Technical pure | |
| Sulfuric acid H2 SO4 | kg | 0.60 | 98% | |
| Water | m3 | 0.25 | River water | |
| Electricity | kW·h | 5.00 | National average electricity | |
| Outputs | Ammonia nitrogen | kg | 0.61 | / |
| Cd | kg | 1.32 × 10−3 | / | |
| Pd | kg | 0.13 | / | |
| As | kg | 2.83 × 10−5 | / | |
| Hg | kg | 4.68 × 10−6 | / | |
| NH4+ | kg | 0.61 | / | |
| Inventory | Unit | Quantity | Specifications | |
|---|---|---|---|---|
| Inputs | Magnesium sulfate heptahydrate MgSO4·7H2O | kg | 1.60 | Technical pure |
| Magnesium oxide MgO | kg | 1.08 | Technical pure | |
| Sulfuric acid H2SO4 | kg | 0.65 | 98% | |
| Water | m3 | 1.73 | River water | |
| Electricity | kW·h | 5.00 | National average electricity | |
| Outputs | SO42− | kg | 0.91 | / |
| Inventory | Unit | Quantity | Specifications | |
|---|---|---|---|---|
| Inputs | Tap water | m3 | 6.13 × 10−4 | / |
| Electricity | kW h | 4.12 × 10−1 | / | |
| Natural gas | Nm3 | 2.59 × 10−1 | 98% | |
| Outputs | Water vapor | kg | 3.84 | / |
| Carbon dioxide CO2 | kg | 2.20 × 10−1 | / | |
| Ammonia NH4 | kg | 1.27 × 10−3 | / | |
| Particulate matter | kg | 3.52 × 10−4 | / | |
| Dust | kg | 4.16 × 10−5 | / | |
| Name of Indicator | Abbreviation (Unit) | LCA Result | Uncertainty | Upper and Lower Limits (95% Confidence Interval) |
|---|---|---|---|---|
| Global Warming Potential | GWP (kg CO2 eq) | 21.08 | 15.54% | [17.8~24.3] |
| Name of Indicator | Abbreviation (Unit) | LCA Result | Uncertainty | Upper and Lower Limits (95% Confidence Interval) |
|---|---|---|---|---|
| Global Warming Potential | GWP (kg CO2 eq) | 62.5 | 18.17% | [51.1~73.8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, C.; Zhou, D.; Xue, B. LCA-Based Carbon Footprint Accounting of Mixed Rare Earth Oxides Production from Ionic Rare Earths. Processes 2022, 10, 1354. https://doi.org/10.3390/pr10071354
Wan C, Zhou D, Xue B. LCA-Based Carbon Footprint Accounting of Mixed Rare Earth Oxides Production from Ionic Rare Earths. Processes. 2022; 10(7):1354. https://doi.org/10.3390/pr10071354
Chicago/Turabian StyleWan, Chen, Dan Zhou, and Bing Xue. 2022. "LCA-Based Carbon Footprint Accounting of Mixed Rare Earth Oxides Production from Ionic Rare Earths" Processes 10, no. 7: 1354. https://doi.org/10.3390/pr10071354






