Use of Typical Wastes as Biochars in Removing Diethyl Phthalate (Det) from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Collection and Processing
2.2. Biomass Pyrolysis and Experimental Process
2.3. Physico-Chemical Characterization
2.4. Surface Area and Porosity
2.5. Morphology and Functional Groups Analysis
2.6. Adsorption and Desorption Experiments
2.7. Analysis of DEP
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Feedstocks
3.2. Characteristics of Biochars
3.2.1. Mass Yield, Proximate and Elemental Composition
3.2.2. Surface Morphology
3.2.3. Change in Surface Functional Groups (FTIR Spectroscopy)
3.2.4. pH, Electric Conductivity, Surface Area, Porosity
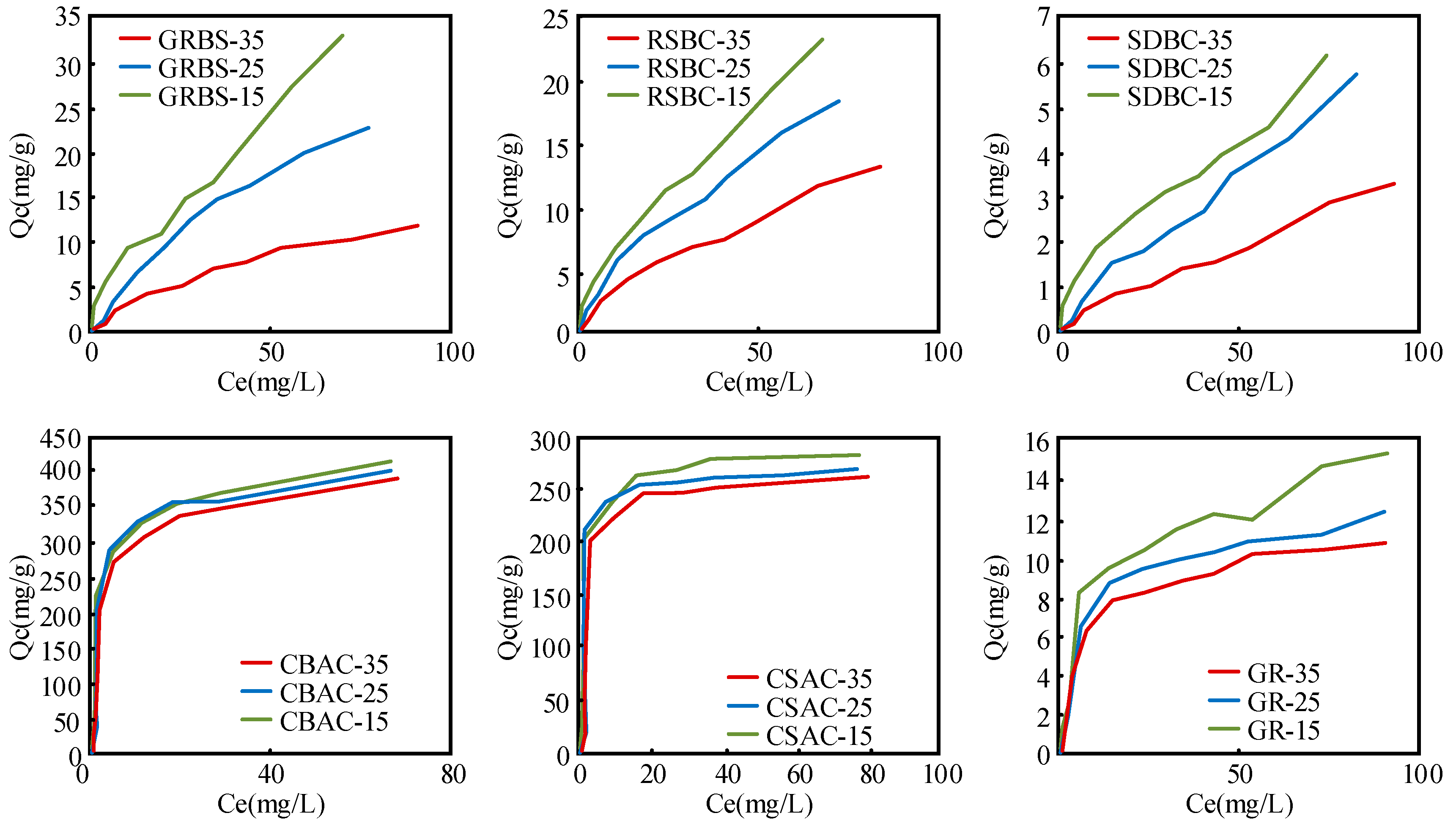
3.3. Adsorption Isotherms of DEP on Biochars
3.4. Effect of pH, Ionic Strength, and Temperature on Adsorption
3.5. Adsorption and Desorption Kinetics
3.6. Adsorption Mechanisms
3.7. Comparison of Adsorption Effect
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shin, H.M.; Dhar, U.; Calafat, A.M.; Nguyen, V.; Schmidt, R.J.; Hertz-Picciotto, I. Temporal trends of exposure to phthalates and phthalate alternatives in california pregnant women during 2007–2013: Comparison with other populations. Environ. Sci. Technol. 2020, 54, 13157–13166. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Van Hecke, K.; Danish, M.; Gaoke, Z.; Verpoort, F. Development of Mixed metal Metal-organic polyhedra networks, colloids, and MOFs and their Pharmacokinetic applications. Sci. Rep. 2017, 7, 832. [Google Scholar] [CrossRef] [Green Version]
- Akram, B.; Ahmad, K.; Khan, J.; Khan, B.A.; Akhtar, J. Low-temperature solution-phase route to sub-10 nm titanium oxide nanocrystals having super-enhanced photoreactivity. N. J. Chem. 2018, 42, 10947–10952. [Google Scholar] [CrossRef]
- Zeng, F.; Cui, K.; Xie, Z.; Wu, L.; Liu, M.; Sun, G.; Lin, Y.; Luo, D.; Zeng, Z. Phthalate esters (PAEs): Emerging organic contaminants in agricultural soils in peri-urban areas around Guangzhou, China. Environ. Pollut. 2008, 156, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.W.; Wen, Z.D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, K.; Zeng, F.; Chen, L.; Cheng, Y.; Li, H.; Li, S.; Zhou, X.; Zhu, F.; Ouyang, G.; et al. Occurrence and distribution of phthalate esters in riverine sediments from the Pearl River Delta region, South China. Mar. Pollut. Bull. 2014, 83, 358–365. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Zhang, H.H.; Zhang, J.; Wang, Q.W.; Yang, G.P. Occurrence, distribution, and ecological risks of phthalate esters in the seawater and sediment of Changjiang River Estuary and its adjacent area. Sci. Total Env. 2018, 619–620, 93–102. [Google Scholar] [CrossRef]
- Marx, J.L. Phthalic acid esters: Biological impact uncertain. Science 1972, 178, 46–47. [Google Scholar] [CrossRef]
- Meng, X.-Z.; Wang, Y.; Xiang, N.; Chen, L.; Liu, Z.; Wu, B.; Dai, X.; Zhang, Y.-H.; Xie, Z.; Ebinghaus, R. Flow of sewage sludge-borne phthalate esters (PAEs) from human release to human intake: Implication for risk assessment of sludge applied to soil. Sci. Total Environ. 2014, 476, 242–249. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.; Paull, G.C.; Van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Wu, X.; Gan, J. Uptake and metabolism of phthalate esters by edible plants. Environ. Sci. Technol. 2015, 49, 8471–8478. [Google Scholar] [CrossRef]
- Hung, C.H.; Yuan, C.; Li, H.W. Photodegradation of diethyl phthalate with PANi/CNT/TiO2 immobilized on glass plate irradiated with visible light and simulated sunlight-effect of synthesized method and pH. J. Hazard. Mater. 2017, 322, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.P.; Zhao, X.K.; Sun, X.J.; Lu, X.L. Oxidative degradation of diethyl phthalate by photochemically-enhanced Fenton reaction. J. Hazard. Mater. 2005, 126, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Roslev, P.; Vorkamp, K.; Aarup, J.; Frederiksen, K.; Nielsen, P.H. Degradation of phthalate esters in an activated sludge wastewater treatment plant. Water Res. 2007, 41, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Z.; Wang, J.; Wang, X.; Niu, X.; Wang, Y.; Shen, Y.; Teng, W.; Fan, J.; Zhang, W.-X. Synthesis of mesoporous silica-carbon microspheres via self-assembly and in-situ carbonization for efficient adsorption of Di-n-butyl phthalate. Chem. Eng. J. 2019, 369, 854–862. [Google Scholar] [CrossRef]
- Liang, D.W.; Zhang, T.; Fang, H.H. Anaerobic degradation of dimethyl phthalate in wastewater in a UASB reactor. Water Res. 2007, 41, 2879–2884. [Google Scholar] [CrossRef]
- Mesdaghinia, A.; Azari, A.; Nodehi, R.N.; Yaghmaeian, K.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Sharafi, K. Removal of phthalate esters (PAEs) by zeolite/Fe3O4: Investigation on the magnetic adsorption separation, catalytic degradation and toxicity bioassay. J. Mol. Liq. 2017, 233, 378–390. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Sun, K.; Xing, B.S. Adsorption of Dialkyl Phthalate Esters on Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 6985–6991. [Google Scholar] [CrossRef]
- Wu, Y.; Si, Y.; Zhou, D.; Gao, J. Adsorption of diethyl phthalate ester to clay minerals. Chemosphere 2015, 119, 690–696. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Ezra, F.S. Role of surface acidity in the adsorption of organic pollutants on the surface of carbon. Environ. Sci. Technol. 1968, 2, 291–297. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, H.; Luo, Y.; Deng, X.; Herbert, S.; Xing, B. Characterization and influence of biochars on nitrous oxide emission from agricultural soil. Environ. Pollut. 2013, 174, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, J.; Chen, B. Adsorption and desorption of phthalic acid esters on graphene oxide and reduced graphene oxide as affected by humic acid. Environ. Pollut. 2018, 232, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Yang, X.; Chen, H.; Müller, K.; Shaheen, S.M.; Rinklebe, J.; Song, H.; Xu, S.; Wu, F.; Wang, H. Effect of biochar aging and co-existence of diethyl phthalate on the mono-sorption of cadmium and zinc to biochar-treated soils. J. Hazard. Mater. 2021, 408, 124850. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Jin, J.; Keiluweit, M.; Kleber, M.; Wang, Z.; Pan, Z.; Xing, B. Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars. Bioresour. Technol. 2012, 118, 120–127. [Google Scholar] [CrossRef]
- Abdul, G.; Zhu, X.; Chen, B. Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 2017, 319, 9–20. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Erin, N.; Yargicoglu, B.Y.S.; Krishna, R. Reddy, Kurt Spokas. Physical and chemical characterization of waste wood derived biochars. Waste Manag. 2015, 36, 256–268. [Google Scholar]
- Décima, M.A.; Marzeddu, S.; Barchiesi, M.; Di Marcantonio, C.; Chiavola, A.; Boni, M.R. A Review on the Removal of Carbamazepine from Aqueous Solution by Using Activated Carbon and Biochar. Sustainability 2021, 13, 11760. [Google Scholar] [CrossRef]
- Marzeddu, S.; Cappelli, A.; Ambrosio, A.; Décima, M.A.; Viotti, P.; Boni, M.R. A Life Cycle Assessment of an Energy-Biochar Chain Involving a Gasification Plant in Italy. Land 2021, 10, 1256. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L.; Huang, Z.; Liu, J.; Xu, Y.; Li, R.; Zhang, M.; Hong, H.; Lin, H. A novel in-situ micro-aeration functional membrane with excellent decoloration efficiency and antifouling performance. J. Mebrane Sci. 2022, 641, 119925. [Google Scholar] [CrossRef]
- Yu, F.; Cheng, Y.J.; Xing, S.Y.; Wang, Y.J.; Ma, J. A comprehensive review on flow-electrode capacitive deionization: Design, active material and environmental application. Sep. Purif. Technol. 2022, 281, 119870. [Google Scholar] [CrossRef]
- Fan, X.L.; Cai, J.; Zhong, M.; Bian, Y.R.; Jiang, X. Mechanistic insights into primary biotransformation of diethyl phthalate in earthworm and significant SOD inhibitory effect of esterolytic products. Chemosphere 2022, 288, 132491. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, H.X.; Guan, Y.B.; Ma, M.; Rennenberg, H. The symbiotic system of sulfate-reducing bacteria and clay-sized fraction of purplish soil strengthens cadmium fixation through iron-bearing minerals. Sci. Total Environ. 2022, 820, 153253. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Liu, Y.; Xu, Y.C.; Li, R.J.; Hong, H.C.; Shen, L.G.; Lin, H.J. Facile synthesis of 2D TiO2@MXene composite membrane with enhanced separation and antifouling performance. J. Membr. Sci. 2021, 640, 119854. [Google Scholar] [CrossRef]
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Munir, M.A.M.; Liu, R. Contrasting effects of operating conditions and biomass particle size on bulk characteristics and surface chemistry of rice husk derived-biochars. J. Anal. Appl. Pyrolysis 2018, 134, 281–292. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Garcia-Delgado, R.A.; Cotoruelo-Minguez, L.M.; Rodriguez, J.J. Equilibrium Study of Single-Solute Adsorption of Anionic Surfactants with Polymeric XAD Resins. Sep. Sci. Technol. 2006, 27, 975–987. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Jafri, N.; Wong, W.Y.; Doshi, V.; Yoon, L.W.; Cheah, K.H. A review on production and characterization of biochars for application in direct carbon fuel cells. Process Saf. Environ. Prot. 2018, 118, 152–166. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrolysis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Sohi, S.P.; Brownsort, P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2013, 5, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Aghoghovwia, M.P.; Hardie, A.G.; Rozanov, A.B. Characterisation, adsorption and desorption of ammonium and nitrate of biochar derived from different feedstocks. Environ. Technol. 2020, 43, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Ruiz, M.P.; Millera, Á.; Alzueta, M.U.; Bilbao, R. Characterization of Biomass Chars Formed under Different Devolatilization Conditions: Differences between Rice Husk and Eucalyptus. Energy Fuels 2008, 17, 1275–1284. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Zhao, J.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar]
- Wang, H.; Fang, C.; Wang, Q.; Chu, Y.; Song, Y.; Chen, Y.; Xue, X. Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv. 2018, 8, 16260–16268. [Google Scholar] [CrossRef] [Green Version]
- Paethanom, A.; Yoshikawa, K. Influence of pyrolysis temperature on rice husk char characteristics and its tar adsorption capability. Energies 2012, 5, 4941–4951. [Google Scholar]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal-organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef]
- Xing, B.; Pignatello, J.J.; Gigliotti, B. Competitive Sorption between Atrazine and Other Organic Compounds in Soils and Model Sorbents. Environ. Sci. Technol. 1996, 31, 2432–2440. [Google Scholar]
- Mohan, S.V.; Shailaja, S.; Krishna, M.R.; Sarma, P.N. Adsorptive removal of phthalate ester (Di-ethyl phthalate) from aqueous phase by activated carbon: A kinetic study. J. Hazard. Mater. 2007, 146, 278–282. [Google Scholar] [CrossRef]
- Shaida, M.A.; Dutta, R.K.; Sen, A.K. Removal of diethyl phthalate via adsorption on mineral rich waste coal modified with chitosan. J. Mol. Liq. 2018, 261, 271–282. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.K.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 degrees C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Jung, B.K.; Hasan, Z.; Jhung, S.H. Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal-organic frameworks. J. Hazard. Mater. 2015, 282, 194–200. [Google Scholar] [CrossRef]
- Ayranci, E.; Bayram, E. Adsorption of phthalic acid and its esters onto high-area activated carbon-cloth studied by in situ UV-spectroscopy. J. Hazard. Mater. 2005, 122, 147–153. [Google Scholar] [CrossRef]
- Cho, H.H.; Huang, H.; Schwab, K. Effects of solution chemistry on the adsorption of ibuprofen and triclosan onto carbon nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.K.; Xiao, S.C.; Yuan, J.H.; Zhao, A.Z. Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresour. Technol. 2011, 102, 10293–10298. [Google Scholar] [CrossRef] [PubMed]
- Means, J.C. Influence of salinity upon sediment-water partitioning of aromatic hydrocarbons. Mar. Chem. 1995, 51, 3–16. [Google Scholar] [CrossRef]
- Turner, A.; Rawling, M.C. The influence of salting out on the sorption of neutral organic compounds in estuaries. Water Res. 2001, 35, 4379–4389. [Google Scholar] [CrossRef]
- Oh, S.; Wang, Q.; Shin, W.S.; Song, D.-I. Effect of salting out on the desorption-resistance of polycyclic aromatic hydrocarbons (PAHs) in coastal sediment. Chem. Eng. J. 2013, 225, 84–92. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.; Karanfil, T. Adsorption of synthetic organic chemicals by carbon nanotubes: Effects of background solution chemistry. Water Res. 2010, 44, 2067–2074. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Z.; Pan, B.; Hong, C.; Jia, K.; Jiang, P.; Zhang, Q.; Pan, B. Equilibrium and heat of adsorption of diethyl phthalate on heterogeneous adsorbents. J. Colloid Interface Sci. 2008, 325, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Huang, F.; Chen, W.; Xing, B.; Zhu, L. Sorption of apolar and polar organic contaminants by waste tire rubber and its chars in single- and bi-solute systems. Environ. Pollut. 2011, 159, 850–857. [Google Scholar] [CrossRef] [PubMed]
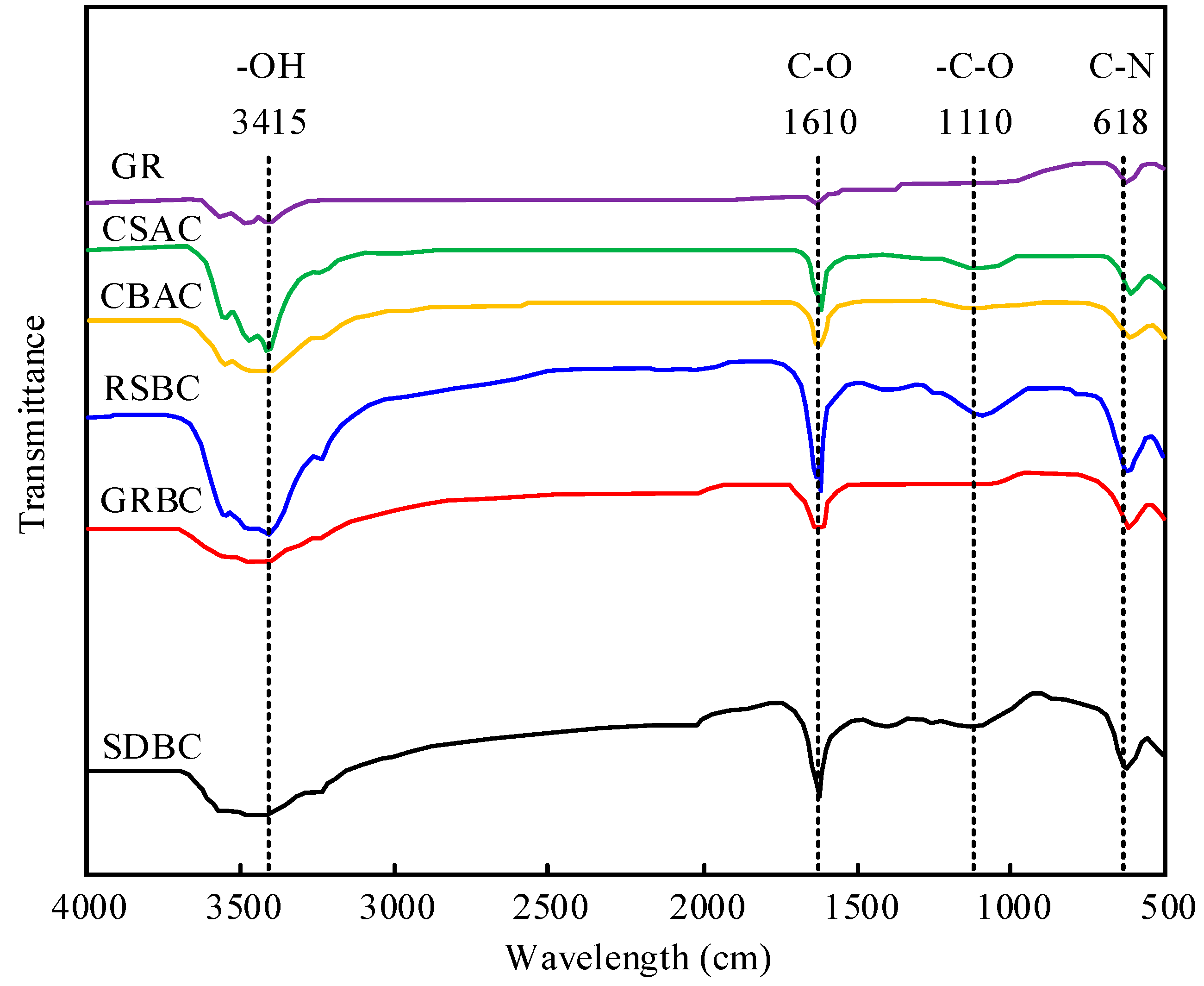


| / | Raw SD | SDBC | Raw GR | GRBC | Raw RS | RSBC | CSAC | CBAC | GR |
|---|---|---|---|---|---|---|---|---|---|
| Yield (%) | -- | 44.26 | -- | 36.58 | -- | 43.7 | -- | -- | -- |
| C (%) | 47.5 | 68.2 | 48.38 | 77.01 | 38.17 | 51.7 | 89.69 | 86.01 | 98.13 |
| H (%) | 0.4 | 2.85 | 4.95 | 2.36 | 5.43 | 2.62 | 0.6 | 0.89 | 0.52 |
| N (%) | 4.32 | 1.28 | 0.34 | 0.12 | 0.64 | 0.6 | 0.01 | 0.19 | 0.3 |
| S (%) | 0.21 | 0.01 | 0.01 | 0.01 | 0.06 | 0.07 | 0.04 | 0.01 | 0.02 |
| O (%) | 10.1 | 4.19 | 39.76 | 9.64 | 52.13 | 14.3 | 7.36 | 2.56 | 0.21 |
| Moisture | 2.41 | 1.23 | 7.5 | 3.2 | 6.43 | 2.92 | -- | -- | -- |
| Ash | 0.7 | 23.47 | 4.87 | 9.85 | 14.23 | 30.6 | 2.31 | 10.34 | 0.84 |
| VM | 94.8 | 69.52 | 68.12 | 14.17 | 73.22 | 21.4 | -- | -- | -- |
| FC | 2.01 | 5.78 | 19.51 | 72.78 | 6.12 | 44.9 | -- | -- | -- |
| H/C | 0.09 | 0.4 | 1.13 | 0.34 | 1.69 | 0.6 | 0.07 | 0.01 | 0.05 |
| O/C | 0.15 | 0.04 | 0.59 | 0.14 | 1.02 | 0.2 | 0.082 | 0.03 | 0.02 |
| (O + N)/C | 0.7 | 0.27 | 0.62 | 0.24 | 1.07 | 0.24 | 0.083 | 0.03 | 0.05 |
| pH | 6.1 | 8.81 | 5.68 | 9.59 | 6.56 | 9.72 | 10.15 | 9.11 | 5.19 |
| EC (dS m−1) | 0.81 | 0.89 | 36.1 | 2.02 | 2.11 | 11.8 | -- | -- | -- |
| / | Raw SD | SDBC | Raw GR | GRBC | Raw RS | RSBC | CSAC | CBAC | GR |
|---|---|---|---|---|---|---|---|---|---|
| SBETβ (m2/g) | 11.07 | 7.35 | 49.63 | 6.74 | 36.53 | 549.90 | 798.50 | 35.11 | |
| Smicro | -- | 2.72 | -- | 30.63 | -- | 15.87 | 773.80 | 934.60 | 35.76 |
| Smeso | -- | 6.64 | -- | 36.71 | -- | 10.23 | 52.68 | 78.01 | 24.05 |
| Vtot (cm3/g) | -- | 0.01 | -- | 0.05 | -- | 0.02 | 0.34 | 0.45 | 0.05 |
| Vmicro | -- | 0.01 | -- | 0.01 | -- | 0.02 | 0.28 | 0.33 | 0.01 |
| Vmeso | -- | 0.01 | -- | 0.05 | -- | 0.02 | 0.07 | 0.11 | 0.04 |
| Davg (nm) | -- | 4.83 | -- | 4.65 | -- | 5.23 | 2.50 | 2.27 | 5.30 |
| Dmicro | -- | 2.38 | -- | 1.56 | -- | 1.96 | 1.41 | 1.30 | 2.35 |
| Samples | Langmuir Model | Freundlich Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qm (mg g−1) | KL (L mg−1) | Radj2 | Kd (L g−1) | KF (mg g−1 (mg L−1)-N) | Nη | Radj2 | Kd | |||||
| Ce = 0.5 mg L−1 | Ce = 5 mg L−1 | Ce = 50 mg L−1 | Ce = 0.5 mg L−1 | Ce = 5 mg L−1 | Ce = 50 mg L−1 | |||||||
| SDBC | 18.39 | 0.53 | 0.98 | 0.09 | 0.09 | 0.07 | 0.17 | 0.79 | 0.98 | 0.20 | 0.12 | 0.08 |
| GRBC | 46.04 | 0.01 | 0.99 | 0.60 | 0.57 | 0.36 | 1.13 | 0.71 | 0.99 | 1.38 | 0.70 | 0.36 |
| RSBC | 31.54 | 0.01 | 0.97 | 0.57 | 0.53 | 0.30 | 1.30 | 0.62 | 0.99 | 1.69 | 0.71 | 0.29 |
| CSAC | 261.04 | 6.96 | 0.96 | 406.01 | 50.70 | 5.21 | 186.01 | 0.10 | 0.84 | 348.00 | 43.60 | 5.46 |
| CBAC | 359.58 | 1.98 | 0.80 | 358.01 | 65.30 | 7.12 | 215.01 | 0.15 | 0.98 | 387.00 | 55.10 | 7.84 |
| GR | 10.94 | 0.37 | 0.93 | 3.42 | 1.42 | 0.21 | 4.42 | 0.22 | 0.96 | 7.57 | 1.27 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Z.; Bi, X.; Jia, H. Use of Typical Wastes as Biochars in Removing Diethyl Phthalate (Det) from Water. Processes 2022, 10, 1369. https://doi.org/10.3390/pr10071369
Chai Z, Bi X, Jia H. Use of Typical Wastes as Biochars in Removing Diethyl Phthalate (Det) from Water. Processes. 2022; 10(7):1369. https://doi.org/10.3390/pr10071369
Chicago/Turabian StyleChai, Zichun, Xianshuang Bi, and Hongbai Jia. 2022. "Use of Typical Wastes as Biochars in Removing Diethyl Phthalate (Det) from Water" Processes 10, no. 7: 1369. https://doi.org/10.3390/pr10071369





