Extraction of Oils and Phytochemicals from Camellia oleifera Seeds: Trends, Challenges, and Innovations
Abstract
:1. Introduction
2. Components of Camellia Seeds
3. Composition of Camellia Seed Oil
3.1. Fatty Acid Composition
3.2. Unsaponifiable Components
| Major Unsaponifiable Matters | Content (mg/kg) | Major Biological Effects | Reference | |
|---|---|---|---|---|
| Squalene | 122.02–751.64 | Anti-oxidant, anti-inflammatory, anti-atherosclerotic, immunity elevation, anti-neoplastic | [7,23] | |
| Sterols | β-amyrin | 607.24–1189.29 | Cardiovascular disease prevention, anti-obesity, anti-diabetic, anti-microbial, anti-inflammatory, immunomodulatory, anti-cancer | [7,24,25] |
| lupeol | 214.64–549.51 | |||
| β-sitosterol | 106.96–293.72 | |||
| cycloartenol | 578.87–1173.90 | |||
| stigmast-7-en-3-ol | 269.73–533.33 | |||
| betulin | 165.57–640.75 | |||
| lanosterol | 715.19–1418.46 | |||
| Tocopherols | α-tocopherol | 115.86–204.60 | Anti-oxidant, anti-inflammatory, anti-cancer, cardiovascular disease prevention | [21,26] |
| γ-tocopherol | 4.53–10.18 | |||
| δ-tocopherol | 0.03–2.86 | |||
| Carotenoids | lycopene | 0.17–10.00 | Anti-oxidant, pro-vitamin A function, improved cognitive function and cardiovascular health, anti-cancer | [27,28] |
| β-carotene | 0.36–21.00 | |||
| lutein | 0.00–1.60 | |||
| Polyphenols | benzoic acid | 2.95–18.87 | Anti-inflammatory, anti-oxidant, anti-tumor, cardiovascular regulation | [29,33,34] |
| p-hydroxybenzoic acid | 0.83–22.56 | |||
| cinnamic acid | 3.72–16.69 | |||
| catechin | 0.62–2.17 | |||
| naringenin | 0.16–6.10 | |||
3.3. Volatile Components
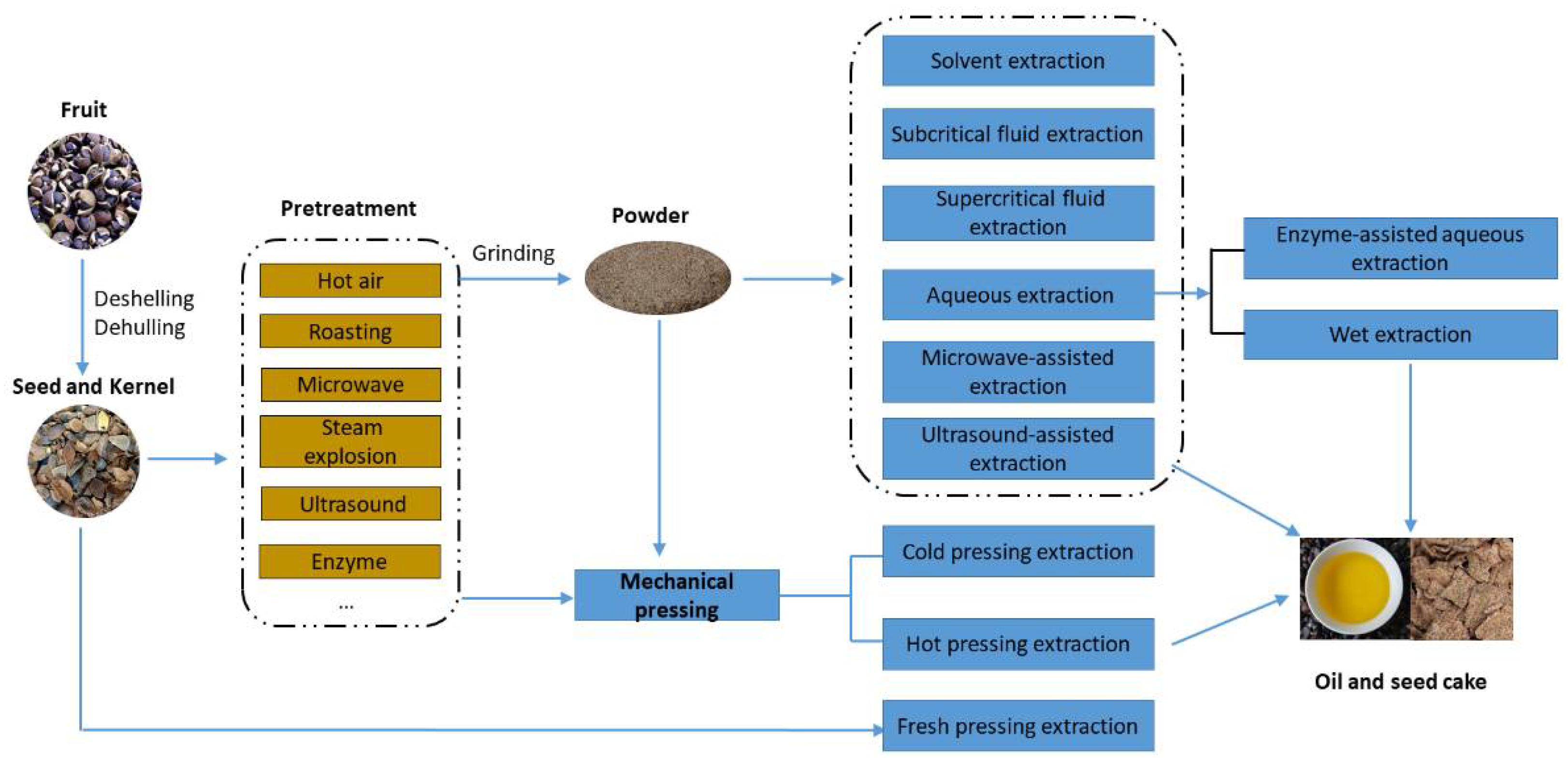
4. Pretreatment Methods
4.1. Drying of Camellia Seeds
4.2. Dehulling of Camellia Seeds
4.3. Roasting or Baking
4.4. Microwave Puffing Pretreatment
4.5. Other Pretreatment Methods
5. Extraction Methods
5.1. Mechanical Pressing Extraction
5.2. Fresh Pressing Extraction
5.3. Solvent Extraction
5.4. Aqueous Extraction Process
5.5. Wet Extraction
5.6. Enzyme-Assisted Aqueous Extraction (EAE)
5.7. Supercritical Fluid Extraction
5.8. Subcritical Fluid Extraction
5.9. Physical Field Enhanced Extraction
5.9.1. Ultrasound-Assisted Extraction
5.9.2. Microwave-Assisted Extraction
6. Effects of Extraction Processes
6.1. Effects on Nutritional Quality
6.2. Effects on Sensory Quality
7. Byproducts Extraction and Utilization
| Products | Extraction Method and Condition | Yield | Reference |
|---|---|---|---|
| Tea saponins | Continuous extraction: 85% ethanol, sample-to-solvent ratio:1:2, 65 °C, 120 min | 86.74% | [152] |
| Single-stage extraction: water, sample-to-solvent ratio: 1:16, 80 °C, 85 min | ~40% | [153] | |
| Continuous extraction: water, sample-to-solvent ratio: 1:16, 80 °C, 60 min | ~90% | [153] | |
| MAE: 300 W, 70% ethanol, sample-to-solvent ratio: 1:7, 30 s | 83% | [66] | |
| SCFE: 30 MPa, volume fraction of entrainer ethanol 75%, 45 °C, 3.5 h | 89.21% | [154] | |
| Tea saponins and oil | 95% n-butanol, extracting times: 4, sample-to-solvent ratio: 1:1.36, 80 °C, 2.57 h | 92.88% (oil) 43.20% (saponins) | [85] |
| 23.43% n-butanol, extracting times: 4, sample-to-solvent ratio: 1:1.20, 76.4 °C | 93.61% (oil) 56.28% (saponins) | [155] | |
| SFE using water: 3 MPa, sample-to-solvent ratio: 1:11, 125 °C, 32 min | 92.06% (oil) 72.20% (saponins) | [125] | |
| Protein | pH 10, sample-to-solvent ratio:1:40, 50 °C, 60 min | 44.57% | [156] |
| Enzyme pretreatment: 2% amylase, 0.7% cellulase, pH 5, 50 °C, 120 min; Extraction: pH 10, sample-to-solvent ratio: 1:25, 45 °C, 80 min | 80.83% | [157] | |
| Steam explosion pretreatment: 0.8–2.3 MPa, 30–120 s; Extraction: pH 10, sample-to-solvent ratio: 1:10, 40 °C, 50 min | 71.01% | [158] | |
| Protein and oil | AEE: 1.5% alkaline protease, sample-to-solvent ratio: 1:5, pH 8, 60 °C, 4 h. | 74.61% (oil) 82.28% (protein) | [111] |
| Ultrasound pretreatment: 300 W, 45 °C, 30 min; AEE: 1.5% alkaline protease, pH 9, sample-to-solvent ratio: 1:5, 60 °C, 3.5 h | 89.70% (oil) 90.64% (protein) | [102] |
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robards, K.; Prenzler, P.; Ryan, D.; Zhong, H. Camellia Oil and Tea Oil. In Gourmet and Health-Promoting Specialty Oils; Robert, M.A., Kamal-Eldin, A., Eds.; AOCS Press: Urbana, IL, USA, 2009; pp. 313–343. [Google Scholar]
- He, S.; Gu, Y. American Camellia Yearbook; American Camellia Society: Fort Valley, GA, USA, 1982; pp. 104–107. [Google Scholar]
- Nguyen, Q.K.; Hoang, V.T.; Nguyen, B.V.; Nguyen, V.T. Surveying results on development status for tea oil Camellia plantation in northern provinces, Vietnam. Vietnam J. Sci. 2006, 3, 169–176. [Google Scholar]
- Yin, D.; Li, S.; Wu, Q.; Feng, C.; Li, B.; Wang, Q.; Wang, L.; Xu, W. Advances in research of six woody oil crops in china. Chin. Bull. Bot. 2018, 53, 110–125. [Google Scholar]
- Tang, L.; Bayer, E.; Zhuang, R. Obtain, properties and utilization of Chinese teaseed oil. Fett 1993, 95, 23–27. [Google Scholar]
- Zhu, G.; Liu, H.; Xie, Y.; Liao, Q.; Lin, Y.; Liu, Y.; Liu, Y.; Xiao, H.; Gao, Z.; Hu, S. Postharvest processing and storage methods for Camellia oleifera seeds. Food Rev. Int. 2020, 36, 319–339. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; del Mar Contreras, M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “FancyTiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef]
- Liang, H.; Hao, B.Q.; Chen, G.C.; Ye, H.; Ma, J. Camellia as an oilseed crop. HortScience 2017, 52, 488–497. [Google Scholar] [CrossRef]
- Chaikul, P.; Sripisut, T.; Chanpirom, S.; Sathirachawan, K.; Ditthawuthikul, N. Melanogenesis inhibitory and antioxidant effects of Camellia oleifera seed oil. Adv. Pharm. Bull. 2017, 7, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Xu, Q.; Dula, B.G.; Wang, J.; Fu, J.; Wang, L.; Qian, B.; Zhou, J.; Wu, J.; Wang, J.; et al. Discrimination of geographical origin of camellia seed oils using electronic nose characteristics and chemometrics. J. Consum. Prot. Food Saf. 2020, 15, 263–270. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, Q.; Pan, J.; Liu, M.; Long, Y.; Ge, F. Effectively improve the quality of camellia oil by the combination of supercritical fluid extraction and molecular distillation (SFE-MD). Lwt 2019, 110, 175–181. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, Y.; Ni, Z.; Tang, F. Determination of benzo [a] pyrene in camellia oil via vortex-assisted extraction using the UPLC-FLD method. Food Sci. Biotechnol. 2017, 26, 15–19. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction methods of oils and phytochemicals from seeds and their environmental and economic impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xie, F.P.; Li, X.; Liu, D.W.; Wang, X.S.; Mao, L.C. Design and test of dehulling machine for Camellia oleifera fruit. J. Hunan Agric. Univ. 2014, 40, 665–668. [Google Scholar]
- He, D.; Xiang, H. Camellia Seed Processing Technology; Chinese Light Industry Press: Beijing, China, 2015; pp. 27–28. [Google Scholar]
- Sun, H.; Fei, X.; Fang, X.; Wang, J. A study on the mechanism of aqueous enzymatic extraction of Camellia oleifera seed oil. Acta Agric. Univ. Jiangxiensis 2011, 33, 1117–1121. [Google Scholar]
- Zhang, W.G.; Jin, G.M. Microwave puffing-pretreated extraction of oil from Camellia oleifera seed and evaluation of its physicochemical characteristics. Int. J. Food Sci. Technol. 2011, 46, 2544–2549. [Google Scholar] [CrossRef]
- He, L.; Zhou, G.Y.; Zhang, H.Y.; Liu, J.A. Research progress on the health function of tea oil. J. Med. Plants Res. 2011, 5, 485–489. [Google Scholar]
- Zhang, Z.M.; Wu, S.X.; Liu, R.X. Effect of production process on nutritional quality of camellia seed oil. Food Sci. 2013, 34, 268–272. [Google Scholar]
- Shi, T.; Zhu, M.; Zhou, X.; Huo, X.; Long, Y.; Zeng, X.; Chen, Y. 1H NMR combined with PLS for the rapid determination of squalene and sterols in vegetable oils. Food Chem. 2019, 287, 46–54. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From preclinical evidence to potential clinical applications. Front. Pharmacol. 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Cooney, R.V. The potential physiological role of γ-tocopherol in human health: A qualitative review. Nutr. Cancer 2020, 72, 808–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Yang, R.; Mao, J.; Jiang, J.; Wang, X.; Zhang, W.; Zhang, Q.; Li, P. Simultaneous determination of tocopherols, carotenoids and phytosterols in edible vegetable oil by ultrasound-assisted saponification, LLE and LC-MS/MS. Food Chem. 2019, 289, 313–319. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; del Mar Contreras, M.; Wang, L. Profiling and quantification of phenolic compounds in Camellia seed oils: Natural tea polyphenols in vegetable oil. Food Res. Int. 2017, 102, 184–194. [Google Scholar] [CrossRef]
- Wei, Z.; Guo, M.; Wang, Y.; Duan, Z.; Yang, K.; Luan, X. Recent advances in research on polyphenol compounds in Camellia oleifera seed oil. Food Sci. 2021, 42, 311–320. [Google Scholar]
- Teixeira, A.M.; Sousa, C. A review on the biological activity of Camellia species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, F.; Chen, L.; Liu, R.; Chang, M.; Wang, X. Identification and in vitro anti-inflammatory activity of different forms of phenolic compounds in Camellia oleifera oil. Food Chem. 2021, 344, 128660. [Google Scholar] [CrossRef]
- Luan, F.; Zeng, J.; Yang, Y.; He, X.; Wang, B.; Gao, Y.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- Xiao, X.; He, L.; Chen, Y.; Wu, L.; Wang, L.; Liu, Z. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel. components. Future Med. Chem. 2017, 9, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Mikoajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Wu, Y.; Wang, Y. Flavor differences of pressed oil-tea camellia seed oils with different heat treatments. China Oils Fats 2020, 45, 14–20. [Google Scholar]
- He, J.; Wu, X.; Yu, Z. Microwave pretreatment of camellia (Camellia oleifera Abel.) seeds: Effect on oil flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Deng, Q.; Yang, Y.; Xiang, X.; Zhou, X.; Tan, C.; Zhou, Q.; Huang, F. Unraveling of the aroma-active compounds in virgin camellia oil (Camellia oleifera Abel) using Gas Chromatography-Mass Spectrometry-Olfactometry, aroma recombination, and omission studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Du, M.H.; Wen, H.; Wang, W.W.; Tang, J.; Shen, L.R. Effects of n-6 PUFA-rich soybean oil, MUFA-rich olive oil and camellia seed oil on weight and cardiometabolic profiles among Chinese women: A 3-month double-blind randomized controlled-feeding trial. Food Funct. 2022, 13, 4375–4383. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhao, X.; Zhao, C.; Yang, H.; Gou, L.; Wang, W.; Guo, H. Pretreatment camellia seeds by protease and application to extraction of camellia oil. Eur. J. Lipid Sci. Technol. 2021, 123, 2000223. [Google Scholar] [CrossRef]
- Wang, F.; Shao, W.; Yang, D. Effect of different drying methods on drying characteristics and quality of Camellia oleifera seeds. J. Food Process. Preserv. 2021, 45, e15976. [Google Scholar] [CrossRef]
- Pongpichaiudom, A.; Songsermpong, S. Characterization of frying, microwave-drying, infrared-drying, and hot-air drying on protein-enriched, instant noodle microstructure, and qualities. J. Food Process. Preserv. 2018, 42, e13560. [Google Scholar] [CrossRef]
- Long, T.; Wu, X.H.; Rong, O.; Yang, L.; Yi, Z.; Li, Y. Drying process of Camellia oleifera seed by combining hot-air with microwave. J. South. Agric. 2016, 47, 1181–1186. [Google Scholar]
- Xing, C.; Li, J.; Jin, Q.; Gu, J.; Wang, X. T The drying characteristics of Camellia oleifera and establishment of hot air drying model. J. Chin. Cereals Oils Assoc. 2012, 27, 38–42. [Google Scholar]
- Chen, G.F.; Song, C.F.; Cui, Z.W. Optimizing of coupled hot-air and microwave drying of tea camellia seeds by response surface analysis. Sci. Technol. Food Ind. 2012, 33, 272–275. [Google Scholar]
- Wu, X.; Long, T.; Wang, Z.; Lan, W. Drying characteristics and models for heat pump drying of Camellia oleifera seed. J. Chin. Cereals Oils Assoc. 2018, 33, 111–117. [Google Scholar]
- Huang, D.; Tao, Y.; Li, W.; Sherif, S.A.; Tang, X. Heat transfer characteristics and kinetics of Camellia Oleifera Seeds during Hot-Air Drying. J. Therm. Sci. Eng. Appl. 2020, 12, 031017. [Google Scholar] [CrossRef]
- Huang, D.; Men, K.; Tang, X.; Li, W.; Sherif, S.A. Microwave intermittent drying characteristics of Camellia oleifera seeds. J. Food Process Eng. 2020, 44, e13608. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Li, C.; Kou, Q.; Li, L. Characteristics and mathematical description of hot-air drying of Camellia oleifera seed. J. South China Univ. Technol. 2010, 38, 116–120. [Google Scholar]
- Motevali, A.; Minaei, S.; Khoshtaghaza, M.H.; Amirnejat, H. Comparison of energy consumption and specific energy requirements of different methods for drying mushroom slices. Energy 2011, 36, 6433–6441. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Zhang, Y.; Liu, Q. Pilot-plant-scale test of cold-pressed oil extraction with twin-screw pressing for camellia seeds. Trans. Chin. Soc. Agric. Eng. 2014, 30, 300–308. [Google Scholar]
- Hu, J.; Wei, Y.; He, D.; Liu, Y. Preparation of natural oil-tea camellia seed oil by dehulling and cold-pressing. China Oils Fats 2009, 34, 16–19. [Google Scholar]
- Li, S.; Liu, X. Cold-pressed oil extraction of camellia seeds. In Proceedings of the 2011 International Conference on New Technology of Agricultural, Zibo, China, 27–29 May 2011; pp. 135–138. [Google Scholar]
- Wang, J.; Zheng, X.; Wan, N.; Lin, G.; You, Y. Fractal character of the pore structure of dehulled rapeseed cake based on scanning electron microscopy image analysis. Trans. Chin. Soc. Agric. Eng. 2008, 24, 16–20. [Google Scholar]
- Long, T.; Wu, X.; Rong, O.; Yang, L. Effects of preheating methods of camellia seed on camellia oil quality. J. Chin. Cereals Oils Assoc. 2017, 32, 79–83. [Google Scholar]
- Zhang, Y.; Li, X.; Lu, X.; Sun, H.; Wang, F. Effect of oilseed roasting on the quality, flavor and safety of oil: A comprehensive review. Food Res. Int. 2021, 150, 110791. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Effect of added caffeic acid and tyrosol on the fatty acid and volatile profiles of camellia oil following heating. J. Agric. Food Chem. 2006, 54, 9551–9558. [Google Scholar]
- Luo, F.; Fei, X. Maillard reaction derived from oil-tea camellia seed through roasting. J. Sci. Food Agric. 2019, 99, 5000–5007. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, X.; Zhou, Y.; Chen, J. Effects of different preheat treatments on volatile compounds of camellia (Camellia oleifera Abel.) seed oil and formation mechanism of key aroma compounds. J. Food Biochem. 2021, 45, e13649. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, L.; Yang, Y.; Ai, B.; Zhong, S.; Xiao, D.; Sheng, Z. Analysis of the volatile organic components of Camellia oleifera Abel. oil from China using headspace-gas chromatography-ion mobility spectrometry. J. Food Process. Preserv. 2021, 45, e15670. [Google Scholar] [CrossRef]
- Yang, K.M.; Hsu, F.L.; Chen, C.W.; Hsu, C.L.; Cheng, M.C. Quality characterization and oxidative stability of camellia seed oils produced with different roasting temperatures. J. Oleo Sci. 2018, 67, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Han, Y.F.; Wang, N.N.; Liu, H.M.; Zheng, Y.Z.; Wang, X.D. Improvement of the oxidative stability of cold-pressed sesame oil using products from the Maillard reaction of sesame enzymatically hydrolyzed protein and reducing sugars. J. Sci. Food Agric. 2020, 100, 1524–1531. [Google Scholar] [CrossRef]
- Wu, S.X.; Zhang, Z.M.; Liu, R.X.; Huang, S.S. Effect of production process on benzo (a) pyrene content in Camellia oil. Adv. Mat. Res. 2012, 554–556, 1099–1102. [Google Scholar]
- Ye, M.; Zhou, H.; Hao, J.; Chen, T.; He, Z.; Wu, F.; Liu, X. Microwave pretreatment on microstructure, characteristic compounds and oxidative stability of Camellia seeds. Ind. Crops Prod. 2021, 161, 113193. [Google Scholar] [CrossRef]
- Zheng, X.; Wan, S.; Li, L.; Zheng, Y. Pilot-scale practice of microwave pretreatment aqueous extraction of camellia seed oil. China Oils Fats 2017, 42, 152–154+157. [Google Scholar]
- Zhang, W.; Zhang, D.; Chen, X. A novel process for extraction of tea oil from Camellia oleifera seed kernels by combination of microwave puffing and aqueous enzymatic oil extraction. Eur. J. Lipid Sci. Technol. 2012, 114, 352–356. [Google Scholar] [CrossRef]
- Liu, R.; Yao, F.; Wu, S.; Huang, S. Effect of microwave treatment on the extraction speed and quality of Camellia seed oil. Adv. Mater. Res. 2012, 549, 984–987. [Google Scholar]
- Huang, S.; Wu, S.; Tan, C. Study on the supercritical carbon dioxide extraction process combined with microwave pretreatment for efficiently extracting high quality camellia seed oil. Sci. Technol. Food Ind. 2014, 35, 253–257+263. [Google Scholar]
- Yao, F. Effect of Microwave Treatment on the Extraction Speed and Quality of Camellia Oil. Master’s Thesis, Changsha University of Science and Technology, Changsha, China, 2012. [Google Scholar]
- Zhang, S.; Pan, Y.G.; Zheng, L.; Yang, Y.; Zheng, X.; Ai, B.; Xu, Z.; Sheng, Z. Application of steam explosion in oil extraction of camellia seed (Camellia oleifera Abel.) and evaluation of its physicochemical properties, fatty acid, and antioxidant activities. Food Sci. Nutr. 2019, 7, 1004–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical continuous oil expression from oilseeds: A review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Evon, P. Twin-screw extrusion technology for vegetable oil extraction: A review. J. Food Eng. 2017, 212, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Fei, X.; Li, K.; Guo, S.; Ye, X.; Wang, Y. The effect of different pretreatments on the efficiency and quality of camellia oil before hydraulic pressing. J. Chin. Cereals Oils Assoc. 2016, 31, 94–99. [Google Scholar]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Hu, Y.; Li, F.; Jin, W.; Godara, V.; Wu, B. Optimization of mechanical oil extraction process from Camellia oleifera seeds regarding oil yield and energy consumption. J. Food Process Eng. 2019, 42, e13157. [Google Scholar] [CrossRef]
- Huang, S.; Hu, Y.; Li, F.; Jin, W.; Wu, B. Multi-objective optimization of mechanical oil extraction from Camellia oleifera seeds using Kriging regression and NSGA-II. J. Food Process Eng. 2020, 43, e13549. [Google Scholar] [CrossRef]
- Meng, J.; Wei, B.; Qiu, L.; Lai, Q.; Tan, C.; Zhou, K.; Wang, L. Effect of different oil preparation processes on the main trace components and oxidative stability of oil-tea camellia seed oil. China Oils Fats 2021, 46, 20–22. [Google Scholar]
- Fei, X. Study on current status, problems and countermeasures of oil-tea camellia seeds oil processing industry. Sci. Technol. Food Ind. 2011, 32, 449–452. [Google Scholar]
- Yu, X.; Li, Q.; Du, S.; Zhang, R.; Xu, C. A novel process for the aqueous extraction of oil from Camellia oleifera seed and its antioxidant activity. Grasas Aceites 2013, 64, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fei, X.; Wang, K.; Yao, X.; Luo, F. Risk analysis of processing methods on benzo(a)pyrene contamination in oil-tea camellia seed oil. China Oils Fats 2013, 38, 64–66. [Google Scholar]
- De Moura, J.M.L.N.; Campbell, K.; Mahfuz, A.; Jung, S.; Glatz, C.E.; Johnson, L. Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. J. Am. Oil Chem. Soc. 2008, 85, 985–995. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Luckenbill-Edds, L.; Shelanski, M.L. Effects of neurotoxic industrial solvents on cultured neuroblastoma cells: Methyl-n-butylketon, n-hexane and derivatives. J. Neuropathol. Exp. Neurol. 1978, 37, 768–789. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.; Jin, Q.; Wang, X. Camellia oil authentication: A comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci. Technol. 2020, 97, 88–99. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Yi, X.; Liu, R.; Xiao, Z.; Wu, H.; Gu, Z.; Li, J.; Li, C. Research on tea oil and tea saponin extracted by n-butyl alcohol from pressed camellia cake. J. Chin. Cereals Oils Assoc. 2016, 31, 67–71. [Google Scholar]
- Yu, L.; Zhang, C.; Luo, Q.; Hu, X. The application of second butyl-acetate in camellia oil extraction. Guangdong Chem. Ind. 2014, 41, 57–58+48. [Google Scholar]
- Wang, L.; Wu, M.; Chen, X.; Wei, X.; Feng, Z. The kinetics and thermodynamics of ultrasonic-assisted camellia oil extraction. J. Chin. Cereals Oils Assoc. 2015, 30, 84–89. [Google Scholar]
- Yang, J.; Li, J.; Wang, M.; Zou, X.; Peng, B.; Yin, Y.; Deng, Z. A novel aqueous extraction for camellia oil by emulsified oil: Frozen/thawed method. Eur. J. Lipid Sci. Technol. 2019, 121, 1800431. [Google Scholar] [CrossRef]
- Gou, W.; Ma, R.; Qin, W.; Fan, G.; Lei, X. Optimization of Oil Extraction from Camellia Seeds by Response Surface Methodology. Food Sci. 2012, 33, 81–84. [Google Scholar]
- Guo, Y.; Tu, K.; Xue, Z.; Ji, C.; Guo, R. Technology for aqueous extraction of camellia seed oil by salt effect. Trans. Chin. Soc. Agric. Eng. 2010, 26, 362–367. [Google Scholar]
- Böttcher, S.; Drusch, S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys. 2016, 11, 91–100. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, W.; Liu, J.; Zhao, W.; Yang, R. Surfactant-assisted aqueous extraction processing of camellia seed oil by cyclic utilization of aqueous phase. Eur. J. Lipid Sci. Technol. 2019, 121, 1800504. [Google Scholar] [CrossRef]
- Li, Q.; Yang, R.; Zhang, W.; Zhu, K.; Hua, X.; Zhao, W. Influence of ethanol on aqueous extraction of oil-tea camellia seed oil. China Oils Fats 2012, 37, 6–9. [Google Scholar]
- Yang, R.; Ni, S.; Zhang, W.; Li, P.; Liu, J.; Xie, B. Summarization on vegetable oil extraction technology by aqueous medium method. Trans. Chin. Soc. Agric. Eng. 2016, 32, 308–314. [Google Scholar]
- Tu, J.; Wu, W.; Yang, J.; Li, J.; Ma, X. A method of producing edible oils with high quality by water. J. Food Process. Preserv. 2017, 41, e13280. [Google Scholar] [CrossRef]
- Lv, M.; Wu, W. Development of a new aqueous procedure for efficiently extracting high quality Camellia oleifera oil. Ind. Crops Prod. 2019, 138, 111583. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, D.; Wang, Y.; Huang, Y.; Li, P.; Li, C. Optimization of wet extraction of high quality oil-tea camellia seed oil. China Oils Fats 2022, 47, 19–23. [Google Scholar]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Valladares-Diestra, K.; de Souza Vandenberghe, L.P.; Soccol, C.R. Oilseed enzymatic pretreatment for efficient oil recovery in biodiesel production industry: A review. Bioenergy Res. 2020, 13, 1016–1030. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Z.; Wu, S.; Huang, S. Optimization and the nutritional components analysis of oil-tea camellia seed oil extracted by aqueous enzymatic extraction process. J. Chin. Cereals Oils Assoc. 2012, 27, 54–61+68. [Google Scholar]
- Feng, H.; Jiang, L.; Li, Y.; Zhang, Y.; Liu, Q.; Sui, X.; Cao, L. Study on impact factors of the ultrasound-assisted enzymatic aqueous extraction of oil from tea seed. Sci. Technol. Food Ind. 2013, 34, 271–272+279. [Google Scholar]
- Wu, J.; Ma, Q.; Hu, C.; He, D. Optimization of ultrasound-assisted aqueous enzymatic extraction of oil and protein from camellia. J. Chin. Cereals Oils Assoc. 2017, 32, 91–95. [Google Scholar]
- Zhou, Y.; Guo, H.; Xiang, J. Effects of composite enzyme on extracting oil-camellia seed oil by aqueous enzymatic method. Food Sci. Technol. Res. 2016, 41, 211–215. [Google Scholar]
- Fang, X.; Wang, K.; Yao, X.; Wang, Y. Aqueous enzymatic extraction of oil-tea camellia (Camellia oleifera) seed oil. For. Res. 2010, 23, 778–781. [Google Scholar]
- Liu, Q.; Zhao, G.; Wang, Y.; Luan, X. Study on aqueous enzymatic extraction of oil from Camellia oleifera seeds. J. Chin. Cereals Oils Assoc. 2011, 26, 36–40. [Google Scholar]
- Sun, H.; Fei, X.; Fang, X. Aqueous enzymatic extraction of oil-tea camellia seed oil. China Oils Fats 2011, 36, 11–15. [Google Scholar]
- Meng, X.; Ge, H.; Ye, Q.; Li, P.; Wang, Z.; Jiang, L. Efficient and response surface optimized aqueous enzymatic extraction of Camellia oleifera (Tea Seed) oil facilitated by concurrent calcium chloride addition. J. Am. Oil Chem. Soc. 2018, 95, 29–37. [Google Scholar] [CrossRef]
- Xiang, J.; Guo, H.; Xiao, L. Technical condition of aqueous enzymatic extraction of oil-camellia seeds. Hunan Agric. Sci. 2015, 2, 82–85. [Google Scholar]
- Zhu, J.; Wang, C.; Luo, F.; Zheng, X. Aqueous enzymatic extraction of oil from Camellia oleifera seed. China Oils Fats 2016, 41, 12–15. [Google Scholar]
- Guo, X.; Huang, W.; Gong, J.; Wang, H.; Li, Z. Preparation technology of protein from Camellia oleifera seed meal made by different production oil methods. Food Mach. 2013, 29, 147–149+175. [Google Scholar]
- Wu, J.; Zhou, L.; Hu, C.; He, D. Aqueous enzymatic extraction of Camellia oleifera seed oil and protein simultaneously. Cereals Oils 2015, 28, 58–61. [Google Scholar]
- Xie, B.; Yang, R.; Gu, J. Effects of pulverization treatment on aqueous enzymatic extraction of Camellia seed oil. Food Mach. 2016, 32, 174–177. [Google Scholar]
- Fang, X.; Fei, X.; Sun, H.; Jin, Y. Aqueous enzymatic extraction and demulsification of camellia seed oil (Camellia oleifera Abel.) and the oil’s physicochemical properties. Eur. J. Lipid Sci. Technol. 2016, 118, 244–251. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Diosady, L.L. Aqueous and enzymatic extraction processes for the production of food-grade proteins and industrial oil from dehulled yellow mustard flour. Food Res. Int. 2013, 52, 547–556. [Google Scholar] [CrossRef]
- Peng, L.; Ye, Q.; Liu, X.; Liu, S.; Meng, X. Optimization of aqueous enzymatic method for Camellia sinensis oil extraction and reuse of enzymes in the process. J. Biosci. Bioeng. 2019, 128, 716–722. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils-a short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X. Analysis of the camellia oil by various extraction process. Sci. Technol. Food Ind. 2012, 33, 307–310. [Google Scholar]
- Zhou, Y.; Gu, C.; Gu, H. Supercritical CO2 extraction of tea seed oil from Camellia seeds and composition analysis of tea seed oil extracts. Adv. Mater. Res. 2012, 538–541, 2372–2376. [Google Scholar]
- Yang, H.; Zhao, M.; Fan, Y.; Deng, Z.; Xiong, H. Comparing quality of camellia oil extracted by different methods. Sci. Technol. Food Ind. 2012, 33, 267–269+274. [Google Scholar]
- Lu, Z.; Fan, L.; Zheng, D.; Liao, Y.; Huang, B.; Chen, L. Technology for supercritical CO2 extraction of Camellia oleifera seed oil and analysis of the fatty acids. J. Fujian Coll. Fore. 2010, 30, 344–348. [Google Scholar]
- Feng, J.; Lei, H.; Ge, F. Modeling of the extraction process of tea seed oil with supercritical carbon dioxide. Braz. J. Chem. Eng. 2015, 32, 941–947. [Google Scholar] [CrossRef]
- Carvalho, P.I.N.; Osorio-Tobón, J.F.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 105, 44–54. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Wang, W.; Yan, Y.; Liu, H.; Qi, K.; Zhu, X.; Wang, X.; Qin, G. Subcritical low temperature extraction technology and its application in extracting seed oils. J. Food Process Eng. 2021, 44, e13805. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Xiao, Z.; Wu, H.; Liu, R.; Zhao, M.; Li, C. Optimization of simultaneous extraction of tea seed oil and saponin with subcritical water applying response surface methodology. J. Chin. Cereals Oils Assoc. 2017, 32, 81–87. [Google Scholar]
- Wu, H.; Li, C.; Li, Z.; Liu, R.; Zhang, A.; Xiao, Z.; Ma, L.; Li, J.; Deng, S. Simultaneous extraction of oil and tea saponin from Camellia oleifera Abel. seeds under subcritical water conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Miao, J.; Che, K.; Xi, R.; He, L.; Chen, X.; Guan, X.; Zhuang, X.; Wen, X.; Gao, Y. Characterization and benzo(a)pyrene content analysis of camellia seed oil extracted by a novel subcritical fluid extraction. J. Am. Oil Chem. Soc. 2013, 90, 1503–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, A.; Roobab, U.; Denoya, G.I.; Inam-Ur-Raheem, M.; Gullón, B.; Lorenzo, J.M.; Barba, F.J.; Zeng, X.A.; Wali, A.; Aadil, R.M. Advances in green processing of seed oils using ultrasound-assisted extraction: A review. J. Food Process. Preserv. 2020, 44, e14740. [Google Scholar] [CrossRef]
- Hu, A.; Feng, Q.; Zheng, J. Solvent extraction of oil-tea camellia seed oil enhanced by ultrasound. China Oils Fats 2009, 34, 17–19. [Google Scholar]
- Zhou, Q.; Fan, C.; Nie, Y.; Qu, X.; Ding, Z. Study on process conditions for ultrasound-assisted extraction of camellia seed oil. J. Chin. Cereals Oils Assoc. 2011, 26, 61–65. [Google Scholar]
- Wu, X.; Li, L. Optimization of ultrasound-assisted extraction of oil from camellia (Camellia oleifera abel) seed. Adv. Mater. Res. 2011, 236–238, 1854–1858. [Google Scholar]
- Ekezie, F.G.C.; Sun, D.W.; Cheng, J.H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Ji, P.; Zhou, J.; Liu, X. Microwave-assisted aqueous extraction of camellia oil. Mod. Food Sci. Technol. 2010, 26, 486–489. [Google Scholar]
- Tang, Z.; Yang, C.; Dong, X.; Zhou, B.; Li, Y. Study on microwave-assisted extraction of residual oil in defatted cake of camellia using ethanol-water as solvent. Food Res. Dev. 2014, 35, 27–30. [Google Scholar]
- Li, Z.; Jin, Q.; Ye, X.; Huang, J.; Liu, R.; Wang, X. Impact of extraction process on quality of oil -tea camellia seed oil. China Oils Fats 2015, 40, 47–51. [Google Scholar]
- Fang, X.; Du, M.; Luo, F.; Jin, Y. Physicochemical properties and lipid composition of camellia seed oil (Camellia oleifera Abel.) extracted using different methods. Food Sci. Technol. Res. 2015, 21, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Nie, M.; Yang, S.; Yao, X.; Fang, X.; Wang, Y. Effects of process methods on physicochemical property and nutrient content of tea oil. Fore. Res. 2010, 23, 165–169. [Google Scholar]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. The inactivation kinetics of polyphenol oxidase and peroxidase in bayberry juice during thermal and ultrasound treatments. Innov. Food Sci. Emerg. Technol. 2018, 45, 169–178. [Google Scholar] [CrossRef]
- Luo, F.; Guo, S.; Fei, X.; Yao, X.; Wang, Y.; Ye, X. The Effects of pressing conditions on volatile organic compounds and the quality of crude camellia oil. J. Chin. Cereals Oils Assoc. 2015, 30, 61–66. [Google Scholar]
- Luo, F.; Chen, Z.; Fei, X.; Wang, C. Effects of different drying methods on the quality of pressed oil-tea camellia seed oil. China Oils Fats 2019, 44, 3–7. [Google Scholar]
- Zhang, S.; Zheng, L.; Binling, A.I.; Zheng, X.; Yang, Y.; Pan, Y.; Sheng, Z. Effect of steam explosion pretreatment on quality of tea seed (Camellia oleifera Abel.) oil obtained by aqueous extraction. Food Sci. 2019, 40, 124–130. [Google Scholar]
- Li, Z.; Jin, Q.; Ye, X.; Wang, Z.; Xia, X.; Wang, X. Changes of bioactive constituents and antioxidant activity of oil-tea camellia seed oil during refining. China Oils Fats 2015, 40, 1–5. [Google Scholar]
- Qin, Y.; Liu, B.; Xue, J.; Wang, L.; Yang, L.; Fang, R.; He, L.; Wang, Y. Study on quality difference of hot- pressed and cold-pressed camellia oil. J. Chin. Cereals Oils Assoc. 2020, 35, 97–104. [Google Scholar]
- Liu, G.; Xu, S.; Wang, X.; Jin, Q.; Xu, X.; Shen, Y.; Xu, G.; Zhang, H. Analysis of the volatile components of tea seed oil (Camellia sinensis O. Ktze) from China using HS-SPME-GC/MS. Int. J. Food Sci. Technol. 2016, 51, 2591–2602. [Google Scholar] [CrossRef]
- Wang, J.; Tang, X.; Chu, Q.; Zhang, M.; Zhang, Y.; Xu, B. Characterization of the volatile compounds in Camellia oleifera seed oil from different geographic origins. Molecules 2022, 27, 308. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, Z.; Niu, Y.; Fang, X.; Cao, W. Characterization of aroma volatiles in camellia seed oils (Camellia oleifera Abel.) by HS-SPME/GC/MS and Electronic Nose combined with multivariate analysis. Food Sci. Technol. Res. 2016, 22, 497–505. [Google Scholar]
- Li, Z.; Yu, L.S.; Huang, J.; She, J.R.; Tang, P.; Liu, J.B.; Yu, N.H.; Fu, G.H.; Li, H.A. Cluster analysis of volatile flavor components of camellia seed oil in different processed methods. Sci. Technol. Food. Ind. 2019, 40, 276–279+284. [Google Scholar]
- Quan, W.; Wang, A.; Gao, C.; Li, C. Applications of Chinese Camellia oleifera and its by-products: A review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, S.; Deng, J.; Li, T.; Ding, C. Antioxidant activities and functional properties of tea seed protein hydrolysates (Camellia oleifera Abel.) influenced by the degree of enzymatic hydrolysis. Food Sci. Biotechnol. 2014, 23, 2075–2082. [Google Scholar] [CrossRef]
- Wang, D.; Huo, R.; Cui, C.; Gao, Q.; Zong, J.; Wang, Y.; Sun, Y.; Hou, R. Anticancer activity and mechanism of total saponins from the residual seed cake of Camellia oleifera Abel. in hepatoma-22 tumor-bearing mice. Food Funct. 2019, 10, 2480–2490. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, T.; Chen, D.; Zhang, N.; Si, B.; Deng, K.; Tu, Y.; Diao, Q. Effects of tea saponin supplementation on nutrient digestibility, methanogenesis, and ruminal microbial flora in dorper crossbred ewe. Animals 2019, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Guo, S.; Du, M.; Fei, X.; Zhong, H. Continuous extraction of tea saponin from oil-tea camellia seed meal. China Oils Fats 2017, 42, 121–124. [Google Scholar]
- Wang, J.; Li, G.; Zhang, Y.; Liao, N.; Wei, Z.; Li, Q. Establishment of continuous multi-stage countercurrent water extraction process of tea saponin from camellia meal. J. Food Sci. Technol. 2021, 39, 153–161. [Google Scholar]
- Wang, L.; Fang, X.; Du, M.; Long, Q. Effects of supercritical CO2 extraction on the quality of oil and physicochemical properties of tea saponin in oil-tea camellia seed cake. China Oils Fats 2020, 45, 109–114. [Google Scholar]
- Liu, R.; Tian, W.; Yi, Y.; Xiao, Z.; Li, C. Optimization of process conditions for simultaneous extraction of camellia oil and saponin by microemulsion method. J. Chin. Cereals Oils Assoc. 2021, 36, 96–99. [Google Scholar]
- He, W.; Chen, J.; Zhao, Y.; Cao, X. Study on the extraction process of protein from Camellia Oleifera seed cake. J. Henan Univ. Sci. Technol. 2019, 40, 13–19. [Google Scholar]
- Zhang, X.; Liu, F. Optimization of enzymatic extraction of protein from camellia seed cake. Cereal Feed Ind. 2013, 8, 37–40. [Google Scholar]
- Zhang, S.; Xu, L.; Zheng, L.; Ai, B.; Zheng, X.; Yang, Y.; Pan, Y.; Sheng, Z. Steam explosion-assisted extraction of oil-tea camellia seed protein and its functional properties. China Oils Fats 2019, 44, 47–53. [Google Scholar]
- Liu, J.; Zhang, W.; Li, P.; Jiang, Z.; Yang, R. Isolation of peanut protein aggregates using aqueous extraction processing combined with membrane separation. Int. J. Food Sci. Technol. 2020, 55, 3203–3214. [Google Scholar] [CrossRef]
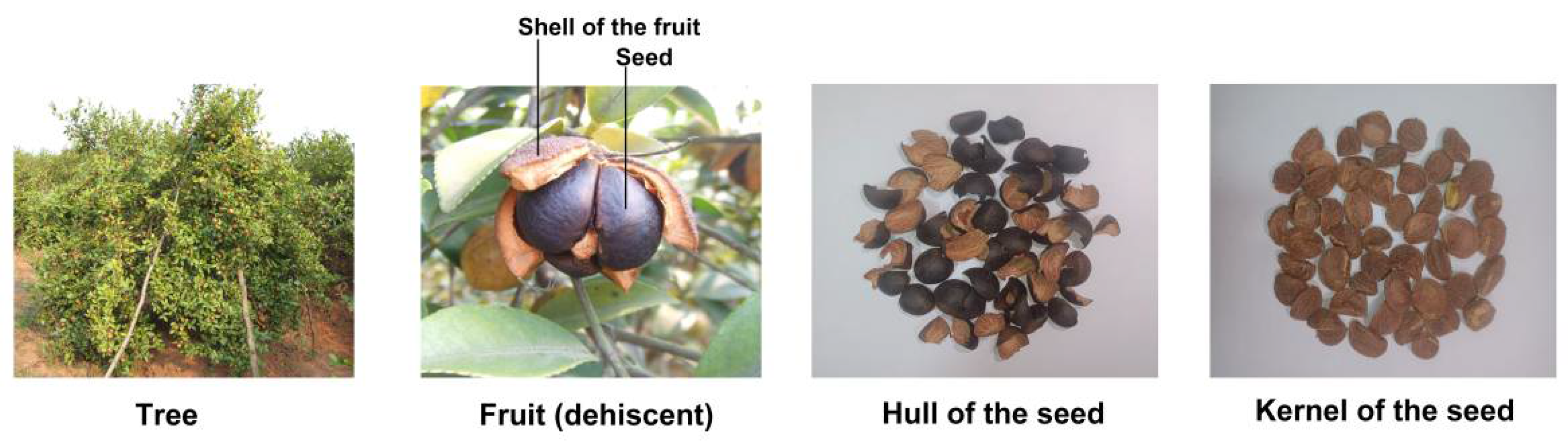

| Fatty Acid | Camellia Seed Oil (%) |
|---|---|
| Tridecanoic acid (C13:0) | 0.0048–0.0066 |
| Myristic acid (C14:0) | 0.0297–0.0617 |
| Pentadecanoic acid (C15:0) | 0.0072–0.0140 |
| Palmitic acid (C16:0) | 7.4541–9.0572 |
| Margaric acid (C17:0) | 0.0635–0.0791 |
| Stearic acid (C18:0) | 1.8946–3.5695 |
| Arachidic acid (C20:0) | 0.0446–0.0987 |
| Behenic acid (C22:0) | 0.0160–0.0307 |
| Tricosanoic acid (C23:0) | 0.0150–0.0510 |
| Lignoceric acid (C24:0) | 0.0305–0.0746 |
| Palmitoleic acid (C16:1) | 0.1200–0.1821 |
| Margaroleic acid (C17:1) | 0.0553–0.0847 |
| Oleic acid (C18:1n9) | 75.9724–79.4949 |
| Asclepic acid (C18:1n11) | 0.5284–1.0959 |
| Gadoleic acid (C20:1) | 0.5268–0.6065 |
| Erucic acid (C22:1) | 0.0259–0.0383 |
| Linoleic acid (C18:2n6) | 7.4679–10.2809 |
| Eicosadienoic acid (C20:2) | 0.0144–0.0213 |
| α-Linolenic acid (C18:3n3) | 0.1869–0.3860 |
| γ-Linolenic acid (C18:3n6) | 0.0356–0.0687 |
| Docosahexaenoic acid (C22:6n3) | 0.0561–0.1047 |
| Saturated fatty acid (SFA) | 9.8917–12.4011 |
| Monounsaturated fatty acid (MUFA) | 77.2590–81.1909 |
| Polyunsaturated fatty acid (PUFA) | 7.8677–10.7537 |
| Extraction Method | Advantages | Disadvantages |
|---|---|---|
| Hot pressing extraction (HPE) | Low investment cost and simple operation | High processing temperature, resulting in low quality of oil and seed, and complex refining process required |
| Cold pressing extraction (CPE) | Simple operation, better quality of oil and seed compared with HPE, as well as simpler refining | Low yield |
| Fresh pressing extraction (FPE) | High yield and superior quality, allows for simultaneous recovery of multiple components | Increased investment cost, additional and tedious operations needed |
| Solvent extraction (SE) | Low investment cost and high yield | High processing temperature, as well as hazardous solvent consumption and residue, resulting in low quality of oil and seed, and requires a complex refining process |
| Aqueous extraction (AE) | Better oil quality, reduced solvent consumption and less refining compared with SE, and allows for simultaneous recovery of multiple components | Low yield and prolonged extraction time, increased investment cost, as well as complex operation in wet conditions and wastewater discharge |
| Wet extraction (WE) | Developed from AE with significantly reduced water consumption | Not viable for simultaneous recovery of multiple components |
| Enzyme-assisted aqueous Extraction (EAE) | Developed from AE with enhanced extraction efficiency and specificity, and milder operation condition | High cost of enzymes |
| Supercritical fluid extraction (SCFE) | High yield and superior quality, environmentally friendly, and mild processing conditions | High pressure, high capital and operating cost, and low throughput |
| Subcritical fluid extraction (SFE) using water | Better quality of oil compared with SE, higher yield than AE, reduced processing time and solvent use | High temperature and increased pressure, high capital and operating cost |
| Subcritical fluid extraction (SFE) using n-butane | Better quality of oil and seed compared with SE, high yield, reduced processing time, and mild processing condition | Increased pressure, high capital and operating cost, requires additional safety management |
| Microwave-assisted extraction (MAE) | Improved yield and reduced processing time | Applies high temperatures and extra energy requirements |
| Ultrasound-assisted extraction (UAE) | Improved yield and reduced processing time | Extra energy requirements, difficult to scale up for commercial application |
| Extraction Method | Squalene (mg/kg) | Phytosterols (mg/kg) | Tocopherol (mg/kg) | Carotenoids (mg/kg) | Polyphenols (mg/kg) a | Reference |
|---|---|---|---|---|---|---|
| CPE | 163 | 1094 | 155 | ND b | [77] | |
| HPE | 87 | 994 | 83 | 16.3 | [77] | |
| SE | 90 | 273 | [119] | |||
| SE-refined | 68 | 726 | 72 | ND | [77] | |
| FPE | 445 | 1115 | 265 | 87.1 | [77] | |
| WE | 222 | 385 (β-sitosterol) | 189 | <10 | [97] | |
| SCFE | 915 (β-sitosterol) | 177 | 1.73 | 511 | [117] | |
| 103 | 301 | [119] | ||||
| SFE | 933 (β-sitosterol) | 168 | 1.76 | 502 | [117] | |
| UAE | 689 (β-sitosterol) | 105 | 1.32 | 417 | [117] | |
| MAE | 325 | 2468 | 288 | 4.52 | [64] | |
| AE | 88 | 255 | [119] | |||
| EAE | 84 | 257 | [119] |
| Pretreatment or Extraction Methods | Characteristic Aroma Compounds | Reference |
|---|---|---|
| Control | hexanal, phenethyl alcohol, phenethyl acetate | [59] |
| Microwave | 3-ethyl-2,5-dimethylpyrazine, methional, furaneol | [59] |
| Frying | 3-ethyl-2,5-dimethylpyrazine, 2,3-diethyl-5-methylpyrazine, methional | [59] |
| Roasting | heptanal, octanal, nonanal, phenylacetaldehyde, (E)-2-octenal, (E)-2-decenal, 2-pentylfuran | [59] |
| Steaming | heptanal, octanal, nonanal, phenylacetaldehyde, (E)-2-octanal, 2-pentylfuran | [59] |
| MPE | octanal, nonanal, (E)-2-decenal, 2-heptenal, octyl formate, hexanal, heptanal, heptanol,2-n-heptylfuran, (E)-2-octenal | [147] |
| Refining | octanal, nonanal, (E)-2-decenal, 2-heptenal, octyl formate, hexanal, heptanal, heptanol, (E)-2-octenal,2-n-heptylfuran | [147] |
| SE | (E)-2-decenal, nonanal, octanal, 2-heptenal, heptanal, heptanol, (E)-2-octenal, octyl formate | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Ma, L.; Yan, Z.; Zhu, Q.; Cai, J.; Wang, S.; Yuan, Y.; Chen, Y.; Deng, S. Extraction of Oils and Phytochemicals from Camellia oleifera Seeds: Trends, Challenges, and Innovations. Processes 2022, 10, 1489. https://doi.org/10.3390/pr10081489
Li G, Ma L, Yan Z, Zhu Q, Cai J, Wang S, Yuan Y, Chen Y, Deng S. Extraction of Oils and Phytochemicals from Camellia oleifera Seeds: Trends, Challenges, and Innovations. Processes. 2022; 10(8):1489. https://doi.org/10.3390/pr10081489
Chicago/Turabian StyleLi, Guihui, Li Ma, Zhipeng Yan, Qinhe Zhu, Jiangtao Cai, Saiyu Wang, Yuan Yuan, Yongzhong Chen, and Senwen Deng. 2022. "Extraction of Oils and Phytochemicals from Camellia oleifera Seeds: Trends, Challenges, and Innovations" Processes 10, no. 8: 1489. https://doi.org/10.3390/pr10081489
APA StyleLi, G., Ma, L., Yan, Z., Zhu, Q., Cai, J., Wang, S., Yuan, Y., Chen, Y., & Deng, S. (2022). Extraction of Oils and Phytochemicals from Camellia oleifera Seeds: Trends, Challenges, and Innovations. Processes, 10(8), 1489. https://doi.org/10.3390/pr10081489





