Calcined Coal Gangue Fines as the Substitute for Slag in the Production of Alkali-Activated Cements and Its Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportion and Sample Preparation
2.3. Test Methods
2.3.1. Compressive Strength
2.3.2. X-ray Diffraction Tests
2.3.3. Scanning Electron Microscope Testing
2.3.4. Thermogravimetric Analysis Tests
2.3.5. Fourier Transform Infrared Tests
3. Results and Discussions
3.1. Hydration Products Analyzed by TGA
3.2. XRD Analysis
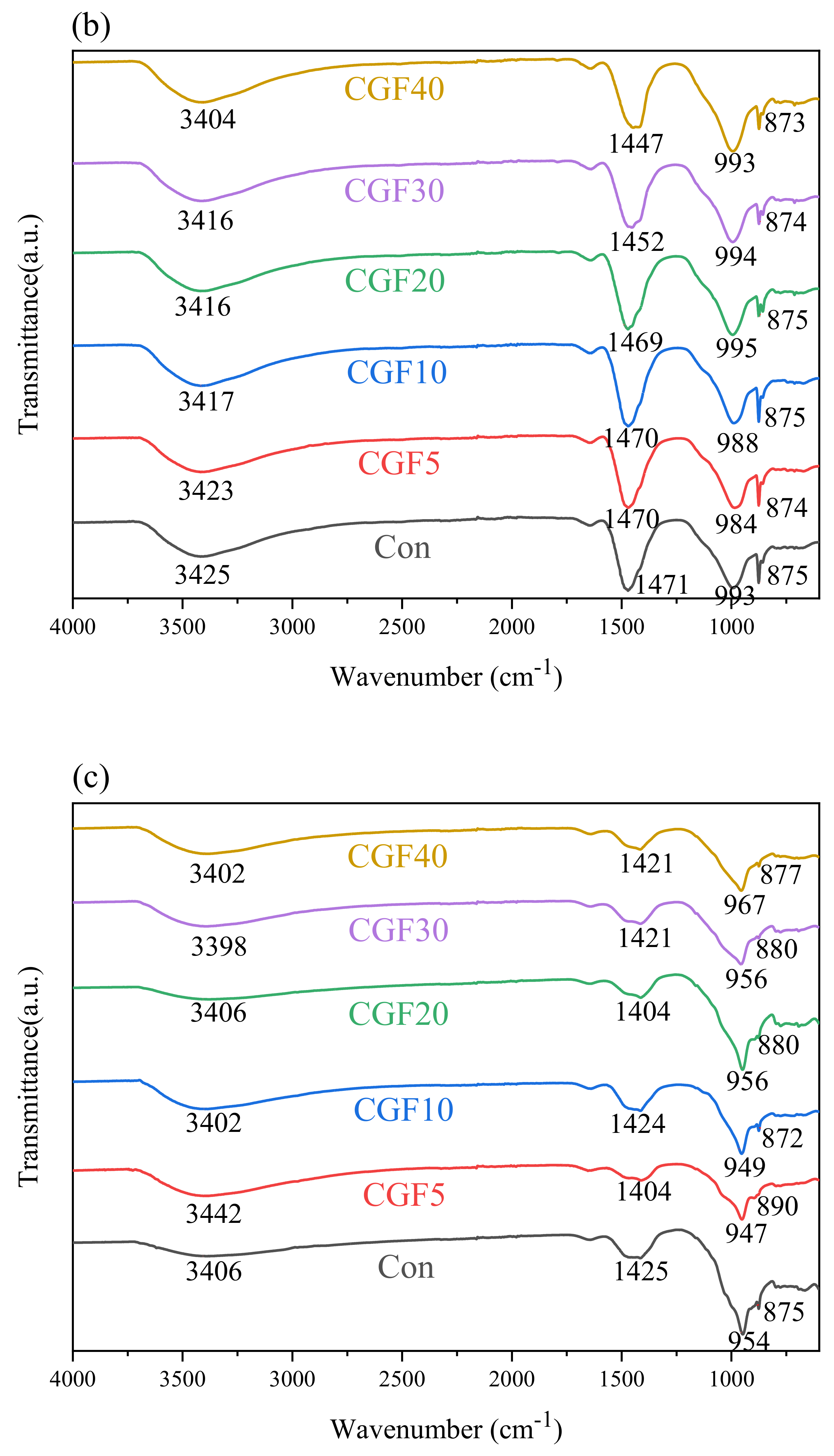
3.3. FTIR Analysis
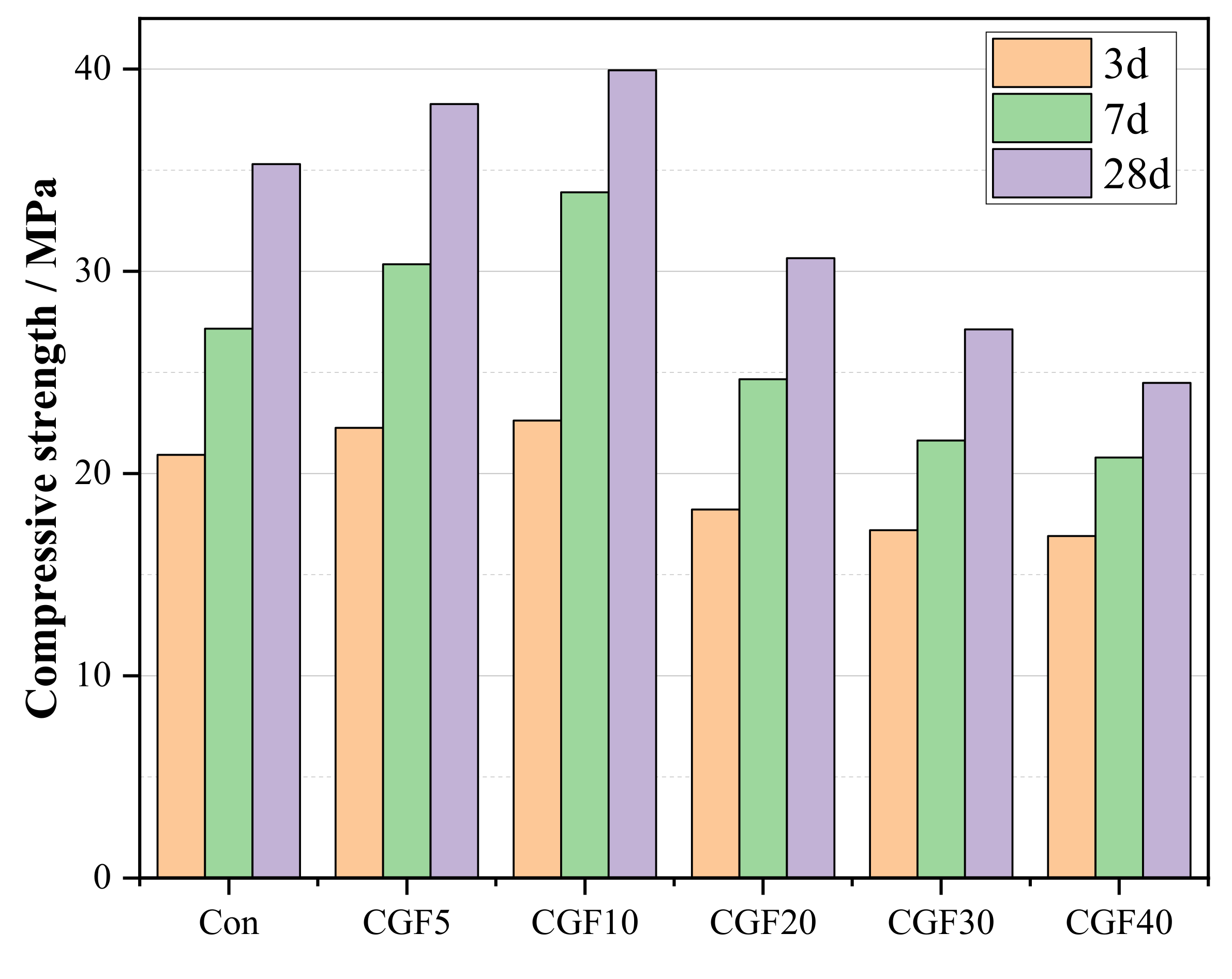
3.4. Compressive Strength
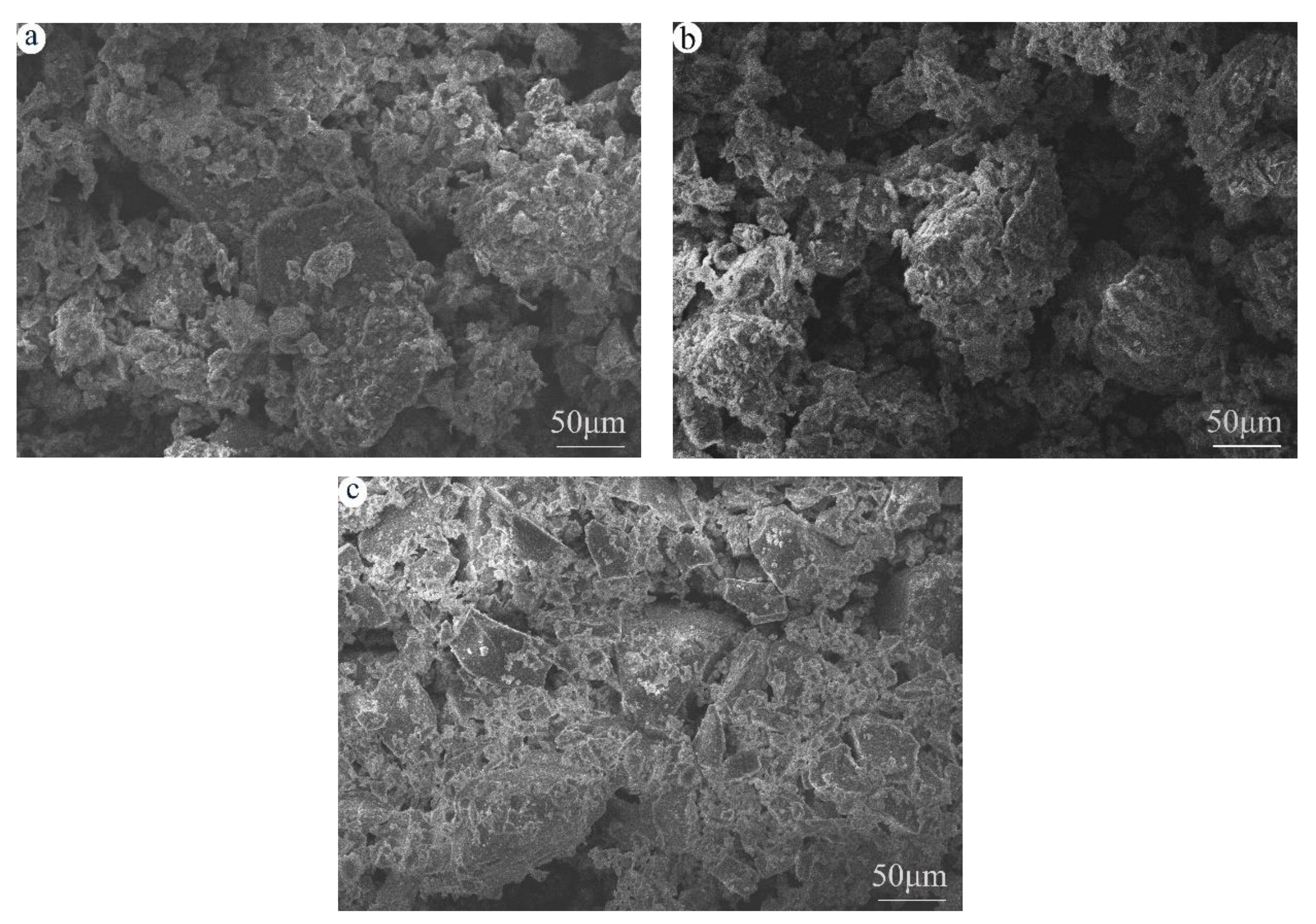
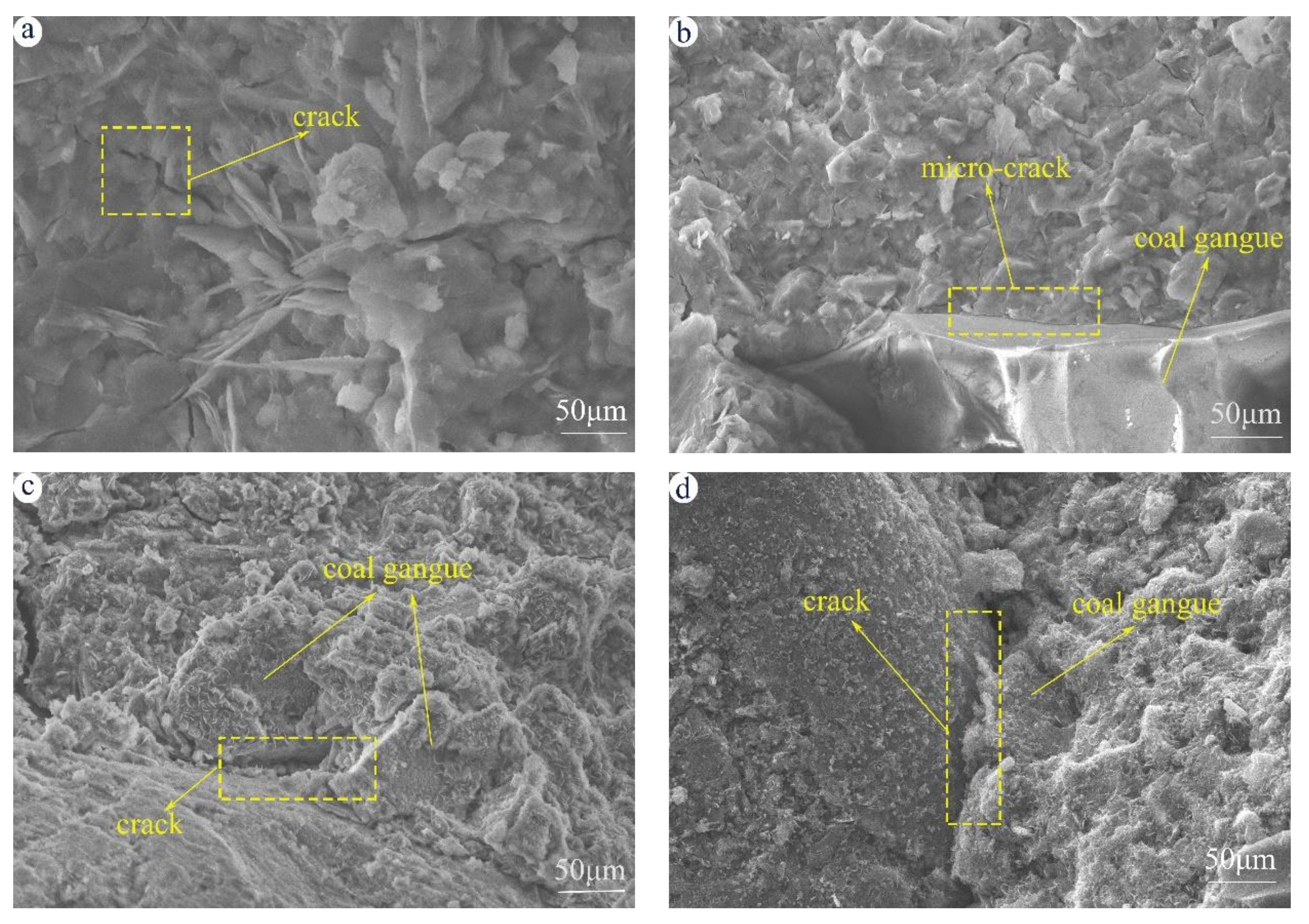
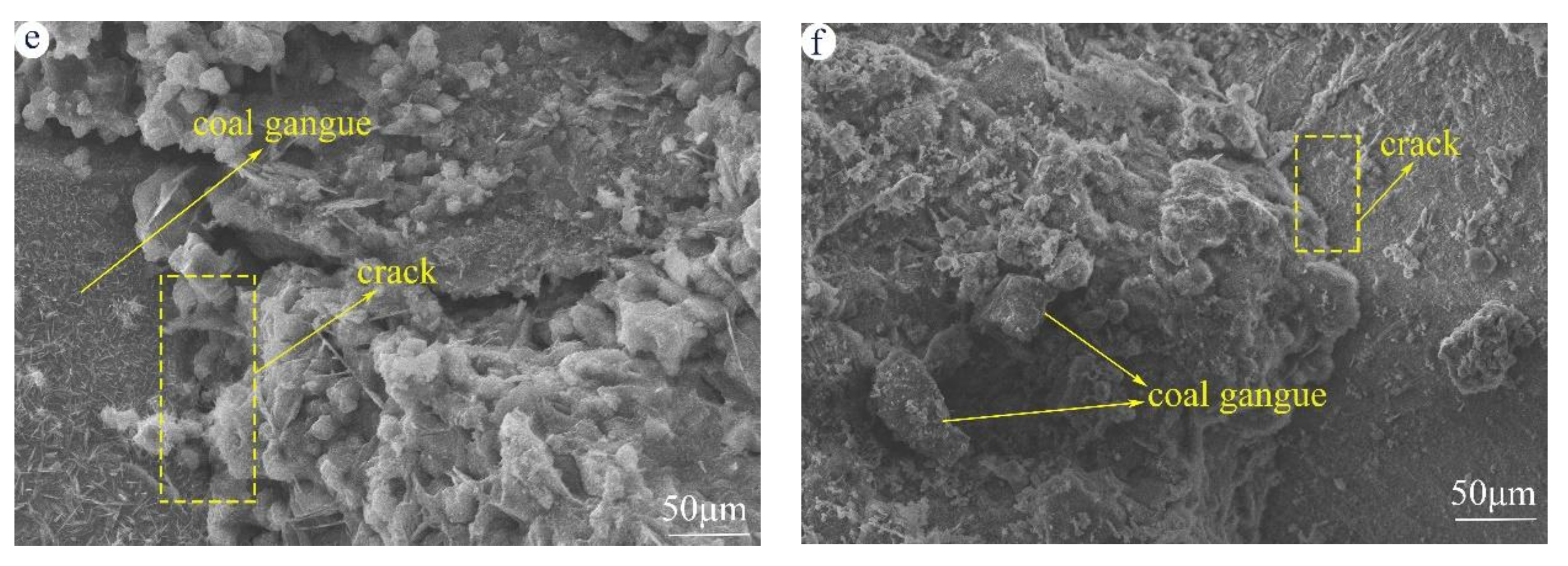
3.5. Microstructure Analyzed by SEM
4. Conclusions
- (1)
- The experimental results confirmed that the metakaolin could be used to substitute slag. CGF10 presents higher mechanical strength compared with Con. The highest compressive strength is achieved at 39.9 MPa after 28 days of curing when metakaolin replaces 10% slag.
- (2)
- High-content metakaolin samples present lower mechanical strength, slower setting, and minor heat release than samples formulated with a lower metakaolin.
- (3)
- The type of gel products formed in AAC does not vary significantly with the content of CGF. However, the increase in the content of metakaolin increases the C-(A)-S-H in gel products.
- (4)
- The internal structure and chemical properties of CGF changed significantly after calcination. In the calcination process, CGF underwent a dehydroxylation reaction, resulting in an increase in its activity. The effect of aggregate filling in the early stage of CGF and the pozzolanic effects can enhances the strength of AAC.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, K.; Zhang, L.; Li, A.; Ullah, F.; Chen, C.; Huang, J.; Zhang, Y. Preparation of high-quality glass-ceramics entirely derived from fly ash of municipal solid waste incineration and coal enhanced with pressure pretreatment. J. Clean. Prod. 2021, 324, 129021. [Google Scholar] [CrossRef]
- Lasek, J.A.; Głód, K.; Słowik, K. The co-combustion of torrefied municipal solid waste and coal in bubbling fluidised bed combustor under atmospheric and elevated pressure. Renew. Energy 2021, 179, 828–841. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Li, K.; Qu, F.; Yan, C.; Wu, Z. Toward understanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2022, 318, 125999. [Google Scholar] [CrossRef]
- Hongqiang, M.A.; Cheng, Y.I.; Chen, H.; Shi, J.; Weijian, L.I.; Guo, Y. Property and Cementation Mechanism of Alkali-activated Coal Gangue-slag Cementitious Materials. Chin. J. Mater. Res. 2018, 32, 898–904. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Wu, C.; Chen, H.; Sun, J.; Liu, J. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, M.; Wang, X.; Li, C.; Jia, H. Preparation of coal gangue-slag-fly ash geopolymer grouting materials. Constr. Build. Mater. 2022, 328, 126997. [Google Scholar] [CrossRef]
- Wang, A.; Hao, F.; Liu, P.; Mo, L.; Liu, K.; Li, Y.; Cao, J.; Sun, D. Separation of calcined coal gangue and its influence on the performance of cement-based materials. J. Build. Eng. 2022, 51, 104293. [Google Scholar] [CrossRef]
- Salguero, F.; Grande, J.A.; Valente, T.; Garrido, R.; de la Torre, M.L.; Fortes, J.C.; Sánchez, A. Recycling of manganese gangue materials from waste-dumps in the Iberian Pyrite Belt—Application as filler for concrete production. Constr. Build. Mater. 2014, 54, 363–368. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Guan, X. Multitechnique investigation of concrete with coal gangue. Constr. Build. Mater. 2021, 301, 124114. [Google Scholar] [CrossRef]
- Zhou, M.; Dou, Y.; Zhang, Y.; Zhang, Y.; Zhang, B. Effects of the variety and content of coal gangue coarse aggregate on the mechanical properties of concrete. Constr. Build. Mater. 2019, 220, 386–395. [Google Scholar] [CrossRef]
- Han, J.Y.; Song, X.Y.; Gao, Z.H. Excitation Effect of Soluble Glass on Composite System with Calcined Coal Gangue and Slag. Appl. Mech. Mater. 2012, 174, 30–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Wang, A.; Sun, D.; Liu, K.; Liu, P.; Chu, Y. Valorization of calcined coal gangue as coarse aggregate in concrete. Cem. Concr. Compos. 2021, 121, 104057. [Google Scholar] [CrossRef]
- Dong, Z.; Xia, J.; Fan, C.; Cao, J. Activity of calcined coal gangue fine aggregate and its effect on the mechanical behavior of cement mortar. Constr. Build. Mater. 2015, 100, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Jiang, W.; Zhang, C.; Li, J.; Wu, S.; Wang, X.; Xu, Y.; Wang, W.; Feng, M. Preparation of solid-waste-based pervious concrete for pavement: A two-stage utilization approach of coal gangue. Constr. Build. Mater. 2022, 319, 125962. [Google Scholar] [CrossRef]
- Rahman, R.; Rakhimov, R.Z.; Rakhimova, N.R.; Ojovan, I. Cementitious Materials for Nuclear Waste Immobilization; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Shi, C.; Fernández-Jiménez, A. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J. Hazard. Mater. 2006, 137, 1656–1663. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, M.; Li, Y.; Chen, Y.; Han, Y.; Wan, S.; Zhou, X.; Hou, H. Comparison of red mud and coal gangue blended geopolymers synthesized through thermal activation and mechanical grinding preactivation. Constr. Build. Mater. 2017, 153, 185–192. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Liu, X.; Sun, H.; Ni, W. Improvement on pozzolanic reactivity of coal gangue by integrated thermal and chemical activation. Fuel 2013, 109, 527–533. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Zhu, M.; Gao, J. Performance of microwave-activated coal gangue powder as auxiliary cementitious material. J. Mater. Res. Technol. 2021, 14, 2799–2811. [Google Scholar] [CrossRef]
- Hao, R.; Li, X.; Xu, P.; Liu, Q. Thermal activation and structural transformation mechanism of kaolinitic coal gangue from Jungar coalfield, Inner Mongolia. China Appl. Clay Sci. 2022, 223, 106508. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.; Dong, H.; Zhang, J.; Sun, C. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int. J. Miner. Process. 2016, 146, 23–28. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Evaluation of slag characteristics on the reaction kinetics and mechanical properties of Na2CO3 activated slag. Constr. Build. Mater. 2017, 131, 334–346. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Wang, J.; Thom, N. Effects of alkali dosage and silicate modulus on autogenous shrinkage of alkali-activated slag cement paste. Cem. Concr. Res. 2021, 141, 106322. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. Influence of metakaolin and limestone on chloride binding of slag activated by mixed magnesium oxide and sodium hydroxide. Cem. Concr. Compos. 2022, 127, 104397. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Xu, Y.; Hu, X.; Wang, Y. Upcycling waste flavedo into a bio-admixture of set retarder and compressive strength enhancer for cement-based materials. J. Clean. Prod. 2022, 332, 130060. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Y.; Zhang, J.; Zhang, C.; Chen, J.; Liu, C. Effect of particle size and thermal activation on the coal gangue based geopolymer. Mater. Chem. Phys. 2021, 267, 124657. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; Mejía de Gutierrez, R. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Farmer, V.C. The Infrared Spectra of Minerals; Mineralogical Society: London, UK, 1974; Volume 4. [Google Scholar]
- Uchino, T.; Sakka, T.; Iwasaki, M. Interpretation of Hydrated States of Sodium Silicate Glasses by Infrared and Raman Analysis. J. Am. Ceram. Soc. 2010, 74, 306–313. [Google Scholar] [CrossRef]
- Gadsden, J.A. Infrared Spectra of Minerals and Related Inorganic Compounds; Butterworths: London, UK, 1975. [Google Scholar]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Ye, T.; Min, X.; Li, X.; Zhang, S.; Gao, Y. Improved holding and releasing capacities of coal gangue toward phosphate through alkali-activation. Chemosphere 2022, 287, 132382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Provis, J.L.; Wang, H.; Bullen, F.; Reid, A. Quantitative kinetic and structural analysis of geopolymers. Part 2. Thermodynamics of sodium silicate activation of metakaolin. Thermochim. Acta 2013, 565, 163–171. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; El-Korchi, T.; Tao, M. A multiscale investigation of reaction kinetics, phase formation, and mechanical properties of metakaolin geopolymers. Cem. Concr. Compos. 2017, 78, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Melo Neto, A.A.; Cincotto, M.A.; Repette, W. Drying and autogenous shrinkage of pastes and mortars with activated slag cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Cheng, Y.; Hongqiang, M.; Hongyu, C.; Jiaxin, W.; Jing, S.; Zonghui, L.; Mingkai, Y. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
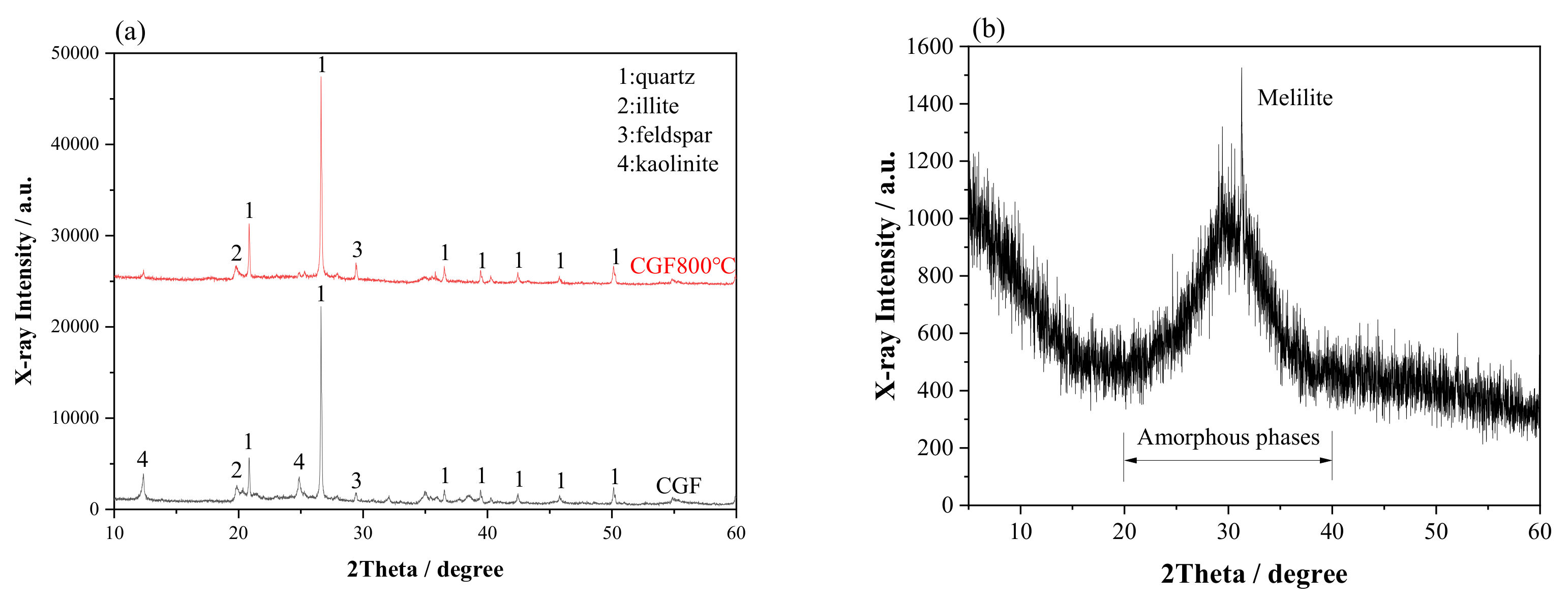
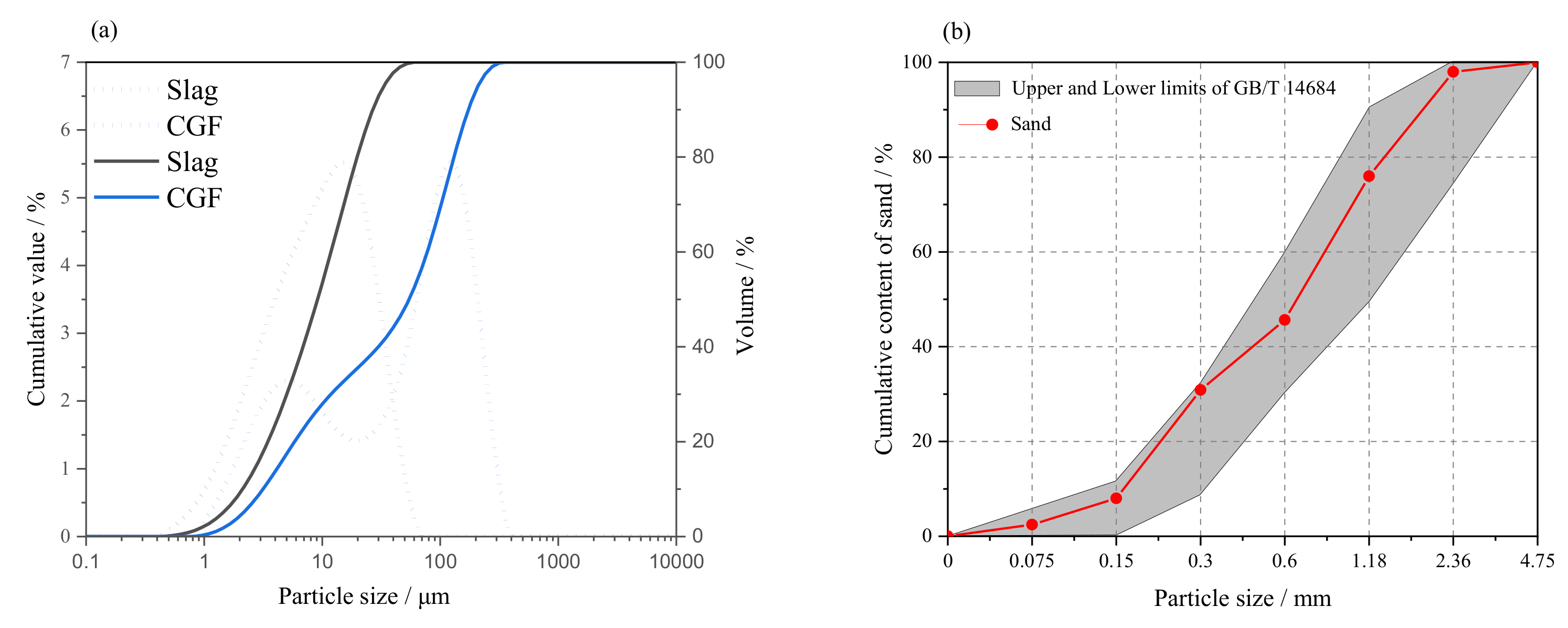
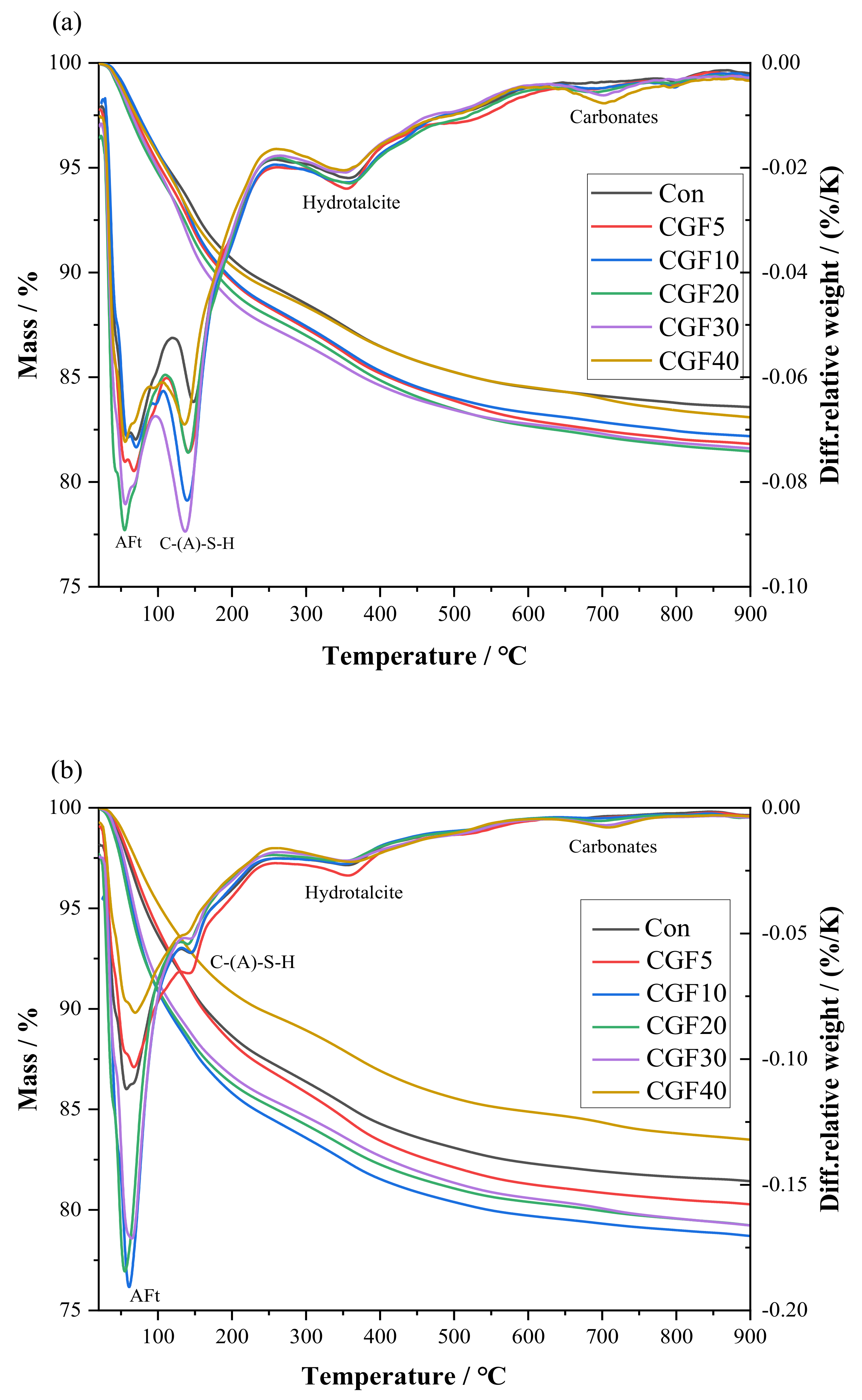
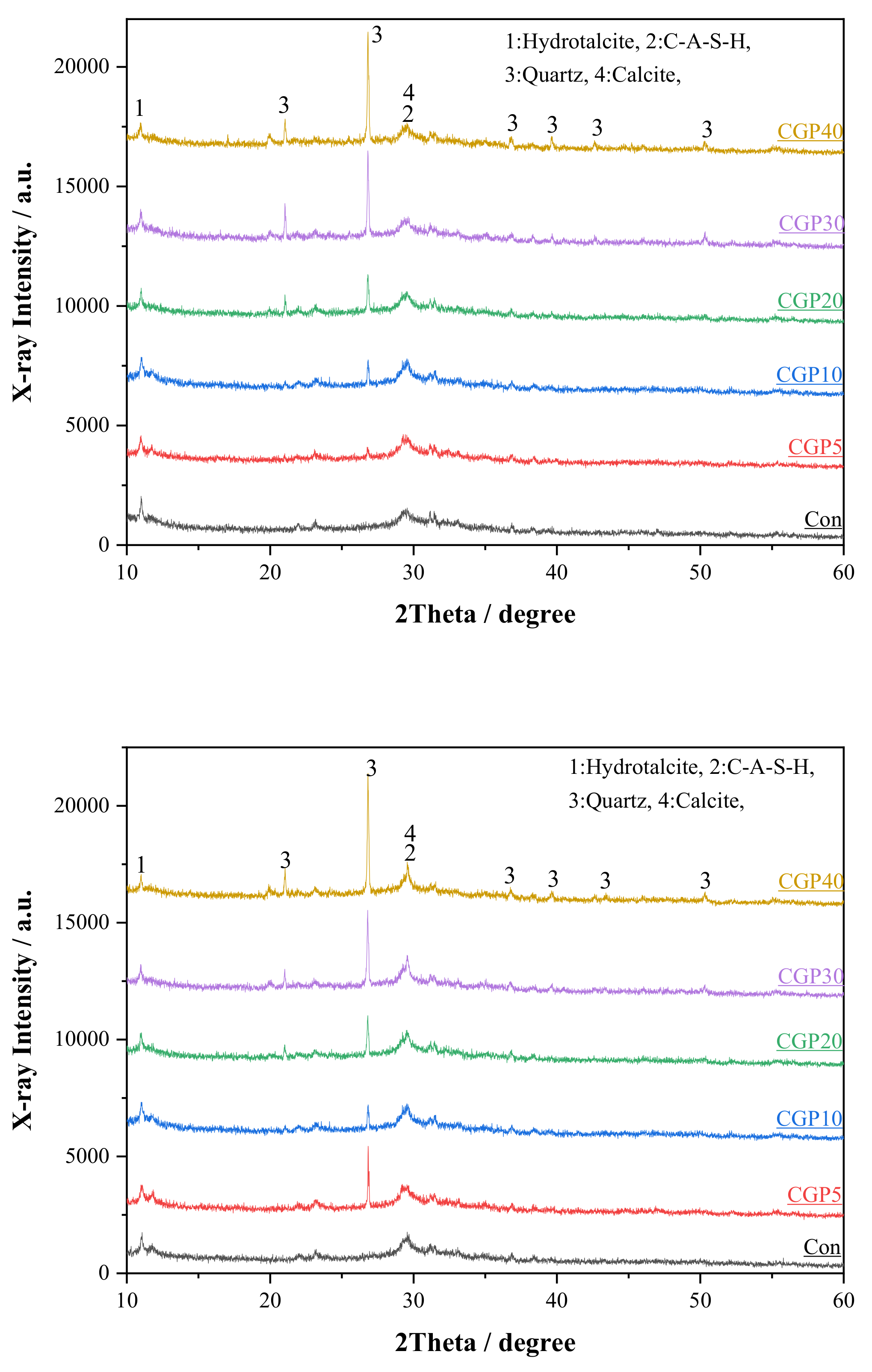

| Materials | Chemical Composition/% (XRF) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | |
| metakaolin | 56.26 | 27.48 | 10.73 | 1.56 | 0.48 | 0.21 | 0.77 | 1.51 |
| slag | 27.37 | 15.47 | 0.30 | 44.95 | 7.58 | 0.33 | 0.35 | 0.73 |
| Sand | Slag | Water | NaOH | Metakaolin | |
|---|---|---|---|---|---|
| Con | 50 | 32.520 | 17.74 | 1.664 | 0 |
| CGF5 | 50 | 30.894 | 17.74 | 1.664 | 1.626 |
| CGF10 | 50 | 29.268 | 17.74 | 1.664 | 3.252 |
| CGF20 | 50 | 26.016 | 17.74 | 1.664 | 6.504 |
| CGF30 | 50 | 22.764 | 17.74 | 1.664 | 9.756 |
| CGF40 | 50 | 19.512 | 17.74 | 1.664 | 13.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, C.; Wu, J.; Gao, M. Calcined Coal Gangue Fines as the Substitute for Slag in the Production of Alkali-Activated Cements and Its Mechanism. Processes 2022, 10, 1557. https://doi.org/10.3390/pr10081557
Liu C, Wang C, Wu J, Gao M. Calcined Coal Gangue Fines as the Substitute for Slag in the Production of Alkali-Activated Cements and Its Mechanism. Processes. 2022; 10(8):1557. https://doi.org/10.3390/pr10081557
Chicago/Turabian StyleLiu, Chenxu, Changbai Wang, Jianyang Wu, and Mengcheng Gao. 2022. "Calcined Coal Gangue Fines as the Substitute for Slag in the Production of Alkali-Activated Cements and Its Mechanism" Processes 10, no. 8: 1557. https://doi.org/10.3390/pr10081557





