Abstract
High efficiency removal of methyl orange (MO) and bromothymol blue (BT) dyes from contaminated water has been reported using magnetic mesoporous nanoparticles modified with cationic polymer brush (poly(2-methacryloyloxy)ethyl] trimethylammonium chloride solution) (Fe3O4-MSNs-PMETAC). Atom transfer radical polymerization (ATRP) was utilized to grow the polymer chains on the magnetic mesoporous silica nanoparticles. The chemical surface modifications were confirmed using IR, TGA, SEM and TEM. The results show that the obtained Fe3O4-MSNs-PMETAC materials were nearly spherical in shape with approximately 30 nm magnetic core, and silica shell thicknesses ranged from 135 to 250 nm. The adsorption performance of the material was found to be unaffected by the pH (3-9) of the media, with a removal efficiency of 100% for both dyes. The adsorption of BT and MO on the surface of Fe3O4-MSNs-PMETAC was found to follow Freundlich and Langmuir models, respectively. Since the synthesized nanocomposite materials exhibit an enhanced properties such as large maximum adsorption capacity, rapid synthesis process, and easy separation from solution, it could be an effective sorbent for the removal of other pollutants such as potentially toxic anionic elements (e.g., arsenate and chromate ions) from water and wastewater.
1. Introduction
The increase in population density leads to an increase economic activities and manufacturing. Consequently, organic pollutants introduced into water sources increase in types and quantity [1,2]. Some of these contaminants are poorly or not biodegradable [3,4]. Continuous exposure to such pollutants has harmful effects on humans [5,6]. Most common industries today such as textiles, plastics and paper depend on dyes, which are one of the most common pollutants [7,8,9,10]. Organic dyes have a complex molecular structure, high toxicity, stability and poor biodegradability, and they can associate with metal ions to form complex pollutants [11,12,13]. Many technologies have been used to remove the organic pollutants from wastewaters such as photocatalysis [14], sedimentation, reverse osmosis, ion-exchange [15], ozonolysis, electrolysis, membrane processes [16], and adsorption [17,18,19]. Adsorption is one of the premier and important technologies that have been utilized for dye removal because it is an effective, economically acceptable procedure, with the ability to regenerate and high retention efficiency without producing secondary contamination [20,21,22]. There are many traditional materials used as adsorbents for removing dyes from aqueous solutions such as activated carbon [23,24], metal–organic framework (MOFs) [25], graphene-based nanocomposites [26], and natural clay [27]. However, most of these materials have limitations due to difficult preparation and disposal, poor selectivity, and cost. In recent years, magnetic nanoparticles (MNPs) have received significant attention for wastewater treatment [28,29,30], due to their magnetic properties that enable them to be removed easily from systems [31,32]. MNPs include nickel, cobalt, iron and their oxides. They have high surface energy, leading to their agglomeration, which affects their adsorption capacity [33,34]. Shen et al. synthesized iron nanoparticles for water treatment to remove metal ions [34]. They found that the removal efficiency of such nanoparticles was high, even though they were chemically unstable. To improve the chemical stability (rust) and prevent aggregation, MNPs may coated with a protective layer [35,36]. The protective layer can be organic (self-assembled monolayer and polymer) or inorganic (e.g., silica shell) [37,38,39]. Mesoporous silica nanoparticles (MSNs) are one of the most used adsorbent nanomaterials in water treatment, due to their stability, affordability, resistance to different environments, low toxicity, high surface area, large pore size, and pore volume [40,41,42,43]. Coating MNPs with mesoporous silica shells could prevent their aggregation, protect them from rust, and enhance the selectivity for contaminants and adsorption capacity [44]. Elmobarak et al. prepared MNPs covered with different thickness of silica shell (5, 8, 10 and 15 nm) to recover oil from oil-in-water emulsion [45]. They found that the best result was obtained when the thickness of the silica layer was 5 nm. Huang et al. synthesized magnetic silica nanoparticles coated with chitosan modified with diethylenetriamine pentaacetate (DTPA) to increase selective adsorption of Pb (II) and methylene blue dye (MB) from multi-metal wastewater based on anion-synergism [46]. The adsorbed amount of Pb (II) in the presence of MB increased from 111.71 to 268.01 mg g−1 and the efficiency of MB removal was increased in the presence of lead as a synergistic effect. Alotaibi et al. have recently reported the preparation of magnetic mesoporous silica nanoparticles coated with poly (2-diethyl aminoethyl methacrylate) (PDEAEMA) brushes. Tertiary amines present in polymer chains were quaternized using 2-iodoethanol to remove anionic dyes effectively [47]. The adsorption efficiency increased after the quaternization process, comparing to the non-quaternized polymer.
To the best of our knowledge, the number of studies using polymer brushes coated magnetic mesoporous nanoparticles as adsorbent is very few. This work shows the synthesis of magnetic mesoporous nanoparticles modified with cationic polymer brush (poly(2-(methacryloyloxy)ethyl] trimethylammonium chloride solution) using atom transfer radical polymerization (ATRP) for removing anionic dyes. The adsorption efficiency of the prepared materials in removing anionic dyes methyl orange (MO) and bromothymol blue (BT) from an aqueous solution has been studied. Adsorption isotherms (Langmuir and Freundlich) and kinetic models (first and second order) were used to fit the experimental data. The nanosystem prepared was characterized by numerous techniques such as FTIR, SEM and TEM.
2. Materials and Methods
2.1. Materials
Ferrous chloride (FeCl2, 99%,) and ferric chloride (FeCl3, 99%) were acquired from Loba Chemie (Mumbai, India). Deionized water was obtained from Elga Pure Nanopore system. Ammonium hydroxide (NH4OH, 28 wt.%), ammonium nitrate (NH4NO3, 99%), 3-aminopropyltriethoxysilane (APTES, >98%), 2-bromo-2-methylpropionyl bromide (BIBB, 98%), N-2,2′-bipyridyl (Bipy, 99%), cetyltrimethylammonium bromide (CTAB, 98%), copper(II) bromide (CuBr2, 99.9%), copper(I) chloride (CuCl, 99.9%), dichloromethane (DCM, HPLC grade), ethanol (HPLC grade), methanol (HPLC grade), hexane (HPLC grade), isopropanol (HPLC grade), tetraethylorthosilicate (TEOS, 98%) and [2-(methacryloyloxy)ethyl]trimethylammonium chloride solution (METAC, 75 wt.% in H2O) were acquired from Sigma-Aldrich (United States). Triethylamine (TEA, 99%) was obtained from Nexgen chemicals (India). Hydrochloric acid (HCl, 36%) was purchased from Fisher Scientific (United States). Sodium hydroxide was obtained from BDH chemicals (United Kingdom). All chemicals were used as received.
2.2. Preparation of the Nanoadsorbent
2.2.1. Synthesis of Magnetic Nanoparticles (Fe3O4)
Magnetic nanoparticles (Fe3O4) were prepared by co-precipitation method. Two separate aqueous solutions of ferrous chloride (FeCl2) and ferric chloride (FeCl3) were prepared with a concentration of 0.1 M in 50 mL. The two solutions were mixed, then 25 mL of ammonium hydroxide (NH4OH) was added under nitrogen atmosphere under stirring at 70 °C for 20 min. The magnetic nanoparticles were collected by filtration, and washed three times with water followed by ethanol.
2.2.2. Magnetic Nanoparticles Embedded with Mesoporous Silica (Fe3O4-MSNs)
Magnetic nanoparticles (0.5 g) were suspended in 180 mL of DI water. To the suspension, 1.0 g of CTAB (C19H42NBr) was added followed by the addition 9 mL of ammonia water (NH4OH) under stirring at 40 °C. A mixture solution of 16 mL n-hexane (C6H14)/4 mL TEOS (Si(OC2H5)4) was added dropwise to the suspension. After stirring for 12 h, the solid product was obtained by filtration, then washed three times with water and ethanol. For the removal template, the solid product was dispersed in a solution of ammonium nitrate (NH4NO3)/ethanol (10 mg/mL) under stirring at 80 °C for 12 h. Fe3O4-MSNs were collected by centrifugation and washed with ethanol three times.
2.2.3. Amino Modified Magnetic Mesoporous Silica (Fe3O4-MSNs-NH2)
Fe3O4-MSNs (2.0 g) were suspended in a mixture of APTES (H2N(CH2)3Si(OC2H5)3)/methanol (0.5 mL/45 mL) at 80 °C for 17 h. Fe3O4-MSNs-NH2 was collected by centrifugation and washed five times with ethanol.
2.2.4. ATRP Initiator Modified Magnetic Mesoporous Silica (Fe3O4-MSNs-Br)
Fe3O4-MSN-NH2 (2.0 g) was dispersed in a mixture of DCM(CH2Cl2)/triethylamine (23 mL/1.5 mL), which was followed by adding a mixture of 2-bromo-2-methylprpionyl bromide((CH3)2CBrCOBr)/DCM (0.5 mL/5 mL) dropwise to the mixture and stirring for 18 h at room temperature. The solid was collected by centrifugation and washed three times with DCM and ethanol.
2.2.5. Polyelectrolyte [2-(methacryloyloxy)ethyl] Trimethylammonium Chloride Brushes Coated Magnetic Mesoporous Silica (Fe3O4-MSNs-PMETAC)
The ATRP initiated nanoparticles (0.5 g) were suspended in a solution of isopropanol (16 mL) and deionized water (4 mL). 2-(Methacryloyloxy)ethyl]trimethylammonium chloride (H2C=C(CH3)CO2CH2CH2N(CH3)3Cl, METAC, 13.8 g), CuBr2 (0.011 g), and of 2,2 bipy (C10H8N2, 0.382 g) were added to the suspension and degassed for 20 min. CuCl (0.1 g) was added to the polymerization mixture and stirred for 3 h under nitrogen atmosphere. The solid was washed three times with DI water.
2.3. Measurement and Characterization
The magnetic nanoparticles were imaged by using SEM (JEOL JSM-6380 LA) and TEM (JEOL JEM-1230). Prior to imaging, the sample was suspended in ethanol (HPLC grade), then one or two drops of the suspension were placed into copper mesh (on a carbon film and dried in an oven at 80 °C overnight. The chemical functional group of the synthesized materials was studied in the region of 4000–400 cm−1 by FTIR Spectroscopy (Thermo Scientific Nicolet IS10). To acquire the IR spectrum of the sample, pellets were prepared by mixing 2 mg of the sample with potassium bromide (KBr), with approximately 0.1 to 1.0%. TGA analyzer (SII TGA 6300) was used to study the thermal stability of the magnetic nanoparticles at heating rate 10 °C/min. UV–vis spectrophotometer (Shimadzu UV-2600) was used to estimate the concentrations of MB and BT before and after adsorption at room temperature.
2.4. Adsorption Studies
By varying the starting dyes concentrations, contact time, and pH, the adsorption capability of anionic dyes onto the surface of Fe3O4-MSNs-PMETAC was investigated. Fe3O4-MSNs-PMETAC (10 mg) was suspended in 10 mL of dye solution of various concentrations and stirred at 25 °C at 200 rpm. UV—vis spectrophotometry at maximum absorbance was used to determine the concentration of dyes after centrifugation. The following expression was used to compute the adsorption capacity:
where V and m denote the volume of contaminant solution in L and the amount of Fe3O4-MSNs-PMETAC in g, respectively. qe denotes the adsorption capacity at equilibrium in mg/g, while C0 and Ce represent the initial concentration of anionic dye in mg/L and dye concentration at time t in mg/L, respectively.
2.4.1. Adsorption Isotherms
Two isotherm models, Langmuir and Freundlich, were used to investigate the dyes’ adsorption behavior on nanomaterials.
The isotherm of the Langmuir model is defined by the Equation (2):
where Ce and qe are the pollutant concentration (mg/L) and equilibrium adsorption capacity (mg/g), respectively. The maximal contaminant adsorption capacity onto nanomaterials (mg/g) is described by qm. The Langmuir constant (L/mg) is represented by KL.
The isotherm of the Freundlich model is defined by the Equation (3):
KF is the Freundlich adsorption constant ((mg/g)/(mg/L)1/n), and 1/n defined as the adsorption intensity and surface heterogeneity.
2.4.2. Adsorption Kinetics
Using pseudo-first-order (4) and pseudo-second-order (5) equations, the dyes’ adsorption kinetics onto Fe3O4-MSNs-PMETAC were calculated.
where qt is the adsorption capacity in mg/g at time t. The rate coefficients of pseudo first-order and pseudo second-order, respectively, are K1 (L/min) and K2 (g/mg·min).
3. Results and Discussion
3.1. General Synthesis Method
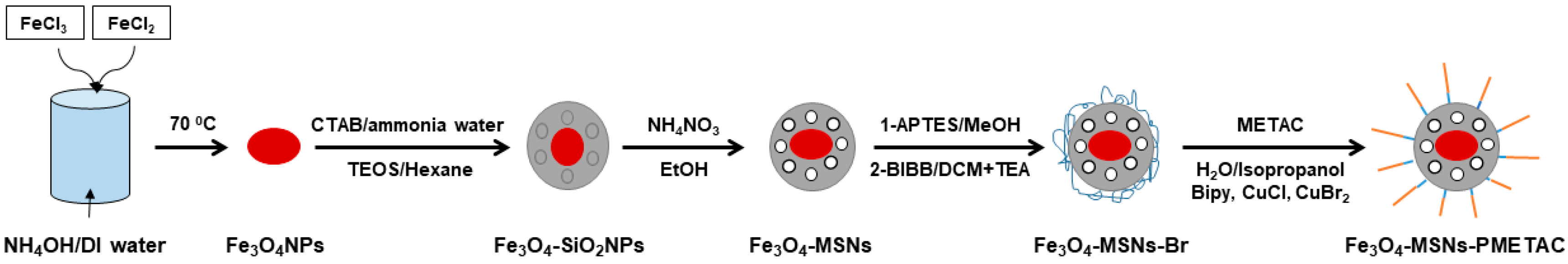
Iron oxide nanoparticles (Fe3O4 NPs) were fabricated by mixing ferrous and ferric salts in basic media. The mixture was heated to 70 °C for 20 min to complete the reduction reaction. The Fe3O4 NPs were then coated with mesoporous silica shell using the Stöber method in the presence of expander agent (hexane) to increase the pore size. After removing the template using an ionic exchange method, Fe3O4-MSNs had been coated with thin layer of organic molecules terminated amine group (APTES). BIBB molecules have been used to react with amine groups, producing Fe3O4-MSNs capped with ATRP initiator. Magnetic mesoporous nanoparticles were modified with cationic polymer brush (poly(2-(methacryloyloxy)ethyl] trimethylammonium chloride solution) using ATRP technique, as shown in Scheme 1.
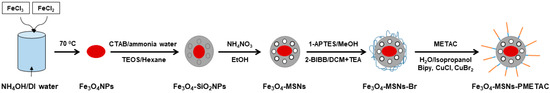
Scheme 1.
The preparation route of Fe3O4-MSNs-PMETAC.
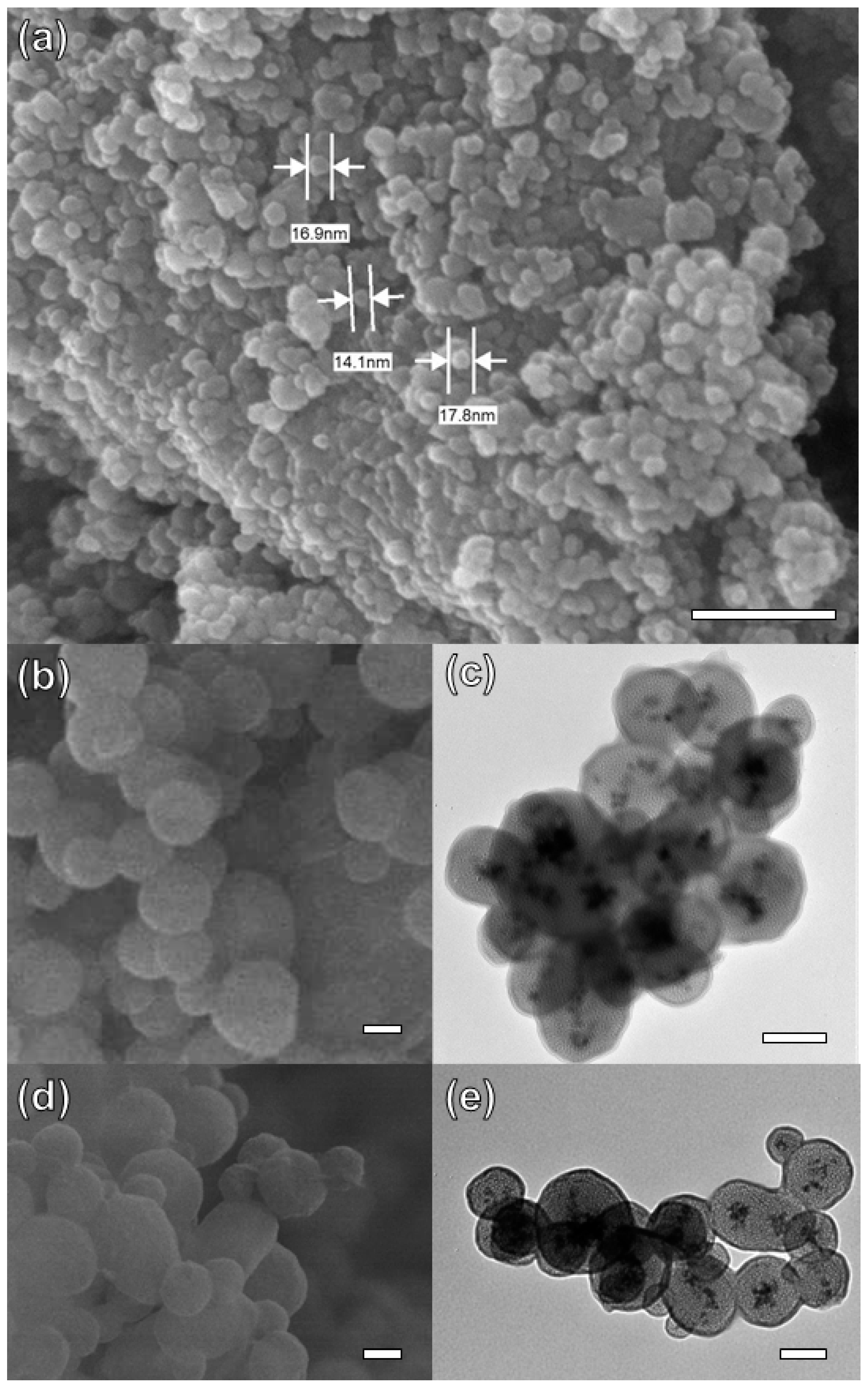
The morphological structure of Fe3O4 NPs and Fe3O4-MSNs-PMETAC was characterized by SEM and TEM. As shown in Figure 1a, the SEM images of Fe3O4 NPs present almost a spherical shape, and size distribution approximately equal to 18 nm. Moreover, it was observed that the iron oxide nanoparticles were aggregated. After covering the Fe3O4 core with a layer of silica, a decrease in the aggregation, and an increase in the particle size was observed. The size of Fe3O4-MSNs was found to be between 120 and 280 nm, as shown in Figure 1b. The TEM image of Fe3O4-MSNs presents a magnetic core of 18 nm and silica shell thicknesses ranged from 135 to 250 nm, (Figure 1c). Moreover, Fe3O4-MSNs exhibited mesoporous structure, with an average pore size of ca. 5 nm. No significant difference has been noticed in SEM images between Fe3O4-MSNs and Fe3O4-MSNs-PMETAC (Figure 1b,d). However, a shell around the core of magnetic nanoparticles was observed in the TEM image (Figure 1e) proving successful capping of PMETAC over the Fe3O4-MSNs.
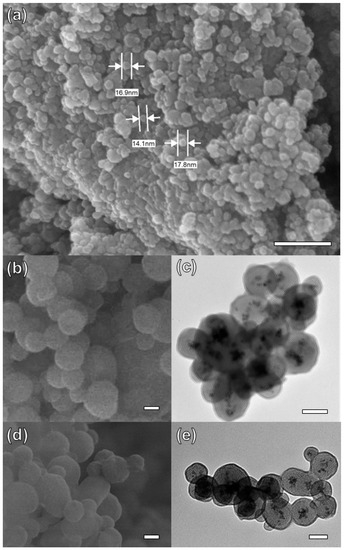
Figure 1.
(a) SEM Fe3O4 (b) SEM Fe3O4 coated with Si (c) TEM Fe3O4 coated with Si (d) SEM Fe3O4 coated with Si and polymer (e) TEM Fe3O4 coated with Si and polymer. Scale bar 100 nm for all images (a–e).
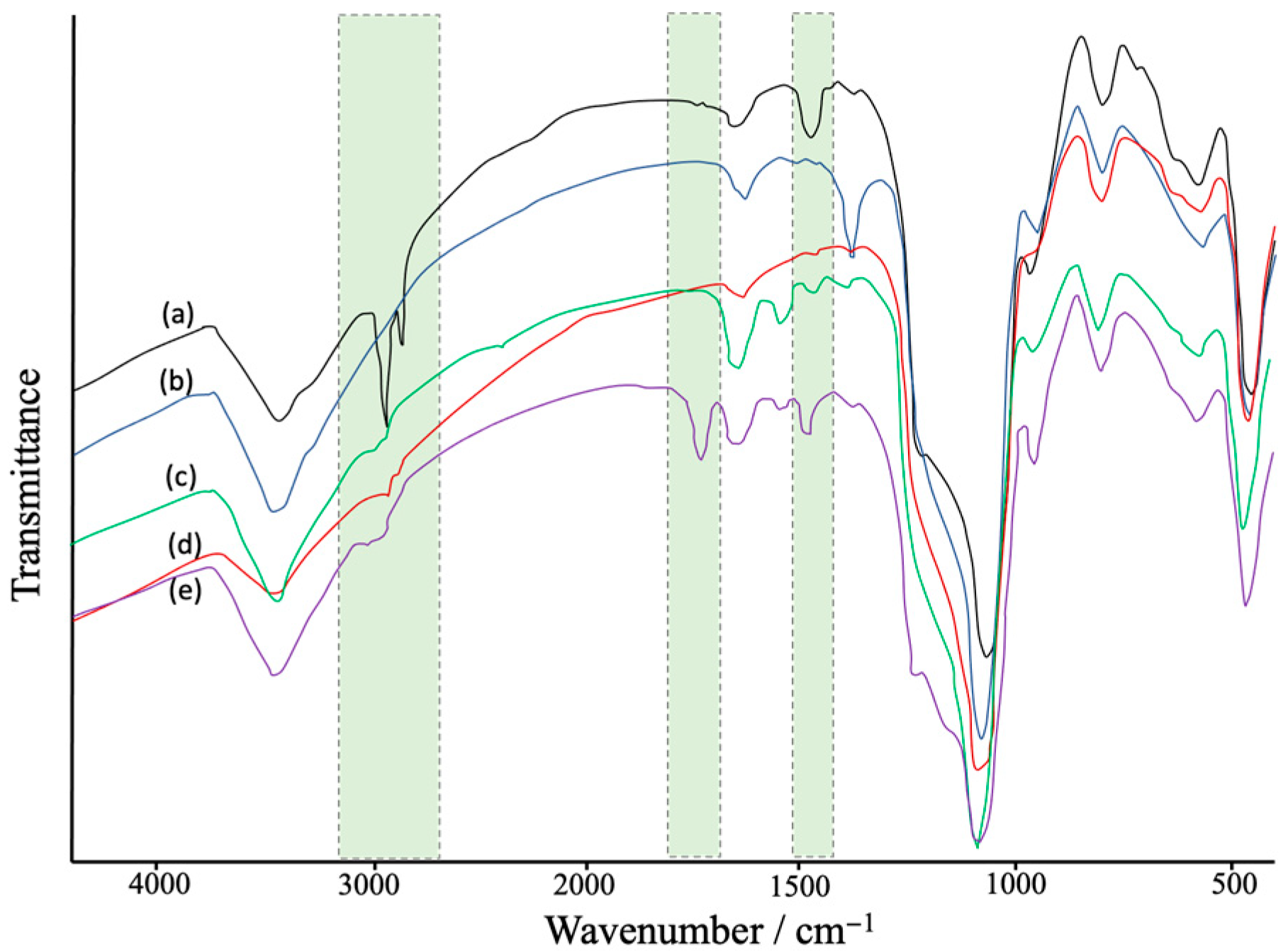
FTIR characterization showed the modification steps of Fe3O4-MSNs (Figure 2). The obtained results show a broad peaks at 1250–1050 cm−1 and 810 cm−1 that were assigned to Si–O band stretching of the silica network [48]. Peaks at ~2900 cm−1 and ~1430 cm−1 were assigned to C-H stretching mode of APTES-BIBB. After the polymerization process, a new peak appeared at ~1750 cm−1, which could be attributed to carbonyl group stretches of the polymer segments [49].
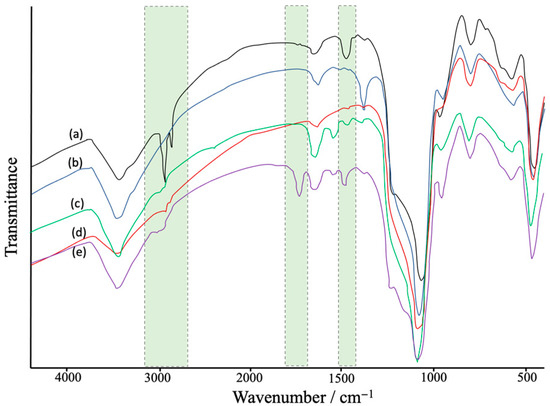
Figure 2.
FTIR spectra: (a) Fe3O4-MSNs with CTAB, (b) CTAB free Fe3O4-MSNs, (c) Fe3O4-MSNs-NH2 (d) Fe3O4-MSNs-Br, and (e) Fe3O4-MSNs-PMETAC.
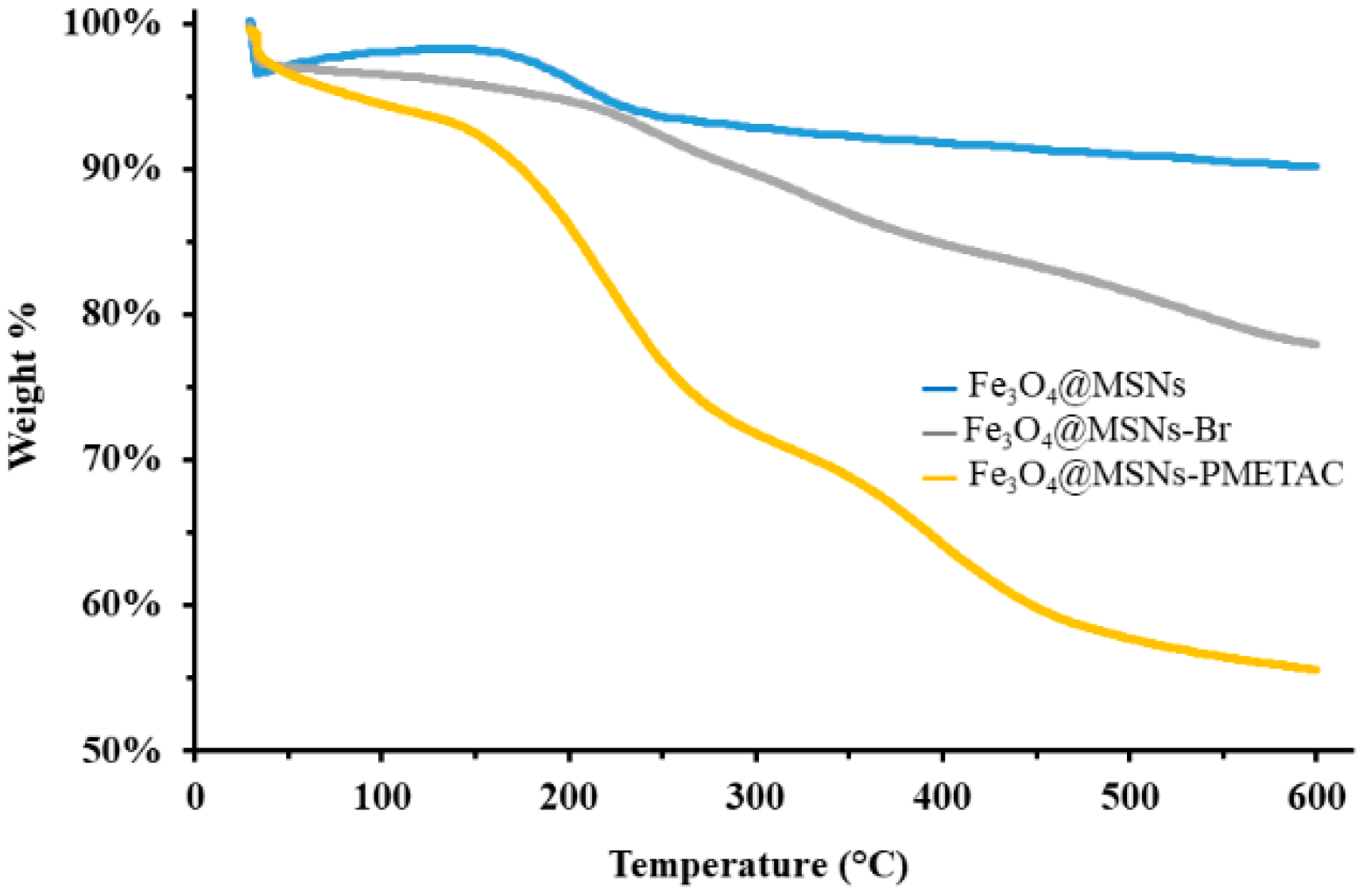
TGA analysis has been conducted to study the thermal stability and weight loss of the functionalized materials. CTAB-free Fe3O4-MSNs showed negligible weight loss with less than 5% at 600 °C, indicating the complete extraction of the template. The ATRP-initiator anchored on the Fe3O4-MSNs shows weight loss of around 20%; whereas the Fe3O4-MSNs-PMETAC shows weight loss of 45%, as demonstrated in Figure 3. The gradual weight increase confirms the successful synthesis of Fe3O4-MSNs-PMETAC.
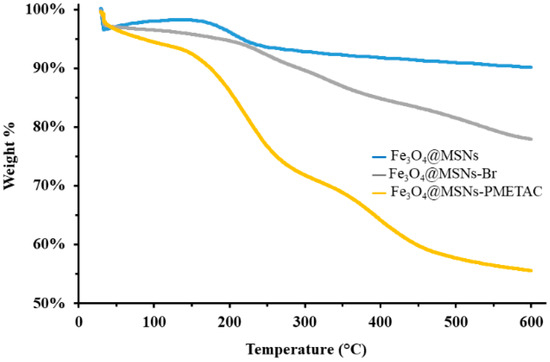
Figure 3.
TGA for CTAB-free Fe3O4-MSNs, Fe3O4-MSNs-Br and Fe3O4-MSNs-PMETAC.
3.2. Adsorption Studies
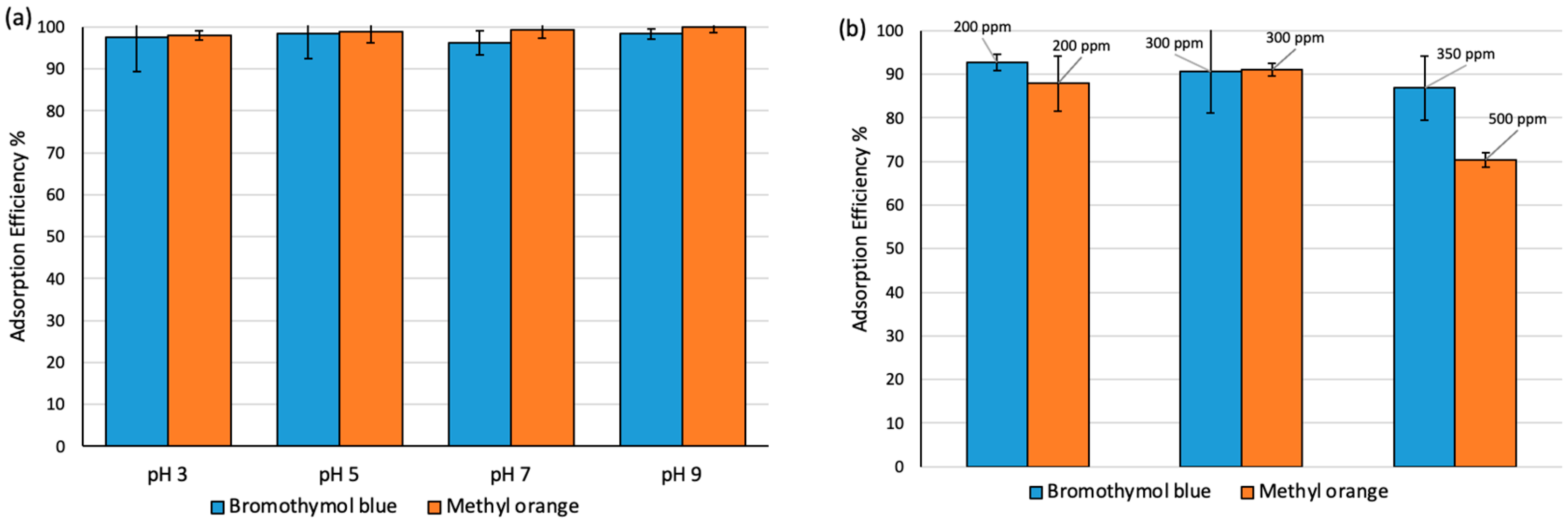
The effect of pH on the extraction efficiency of MO and BT at 350 ppm waw studied at different pH values between 3 to 9. The dosage of adsorbent was (10 mg/10 mL) with 3 h contact time at 25 °C. As shown in Figure 4a, the extraction efficiency of both dyes on Fe3O4-MSNs-PMETAC was observed to be ca. 100% at all studied pH levels. Furthermore, the effect of analytes concentration on the performance of Fe3O4-MSNs-PMETAC was studied using different dye concentrations (200, 300, and 500 ppm) at 25 °C and pH 7. As presented in Figure 4b, the amount of MO uptake by Fe3O4-MSNs-PMETAC decreased gradually as the concentration of dye increased from 300 to 500 ppm. On the other hand, a slight reduction on the extraction efficiency of the material for removal of BT has been observed.
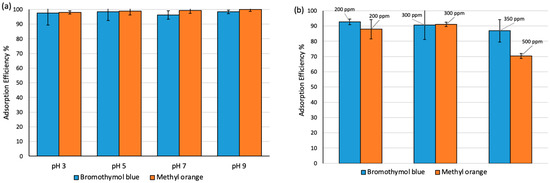
Figure 4.
(a) Effect of pH on the extraction efficiency of MO and BT by Fe3O4-MSNs-PMETAC (contact time 3 h at 25 °C and concentration is 100 ppm). (b) Adsorption Efficiency (%) at different concentrations 200, 300, 350 and 500 ppm by Fe3O4-MSNs-PMETAC (pH = 7 and 80 min at 25 °C).
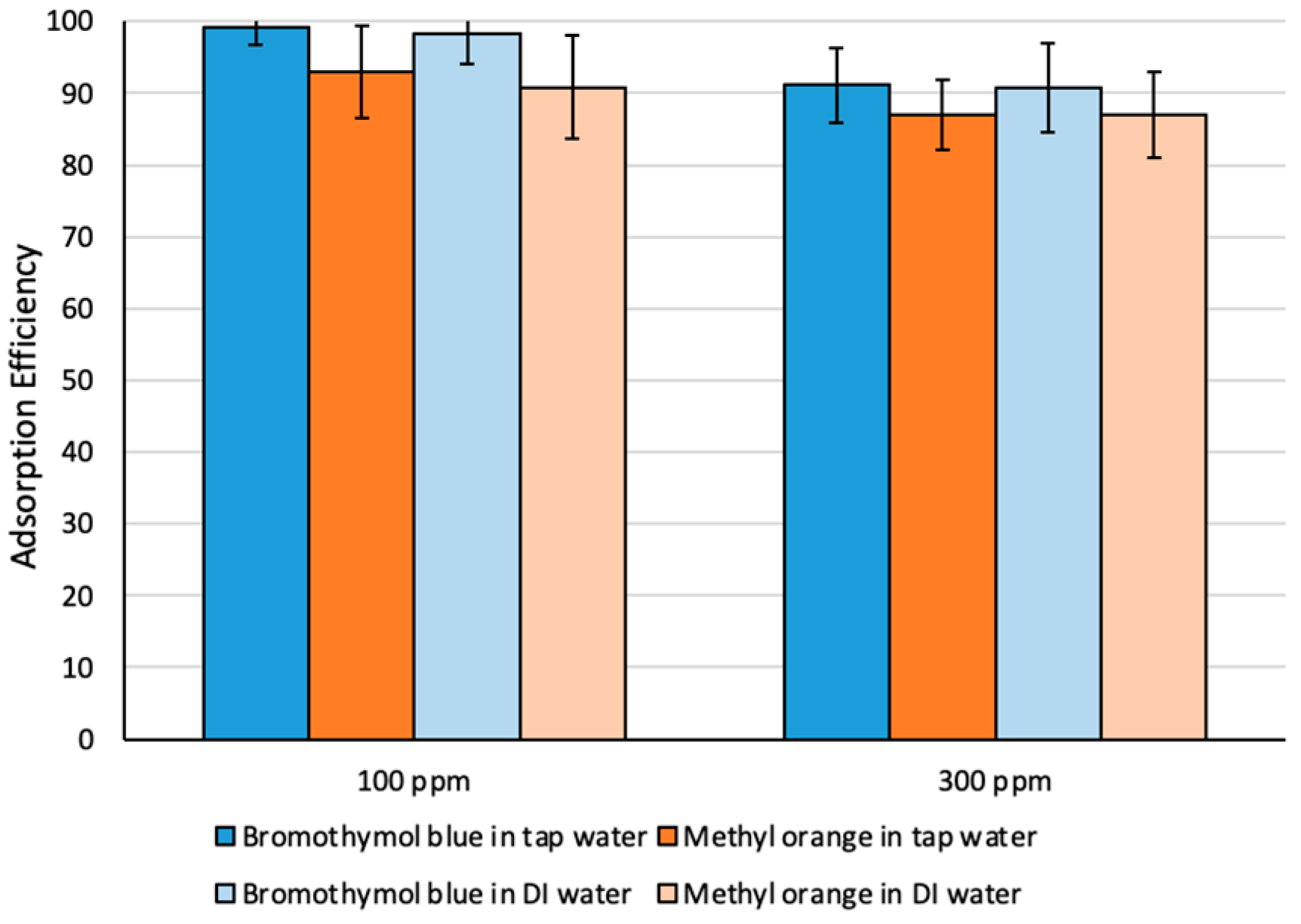
Adsorption efficiency has also been investigated using tap water spiked with different dye concentrations (Figure 5). More than 95% extraction efficiency was achieved, indicating that performance of Fe3O4-MSNs-PMETAC was not affected by the presence of the competitive ions.
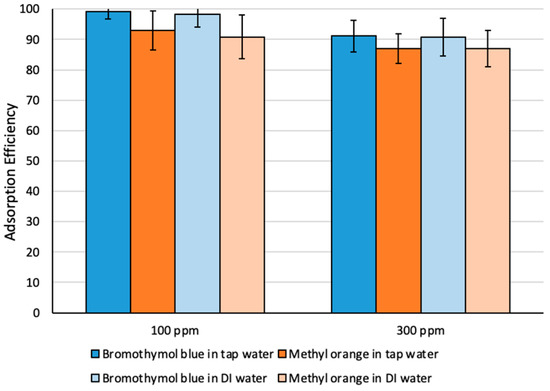
Figure 5.
Adsorption efficiency (%) of methyl orange and bromothymol blue dyes using different water types.
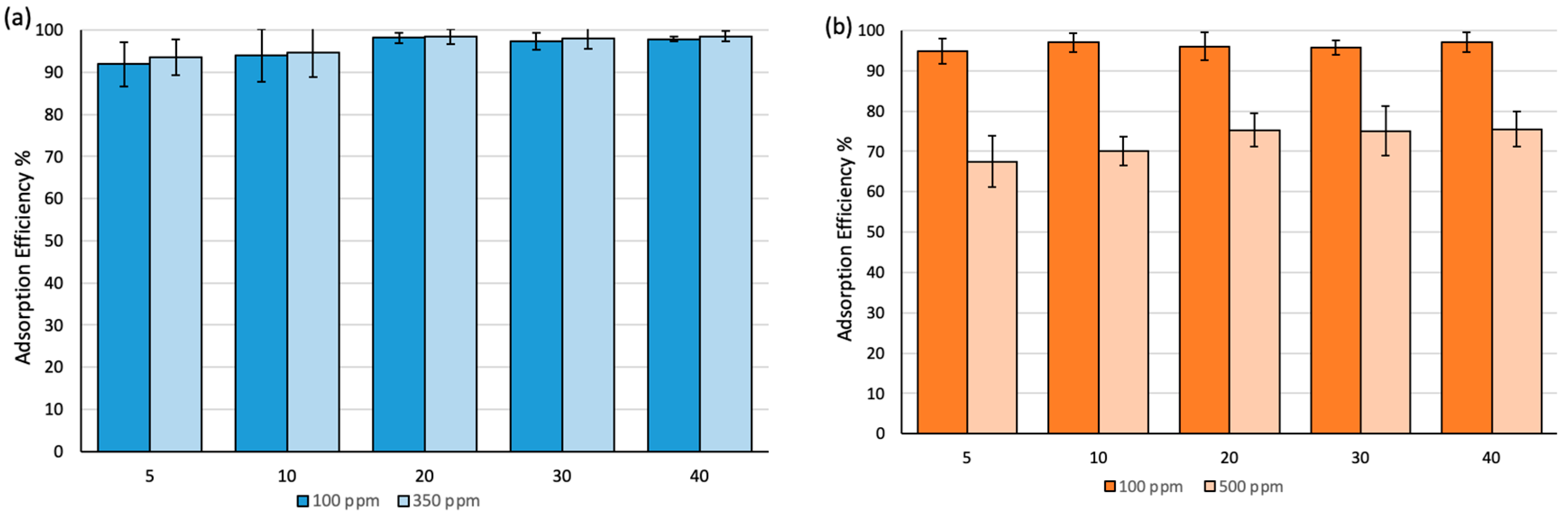
The effect of time variation on the adsorption efficiency has been studied over a 40 min time frame at different concentrations, as shown in Figure 6. The efficiency was ca. 90% in 5 min after exposure time at 100 ppm and 350 ppm of BT and reached equilibrium after 20 min of exposure time with almost 98% removal efficiency. Similar behavior was observed when MO was used (Figure 6b). However, the removal efficiency decreased to ~70% at 500 ppm.
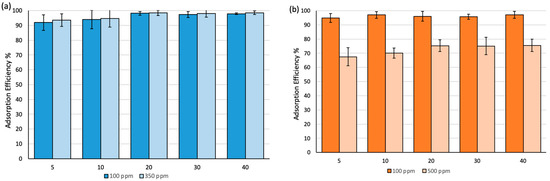
Figure 6.
Adsorption efficiency (%) at different exposure times: (a) Fe3O4-MSNs-PMETAC in bromothymol blue solution at pH = 7 and 25 °C, (b) Fe3O4-MSNs-PMETAC in methyl orange solution at pH = 7 and 25 °C.
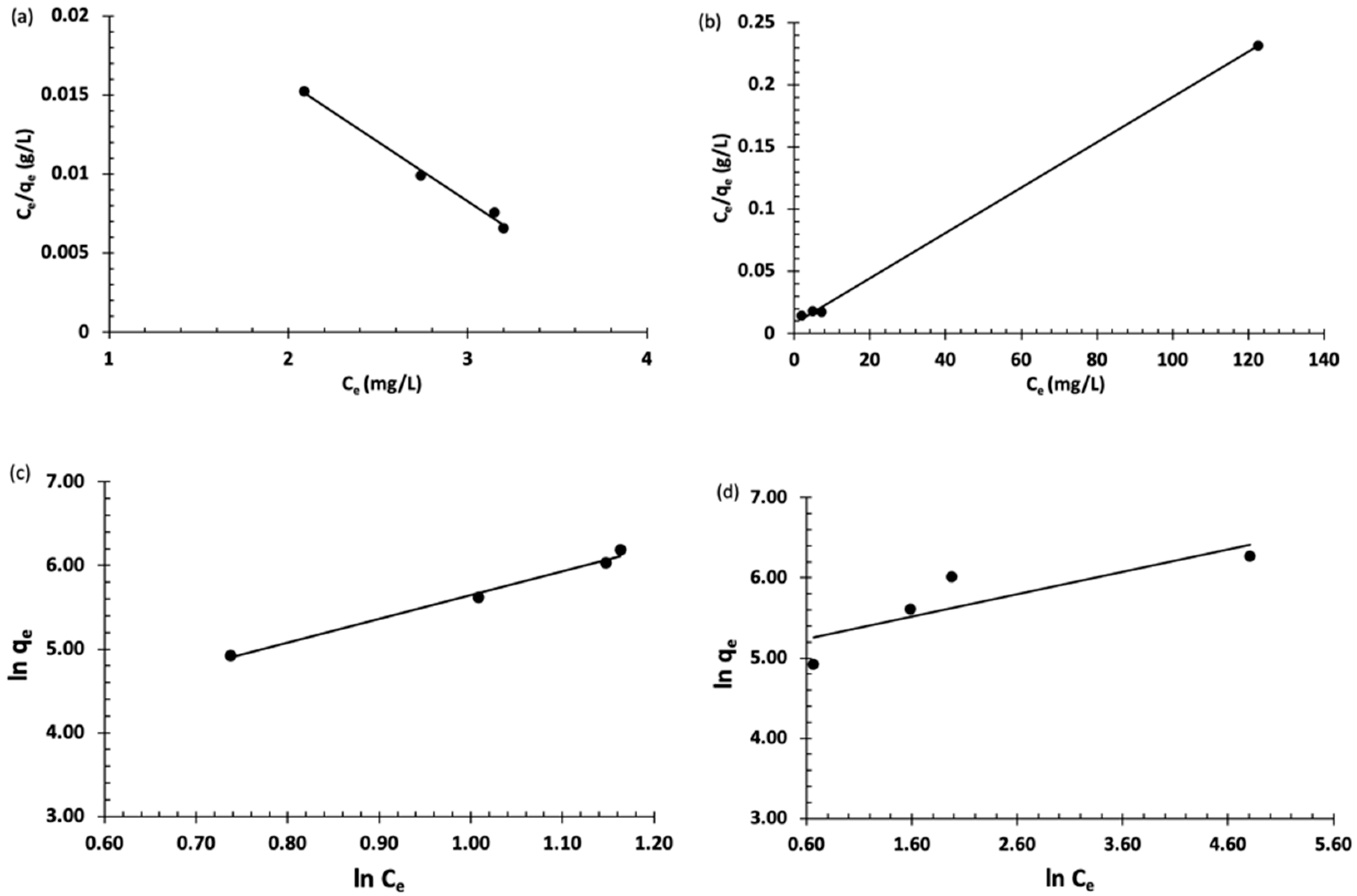
Langmuir and Freundlich’s isotherms are the most commonly used for modeling adsorption data. The Langmuir isotherm is used to describe a monolayer adsorption of the analyte on a homogeneous site, whereas Freundlich is applied to describe a multilayer adsorption on heterogeneous sites. These models were used to explain the distribution of the organic dyes between the adsorbent (Fe3O4-MSNs-PMETAC) and liquid phases (Figure 7). The adsorption of BT on the surface of Fe3O4-MSNs-PMETAC was found to follow the Freundlich model better than Langmuir’s model as indicated by the linear regression coefficient (R2) value. On the other hand, the adsorption data of MO was found to fit well with the Langmuir model with maximum adsorption capacity of 547.89 mg/g.
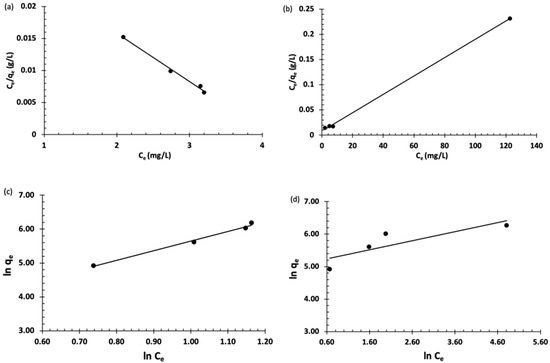
Figure 7.
(a,b) Langmuir isotherm for the adsorption of bromothymol blue and methyl orange, respectively, at pH = 7 and 25 °C. (c,d) Freundlich isotherm for the adsorption of bromothymol blue and methyl orange at pH = 7 and 25 °C.
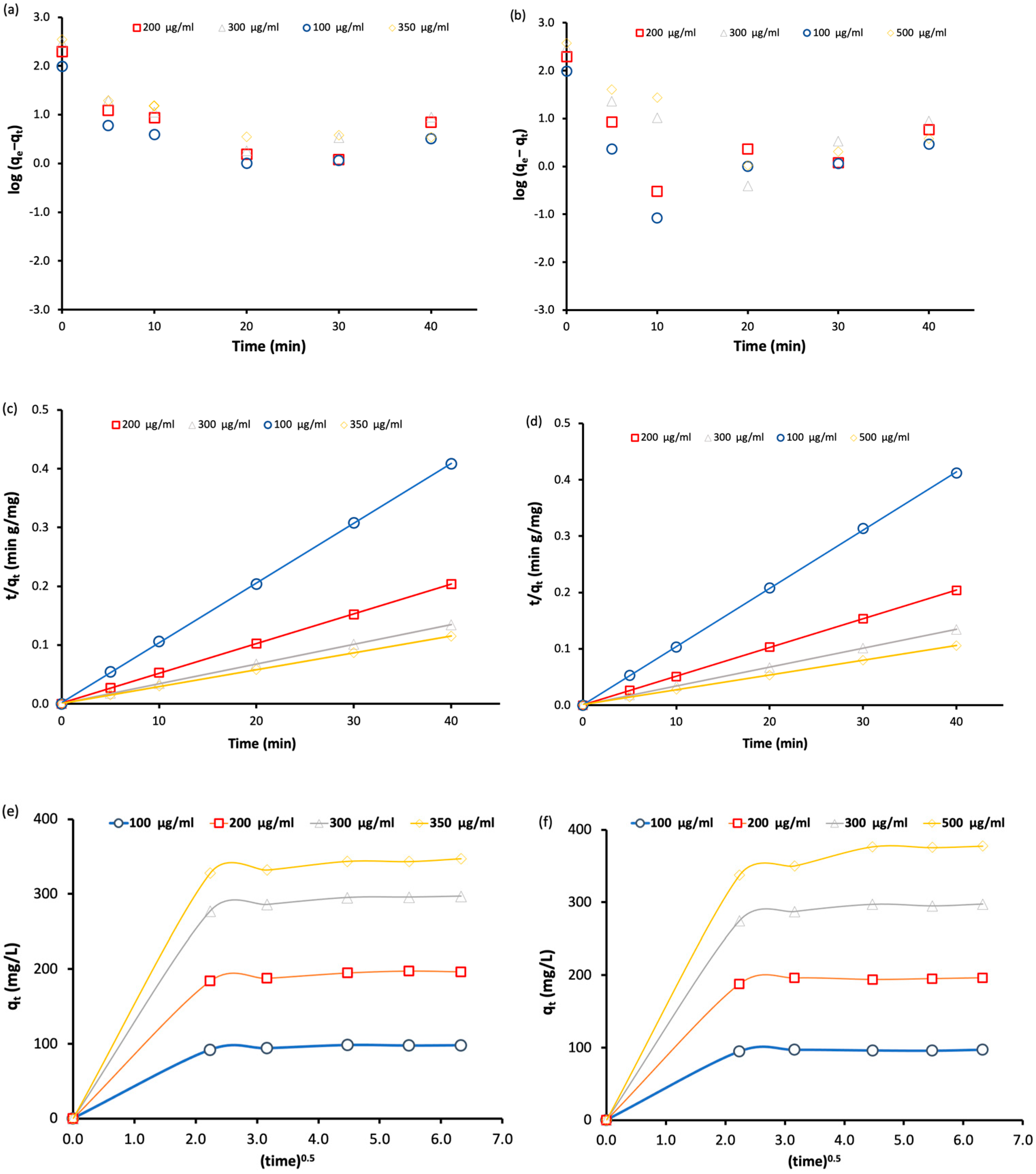
To determine the adsorption mechanism of BT and MO by Fe3O4-MSNs-PMETAC, three kinetic models were used: pseudo first-order, pseudo second-order and intraparticle diffusion model. Figure 8 shows the plot curves of the adsorption data of BT and MO fitted with three different models at different dyes’ concentration. In all cases, the adsorption data fitted well with pseudo second-order model at all dye concentrations as indicated by the correlation coefficients. When the intraparticle diffusion model was applied, the adsorption of both dyes (BT and MO) onto the surface of the adsorbent generally involved one fast step adsorption mechanism.
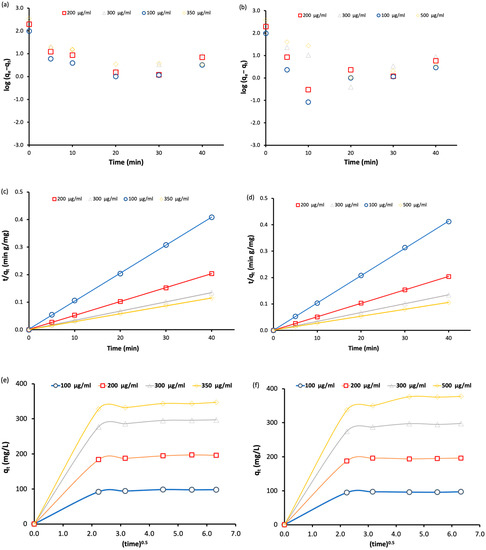
Figure 8.
(a,b) Pseudo-first-order kinetics for the adsorption of bromothymol blue and methyl orange, respectively, at pH = 7 and 25 °C. (c,d) Pseudo-second-order kinetics for the adsorption of bromothymol blue and methyl orange at pH = 7 and 25 °C. (e,f) Intraparticle diffusion models for the adsorption of bromothymol blue and methyl orange at pH = 7 and 25 °C.
A variety of materials have been developed to remove the MO and BT dyes from water. Fe3O4-MSNs-PMETAC performance in comparison to various adsorbents is shown in Table 1. As can be shown, Fe3O4-MSNs-PMETAC has greater adsorption capabilities than many other adsorbents employed in earlier investigations. Fe3O4-MSNs-PMETAC could be a promising candidate for efficient removal of anionic dyes, due to easy production and strong adsorption capability.

Table 1.
Comparison of the amount of methyl orange (MO) and bromothymol blue (BT) adsorbed on different materials.
4. Conclusions
In the present work, magnetic mesoporous nanoparticles were synthesized and modified with cationic polymer brush (poly(2-(methacryloyloxy)ethyl] trimethylammonium chloride solution). The performance of the prepared material was then evaluated for the removal of two types of ionic dyes (MO and BT). The impact of different parameters such as analyte dosage, contact time and pH level were studied. The results confirm the ability of Fe3O4-MSNs-PMETAC to remove MB and BT ions from contaminated water samples with 100% extraction efficiency. The linear regression coefficient value for the studied isotherms suggested that the adsorption behavior of BT by Fe3O4-MSNs-PMETAC is better approximated by the Freundlich isotherm, whereas in the case of MO, it follows the Langmuir isotherm. The kinetic behavior of both dyes was found to be a second-order process. These results indicate that the prepared material could introduce a new direction of producing molecularly designed adsorbents that involve the combination of magnetic mesoporous nanoparticles with the highly resourceful field of polymer brushes with tunable properties for the removal of pollutants from aqueous solutions.
Author Contributions
L.A., H.A. performed the experiments and analyzed the data. K.A., A.B., R.A. and A.A. designed the experiments, performed the experiments, analyzed the data, wrote the draft paper, and revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by Researchers Supporting Project number (RSP-2021/239), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Sikosana, M.L.; Sikhwivhilu, K.; Moutloali, R.; Madyira, D.M. Municipal wastewater treatment technologies: A review. Procedia Manuf. 2019, 35, 1018–1024. [Google Scholar] [CrossRef]
- Lannelongue, G.; Gonzalez-Benito, J.; Quiroz, I. Environmental management and labour productivity: The moderating role of capital intensity. J. Environ. Manag. 2017, 190, 158–169. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Brozinski, J.-M.; Lahti, M.; Meierjohann, A.; Oikari, A.; Kronberg, L. The Anti-Inflammatory Drugs Diclofenac, Naproxen and Ibuprofen are found in the Bile of Wild Fish Caught Downstream of a Wastewater Treatment Plant. Environ. Sci. Technol. 2013, 47, 342–348. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Yang, H. Assessing water scarcity by simultaneously considering environmental flow requirements, water quantity, and water quality. Ecol. Indic. 2016, 60, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Aghdasinia, H.; Mohammadi, R.; Ramavandi, B. Modification of bio-hydroxyapatite generated from waste poultry bone with MgO for purifying methyl violet-laden liquids. Environ. Sci. Pollut. Res. 2020, 27, 44218–44229. [Google Scholar] [CrossRef]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar] [CrossRef]
- Esvandi, Z.; Foroutan, R.; Peighambardoust, S.J.; Akbari, A.; Ramavandi, B. Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surfaces Interfaces 2020, 21, 100754. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Huan, Y.; Hao, C.; Chen, W. Acrylic acid functionalized graphene oxide: High-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 2020, 261, 127736. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Wang, Y.; Liu, Z.; Li, Y.; Yang, H.; Xu, Z.-L. High efficient dye removal with hydrolyzed ethanolamine-Polyacrylonitrile UF membrane: Rejection of anionic dye and selective adsorption of cationic dye. Chemosphere 2020, 259, 127390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, X.; Huang, Y.; Keller, A.A.; Lan, J. Competitive removal of Pb2+ and malachite green from water by magnetic phosphate nanocomposites. Water Res. 2019, 150, 442–451. [Google Scholar] [CrossRef]
- Qiao, X.-Q.; Zhang, Z.-W.; Li, Q.-H.; Hou, D.; Zhang, Q.; Zhang, J.; Li, D.-S.; Feng, P.; Bu, X. In situ synthesis of n–n Bi2MoO6 & Bi2S3 heterojunctions for highly efficient photocatalytic removal of Cr(vi). J. Mater. Chem. A 2018, 6, 22580–22589. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Peralta, M.E.; Ocampo, S.; Funes, I.G.; Medina, F.O.; Parolo, M.E.; Carlos, L. Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics 2020, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Amari, A.; Alzahrani, F.; Alsaiari, N.; Katubi, K.; Rebah, F.; Tahoon, M. Magnetic Metal Organic Framework Immobilized Laccase for Wastewater Decolorization. Processes 2021, 9, 774. [Google Scholar] [CrossRef]
- Katubi, K.; Alsaiari, N.; Alzahrani, F.; Siddeeg, S.M.; Tahoon, M.A. Synthesis of Manganese Ferrite/Graphene Oxide Magnetic Nanocomposite for Pollutants Removal from Water. Processes 2021, 9, 589. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Li, H.; Zhao, C.; Zhao, R.; Li, S. Highly selective uranium adsorption on 2-phosphonobutane-1,2,4-tricarboxylic acid-decorated chitosan-coated magnetic silica nanoparticles. Chem. Eng. J. 2020, 388, 124349. [Google Scholar] [CrossRef]
- Wang, P.; Shen, T.; Li, X.; Tang, Y.; Li, Y. Magnetic Mesoporous Calcium Carbonate-Based Nanocomposites for the Removal of Toxic Pb(II) and Cd(II) Ions from Water. ACS Appl. Nano Mater. 2020, 3, 1272–1281. [Google Scholar] [CrossRef]
- Naushad, M.; Vasudevan, S.; Sharma, G.; Kumar, A.; Alothman, Z. Adsorption kinetics, isotherms, and thermodynamic studies for Hg2+ adsorption from aqueous medium using alizarin red-S-loaded amberlite IRA-400 resin. Desalination Water Treat. 2016, 57, 18551–18559. [Google Scholar] [CrossRef]
- Hadi, P.; Guo, J.; Barford, J.; McKay, G. Multilayer Dye Adsorption in Activated Carbons—Facile Approach to Exploit Vacant Sites and Interlayer Charge Interaction. Environ. Sci. Technol. 2016, 50, 5041–5049. [Google Scholar] [CrossRef] [PubMed]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated Carbon for Dyes Removal: Modeling and Understanding the Adsorption Process. J. Chem. 2020, 2020, 2096834. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, M.; Zhao, X.; Song, S.; Gao, Z. Ultra-High-Capacity Adsorption of Rhodamine B in a Carboxyl-Functionalized Metal–Organic Framework via Surface Adsorption. J. Chem. Eng. Data 2020, 66, 669–676. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Singh, S.P.; Badhulika, S. Reusable, few-layered-MoS2 nanosheets/graphene hybrid on cellulose paper for superior adsorption of methylene blue dye. New J. Chem. 2020, 44, 5489–5500. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Hassan, M.S.; Alam, M.M.; Zabin, S.A.; Alsenani, N.I.; Baghdadi, N.E. Natural Clay as a Low-Cost Adsorbent for Crystal Violet Dye Removal and Antimicrobial Activity. Nanomaterials 2021, 11, 2789. [Google Scholar] [CrossRef]
- Hanafy, A.; Hegab, I. Effects of egg weight and light sources during incubation period on embryonic development and post-hatch growth of Japanese quail (Coturnix japonica). Eur. Poult. Sci. 2019. [Google Scholar] [CrossRef]
- Shurair, M.; Almomani, F.; Bhosale, R.; Khraisheh, M.; Qiblawey, H. Harvesting of intact microalgae in single and sequential conditioning steps by chemical and biological based-flocculants: Effect on harvesting efficiency, water recovery and algal cell morphology. Bioresour. Technol. 2019, 281, 250–259. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Du, Z.-D.; Cui, Y.-Y.; Yang, C.-X.; Yan, X.-P. Synthesis of magnetic amino-functionalized microporous organic network composites for magnetic solid phase extraction of endocrine disrupting chemicals from water, beverage bottle and juice samples. Talanta 2020, 206, 120179. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Abdalla, M.A.; Ahamad, T.; Alothman, Z.; Alshehri, S.M.; Ghfar, A.A. Efficient removal of toxic metal ions from wastewater using a recyclable nanocomposite: A study of adsorption parameters and interaction mechanism. J. Clean. Prod. 2017, 156, 426–436. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Oliveira, R.V.M.; Lima, J.R.A.; Cunha, G.D.C.; Romão, L.P.C. Use of eco-friendly magnetic materials for the removal of polycyclic aromatic hydrocarbons and metals from environmental water samples. J. Environ. Chem. Eng. 2020, 8, 104050. [Google Scholar] [CrossRef]
- Burnett, A.L.; Chang, A.G.; Crone, J.K.; Huang, P.; Sezen, S.F. Noncholinergic Penile Erection in Mice Lacking the Gene for Endothelial Nitric Oxide Synthase. J. Androl. 2002, 23, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Kamgar, A.; Hassanajili, S.; Karimipourfard, G. Fe3O4@SiO2@MPS core/shell nanocomposites: The effect of the core weight on their magnetic properties and oil separation performance. J. Environ. Chem. Eng. 2018, 6, 3034–3040. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; AlHazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic Acid-Terminated Poly (2-Diethyl Amino Ethyl Methacrylate) Brush-Gated Magnetic Mesoporous Nanoparticles as a Smart Drug Delivery System. Polymers 2020, 13, 59. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Alswieleh, A.M. Efficient Removal of Dyes from Aqueous Solution by Adsorption on L-Arginine-Modified Mesoporous Silica Nanoparticles. Processes 2022, 10, 1079. [Google Scholar] [CrossRef]
- Beagan, A.; Alotaibi, K.; Almakhlafi, M.; Algarabli, W.; Alajmi, N.; Alanazi, M.; Alwaalah, H.; Alharbi, F.; Alshammari, R.; Alswieleh, A. Amine and sulfonic acid functionalized mesoporous silica as an effective adsorbent for removal of methylene blue from contaminated water. J. King Saud Univ. Sci. 2022, 34, 101762. [Google Scholar] [CrossRef]
- Alswieleh, A.M. Remediation of cationic and anionic dyes from water by histidine modified mesoporous silica. Int. J. Environ. Anal. Chem. 2021, 1–13. [Google Scholar] [CrossRef]
- Arica, T.A.; Ayas, E.; Arica, M.Y. Magnetic MCM-41 silica particles grafted with poly(glycidylmethacrylate) brush: Modification and application for removal of direct dyes. Microporous Mesoporous Mater. 2017, 243, 164–175. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Almomani, F. Functionalization of silica-coated magnetic nanoparticles as powerful demulsifier to recover oil from oil-in-water emulsion. Chemosphere 2021, 279, 130360. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef]
- Alotaibi, K.M.; Almethen, A.A.; Beagan, A.M.; Al-Swaidan, H.M.; Ahmad, A.; Bhawani, S.A.; Alswieleh, A.M. Quaternization of Poly(2-diethyl aminoethyl methacrylate) Brush-Grafted Magnetic Mesoporous Nanoparticles Using 2-Iodoethanol for Removing Anionic Dyes. Appl. Sci. 2021, 11, 10451. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar] [CrossRef]
- Alswieleh, A.M.; Beagan, A.M.; Alsheheri, B.M.; Alotaibi, K.M.; Alharthi, M.D.; Almeataq, M.S. Hybrid Mesoporous Silica Nanoparticles Grafted with 2-(tert-butylamino)ethyl Methacrylate-b-poly(ethylene Glycol) Methyl Ether Methacrylate Diblock Brushes as Drug Nanocarrier. Molecules 2020, 25, 195. [Google Scholar] [CrossRef] [Green Version]
- Linde, M.P.; Marquez, K. Alkali Lignin from Rice (Oryza sativa L.) Husk as Adsorbent for Aqueous Methyl Orange and Bromothymol Blue: Analysis of the Adsorption Kinetics and Mechanism. KIMIKA 2021, 32, 19–33. [Google Scholar] [CrossRef]
- Adewuyi, A.; Oderinde, R.A. Removal of phenolphthalein and methyl orange from laboratory wastewater using tetraethylammonium modified kaolinite clay. Curr. Res. Green Sustain. Chem. 2022, 5, 100320. [Google Scholar] [CrossRef]
- Lubbad, S.H.; Abu Al-Roos, B.K.; Kodeh, F.S. Adsorptive-removal of Bromothymol Blue as Acidic-dye Probe from Water Solution Using Latvian Sphagnum Peat Moss: Thermodynamic Assessment, Kinetic and Isotherm Modeling. Curr. Green Chem. 2019, 6, 53–61. [Google Scholar] [CrossRef]
- Tahari, N.; de Hoyos-Martinez, P.L.; Izaguirre, N.; Houwaida, N.; Abderrabba, M.; Ayadi, S.; Labidi, J. Preparation of chitosan/tannin and montmorillonite films as adsorbents for Methyl Orange dye removal. Int. J. Biol. Macromol. 2022, 210, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Tabti, A.; Benchikh, I.; Serier, M.; Launay, F.; Djafri, F. Adsorption of Bromothymol Blue (BTB) Dye Using Four Zeolites as Adsorbent. Kem. U Ind. 2021, 70, 243–250. [Google Scholar] [CrossRef]
- Riaz, Q.; Ahmed, M.; Zafar, M.N.; Zubair, M.; Nazar, M.F.; Sumrra, S.H.; Ahmad, I.; Hosseini-Bandegharaeic, A. NiO nanoparticles for enhanced removal of methyl orange: Equilibrium, kinetics, thermodynamic and desorption studies. Int. J. Environ. Anal. Chem. 2022, 102, 84–103. [Google Scholar] [CrossRef]
- Ghaedi, M.; Khajehsharifi, H.; Yadkuri, A.H.; Roosta, M.; Asghari, A. Oxidized multiwalled carbon nanotubes as efficient adsorbent for bromothymol blue. Toxicol. Environ. Chem. 2012, 94, 873–883. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Zhang, Q.; Pu, Y.; Wang, J.; Cao, L.; Zhou, S.; Li, Q.; Qiao, C. ZrMOX Particles for Enhanced Removal of Methyl Orange from Wastewater: Preparation, Characterization, and Adsorption Study. Adsorpt. Sci. Technol. 2022, 2022, 9685352. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nazari, E.; Sahraie, R.; Purkait, M.K. Kinetic and isotherm study of Bromothymol Blue and Methylene blue removal using Au-NP loaded on activated carbon. Desalination Water Treat. 2014, 52, 5504–5512. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).