Influence of Pretreatments and Freeze-Drying Conditions of Strawberries on Drying Kinetics and Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Pretreatment
2.2. Drying Method
2.3. Modeling of Drying Curves
2.4. Measurement of Color Coordinates
2.5. Water Activity
2.6. Total Phenolic Content (TPC) and Antioxidant Activity (AA)
2.7. Ascorbic Acid Content
2.8. Statistical Analysis
3. Results and Discussion
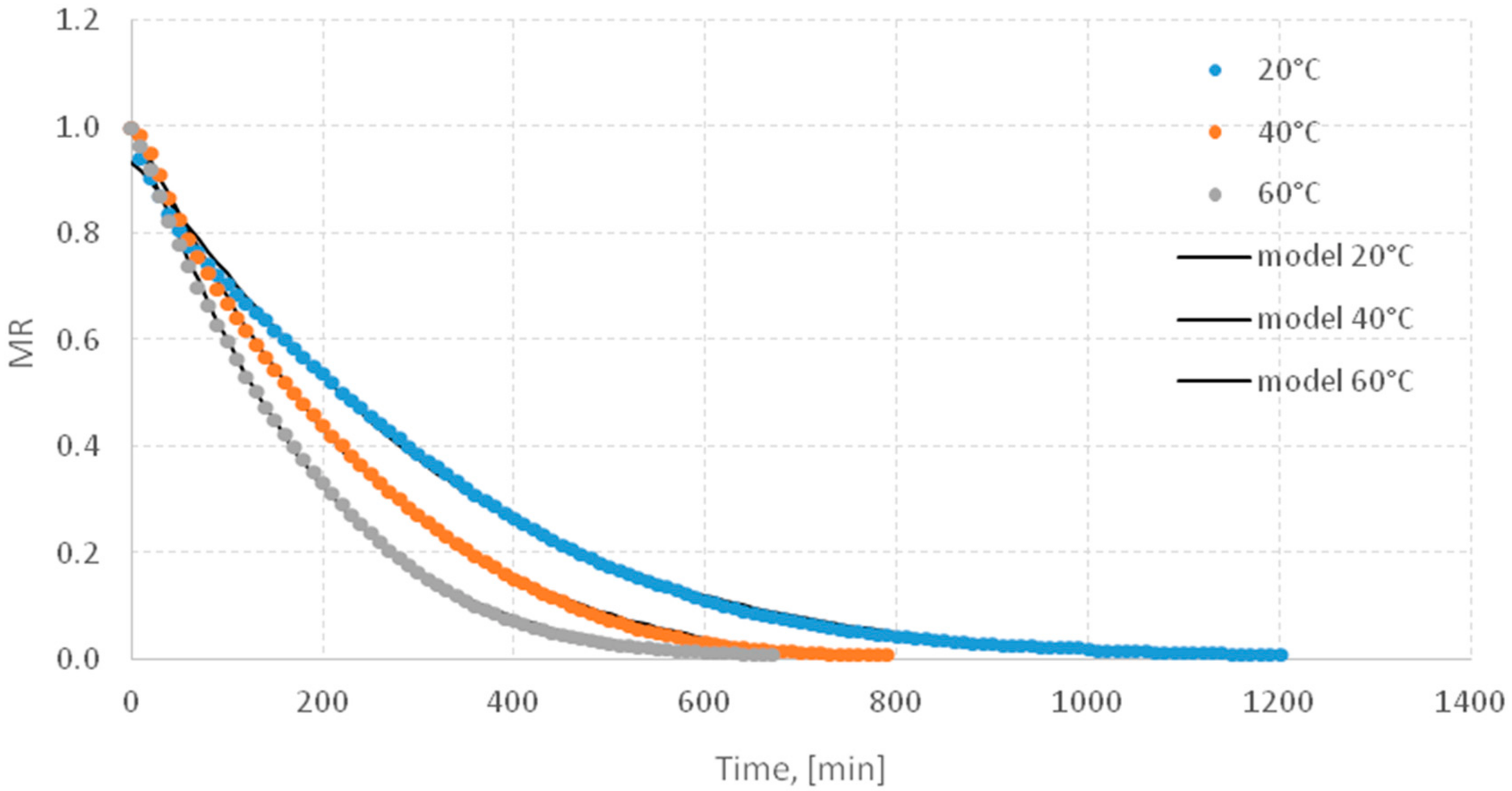
3.1. Drying Kinetics
3.2. Color Changes and Water Activity
3.3. TPC and AA
3.4. Ascorbic Acid Changes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The Strawberry: Composition, Nutritional Quality, and Impact on Human Health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Karakasova, L.; Ampova, J.; Babanovska-Milenkovska, F.; Durmishi, N.; Stamatovska, V. Comparison of Quality Characteristics of Fresh and of Dried Strawberries. In Proceedings of the 2017 UBT International Conference, University for Business and Technology, Pristina, Kosovo, 28 October 2017. [Google Scholar]
- Muzaffar, H.; Rouf, A.; Kanojia, V.; Muzaffar, Z.; Noor, F. Dehydration of Strawberry—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1216–1224. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry As a Functional Food: An Evidence-Based Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Crecente-Campo, J.; Nunes-Damaceno, M.; Romero-Rodríguez, M.A.; Vázquez-Odériz, M.L. Color, Anthocyanin Pigment, Ascorbic Acid and Total Phenolic Compound Determination in Organic versus Conventional Strawberries (Fragaria × ananassa Duch, Cv Selva). J. Food Compos. Anal. 2012, 28, 23–30. [Google Scholar] [CrossRef]
- Alonzo-Macías, M.; Cardador-Martínez, A.; Mounir, S.; Montejano-Gaitán, G.; Allaf, K. Comparative Study of the Effects of Drying Methods on Antioxidant Activity of Dried Strawberry (Fragaria Var. Camarosa). J. Food Res. 2013, 2, 92. [Google Scholar] [CrossRef]
- Dziki, D. Recent Trends in Pretreatment of Food before Freeze-Drying. Processes 2020, 8, 1661. [Google Scholar] [CrossRef]
- Anjaneyulu, A.; Sharangi, A.B.; Upadhyay, T.K.; Alshammari, N.; Saeed, M.; Al-Keridis, L.A. Physico-Chemical Properties of Red Pepper (Capsicum annuum L.) as Influenced by Different Drying Methods and Temperatures. Processes 2022, 10, 484. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Janiszewska-Turak, E.; Biernacka, B. Wild Strawberry Fragaria vesca L.: Kinetics of Fruit Drying and Quality Characteristics of the Dried Fruits. Processes 2020, 8, 1265. [Google Scholar] [CrossRef]
- Magied, M.M.A.; Ali, M.R. Effect of Drying Method on Physical Properties and Bioactive Compounds of Red Chili Pepper “Capsicum annuum L.”. Curr. Nutr. Food Sci. 2017, 13, 43–49. [Google Scholar] [CrossRef]
- Dziki, D.; Polak, R.; Rudy, S.; Krzykowski, A.; Gawlik-Dziki, U.; Różyło, R.; Miś, A.; Combrzyński, M. Simulation of the Process Kinetics and Analysis of Physicochemical Properties in the Freeze Drying of Kale. Int. Agrophysics 2018, 32, 49–56. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; 5th revision; Cunniff, P., Ed.; AOAC: Washington, DC, USA, 1999. [Google Scholar]
- Sarimeseli, A. Microwave Drying Characteristics of Coriander (Coriandrum sativum L.) Leaves. Energy Convers. Manag. 2011, 52, 1449–1453. [Google Scholar] [CrossRef]
- Demir, V.; Gunhan, T.; Yagcioglu, A.K.; Degirmencioglu, A. Mathematical Modelling and the Determination of Some Quality Parameters of Air-Dried Bay Leaves. Biosyst. Eng. 2004, 88, 325–335. [Google Scholar] [CrossRef]
- Henderson, S.M.; Pabis, S. Grain Drying Theory: Temperature Effect on Drying Coefficient. J. Agric. Eng. Res. 1961, 6, 169–174. [Google Scholar]
- Dandamrongrak, R.; Young, G.; Mason, R. Evaluation of Various Pre-Treatments for the Dehydration of Banana and Selection of Suitable Drying Models. J. Food Eng. 2002, 55, 139–146. [Google Scholar] [CrossRef]
- Wang, C.Y.; Singh, R.P. Use of Variable Equilibrium Moisture Content in Modeling Rice Drying. Trans. ASAE 1978, 11, 668–672. [Google Scholar]
- Soysal, Y.; Öztekin, S.; Eren, Ö. Microwave Drying of Parsley: Modelling, Kinetics, and Energy Aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A New Model for Single-Layer Drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Serin, S.; Turhan, K.N.; Turhan, M. Correlation between Water Activity and Moisture Content of Turkish Flower and Pine Honeys. Food Sci. Technol. 2018, 38, 238–243. [Google Scholar] [CrossRef]
- Romankiewicz, D.; Hassoon, W.H.; Cacak-Pietrzak, G.; Sobczyk, M.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The Effect of Chia Seeds (Salvia hispanica L.) Addition on Quality and Nutritional Value of Wheat Bread. J. Food Qual. 2017, 2017, 7352631. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Kaszuba, K.; Piwowarczyk, K.; Świeca, M.; Dziki, D.; Czyż, J. Onion Skin—Raw Material for the Production of Supplement That Enhances the Health-Beneficial Properties of Wheat Bread. Food Res. Int. 2015, 73, 97–106. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sujka, K.; Cacak-Pietrzak, G.; Sułek, A.; Murgrabia, K.; Dziki, D. Buckwheat Hull-Enriched Pasta: Physicochemical and Sensory Properties. Molecules 2022, 27, 4065. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Raju, R.; Mrad, M.; Reddell, P.; Münch, G. The Reciprocal EC50 Value as a Convenient Measure of the Potency of a Compound in Bioactivity-Guided Purification of Natural Products. Fitoterapia 2020, 143, 104598. [Google Scholar] [CrossRef] [PubMed]
- Fontannaz, P.; Kilinç, T.; Heudi, O. HPLC-UV Determination of Total Ascorbic Acid in a Wide Range of Fortified Food Products. Food Chem. 2006, 94, 626–631. [Google Scholar] [CrossRef]
- Rudy, S.; Dziki, D.; Krzykowski, A.; Gawlik-Dziki, U.; Polak, R.; Różyło, R.; Kulig, R. Influence of Pre-Treatments and Freeze-Drying Temperature on the Process Kinetics and Selected Physico-Chemical Properties of Cranberries (Vaccinium macrocarpon Ait.). LWT Food Sci. Technol. 2015, 63, 497–503. [Google Scholar] [CrossRef]
- Suramya, M.; Hemantha, J. Mathematical Modeling of Drying Characteristics of Chilli in Hot Air Oven and Fluidized Bed Dryers. Agric. Eng. Int. CIGR J. 2013, 15, 154–166. [Google Scholar]
- Zarein, M. Kinetic Drying and Mathematical Modeling of Apple Slices on Dehydration Process. J. Food Process. Technol. 2013, 4, 7. [Google Scholar] [CrossRef]
- Darıcı, S.; Şen, S. Experimental Investigation of Convective Drying Kinetics of Kiwi under Different Conditions. Heat Mass Transf. 2015, 51, 1167–1176. [Google Scholar] [CrossRef]
- Kowalska, J.; Kowalska, H.; Marzec, A.; Brzeziński, T.; Samborska, K.; Lenart, A. Dried Strawberries as a High Nutritional Value Fruit Snack. Food Sci. Biotechnol. 2018, 27, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Shishehgarha, F.; Makhlouf, J.; Ratti, C. Freeze-Drying Characteristics of Strawberries. Dry. Technol. 2002, 20, 131–145. [Google Scholar] [CrossRef]
- Sandulachi, E.; Tatarov, P. Water Activity Concept and Its Role in Strawberries. Food Chem. J. Mold. 2012, 7, 103–115. [Google Scholar] [CrossRef]
- Escriche, I.; Chiralt, A.; Moreno, J.; Serra, J.A. Influence of Blanching-Osmotic Dehydration Treatments on Volatile Fraction of Strawberries. J. Food Sci. 2000, 65, 1107–1111. [Google Scholar] [CrossRef]
- Gulati, T.; Datta, A.K. Mechanistic understanding of case-hardening and texture development during drying of food materials. J. Food Eng. 2015, 166, 119–138. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of Drying Methods with the Application of Vacuum Microwaves on the Bioactive Compounds, Color, and Antioxidant Activity of Strawberry Fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Orak, H.; Aktas, T.; Yagar, H.; İsbilir, S.S.; Ekinci, N.; Sahin, F.H. Effects of Hot Air and Freeze Drying Methods on Antioxidant Activity, Colour and Some Nutritional Characteristics of Strawberry Tree (Arbutus unedo L.) Fruit. Food Sci. Technol. Int. 2012, 18, 391–402. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; del Camacho, M.M.; Martínez-Navarrete, N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods 2019, 9, 32. [Google Scholar] [CrossRef]



| Number | Model Name | Equation |
|---|---|---|
| 1 | Newton [14] | |
| 2 | Page [15] | |
| 3 | Henderson and Pabis [16] | |
| 4 | Logarithmic [17] | |
| 5 | Wang and Singh [18] | |
| 6 | Logistic [19] | |
| 7 | Midilli [20] |
| Model Name | Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 40 °C | 60 °C | |||||||
| R2 | RMSE | χ2 | R2 | RMSE | χ2 | R2 | RMSE | χ2 | |
| Newton | 0.9947 | 0.0193 | 0.0004 | 0.9906 | 0.0277 | 0.0008 | 0.9931 | 0.0234 | 0.00055 |
| Page | 0.9959 | 0.0168 | 0.0003 | 0.9982 | 0.0120 | 0.0001 | 0.9994 | 0.0069 | 0.00005 |
| Henderson and Pabis | 0.9947 | 0.0193 | 0.0004 | 0.9939 | 0.0222 | 0.0005 | 0.9959 | 0.0182 | 0.00034 |
| Logarithmic | 0.9974 | 0.0134 | 0.0002 | 0.9989 | 0.0096 | 0.0001 | 0.9987 | 0.0103 | 0.00011 |
| Wang and Singh | 0.9700 | 0.0457 | 0.0021 | 0.9922 | 0.0252 | 0.0007 | 0.9858 | 0.0337 | 0.00117 |
| Logistic | 0.9988 | 0.0093 | 0.0001 | 0.9986 | 0.0106 | 0.0001 | 0.9996 | 0.0055 | 0.00003 |
| Midilli | 0.9983 | 0.0108 | 0.0001 | 0.9992 | 0.0079 | 0.0001 | 0.9996 | 0.0054 | 0.00003 |
| Model Name | Temperature | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 °C | 40 °C | 60 °C | |||||||
| R2 | RMSE | χ2 | R2 | RMSE | χ2 | R2 | RMSE | χ2 | |
| Newton | 0.9434 | 0.0781 | 0.0062 | 0.9462 | 0.0761 | 0.0059 | 0.9449 | 0.0767 | 0.0061 |
| Page | 0.9939 | 0.0257 | 0.0007 | 0.9973 | 0.0169 | 0.0003 | 0.9969 | 0.0183 | 0.0004 |
| Henderson and Pabis | 0.9626 | 0.0634 | 0.0042 | 0.9644 | 0.0619 | 0.0040 | 0.9619 | 0.0638 | 0.0043 |
| Logarithmic | 0.989 | 0.0344 | 0.0013 | 0.989 | 0.0344 | 0.0013 | 0.9895 | 0.0335 | 0.0012 |
| Wang and Singh | 0.9915 | 0.0303 | 0.001 | 0.9919 | 0.0295 | 0.0009 | 0.992 | 0.0293 | 0.0009 |
| Logistic | 0.9987 | 0.0117 | 0.0001 | 0.9991 | 0.0100 | 0.0001 | 0.999 | 0.0103 | 0.0001 |
| Midilli | 0.9988 | 0.0111 | 0.0001 | 0.9992 | 0.0094 | 0.0001 | 0.9989 | 0.0107 | 0.0001 |
| Temperature | Equation | Coefficient | |||
|---|---|---|---|---|---|
| a | k | n | b | ||
| 20 °C | Newton | 0.003404 | |||
| Page | 0.002308 | 1.065620 | |||
| Henderson and Pabis | 0.998825 | 0.003400 | |||
| Logarithmic | 1.012891 | 0.003044 | −0.035153 | ||
| Wang and Singh | −0.002270 | 0.000001 | |||
| Logistic | 1.977956 | 0.004454 | 1.111585 | ||
| Midilli | 0.932817 | 0.001204 | 1.161019 | −0.000007 | |
| 40 °C | Newton | 0.004463 | |||
| Page | 0.001710 | 1.170589 | |||
| Henderson and Pabis | 1.059556 | 0.004717 | |||
| Logarithmic | 1.088395 | 0.003997 | −0.059746 | ||
| Wang and Singh | −0.003136 | 0.000002 | |||
| Logistic | 2.071080 | 0.006215 | 1.083810 | ||
| Midilli | 0.998372 | 0.002219 | 1.115782 | −0.000036 | |
| 60 °C | Newton | 0.005747 | |||
| Page | 0.002469 | 1.157272 | |||
| Henderson and Pabis | 1.054714 | 0.006047 | |||
| Logarithmic | 1.071121 | 0.005403 | −0.038136 | ||
| Wang and Singh | −0.003930 | 0.000004 | |||
| Logistic | 2.230434 | 0.007794 | 1.237159 | ||
| Midilli | 0.992444 | 0.002541 | 1.147397 | −0.000016 | |
| Temperature | Equation | Coefficient | |||
|---|---|---|---|---|---|
| a | k | n | b | ||
| 20 °C | Newton | 0.006005 | |||
| Page | 0.000557 | 1.444429 | |||
| Henderson and Pabis | 1.143041 | 0.006796 | |||
| Logarithmic | 1.335773 | 0.004172 | −0.260742 | ||
| Wang and Singh | −0.004311 | 0.000005 | |||
| Logistic | 1.148484 | 0.014077 | 0.172749 | ||
| Midilli | 0.967159 | 0.000192 | 1.638827 | −0.000050 | |
| 40 °C | Newton | 0.007220 | |||
| Page | 0.000402 | 1.562833 | |||
| Henderson and Pabis | 1.138669 | 0.008139 | |||
| Logarithmic | 1.314926 | 0.005141 | −0.240099 | ||
| Wang and Singh | −0.005199 | 0.000007 | |||
| Logistic | 1.166419 | 0.016613 | 0.185766 | ||
| Midilli | 0.970477 | 0.000286 | 1.621971 | −0.000050 | |
| 60 °C | Newton | 0.007865 | |||
| Page | 0.000518 | 1.544514 | |||
| Henderson and Pabis | 1.129497 | 0.008817 | |||
| Logarithmic | 1.359394 | 0.005180 | −0.297120 | ||
| Wang and Singh | −0.005643 | 0.000008 | |||
| Logistic | 1.145264 | 0.018421 | 0.174070 | ||
| Midilli | 0.964061 | 0.000309 | 1.632707 | −0.000068 | |
| MD * | DT | Color Parameters | Water Activity | |||
|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | |||
| FF | 30.30 ± 1.40 a *** | 16.50 ± 0.20 a | 8.80 ± 0.50 a | - | - | |
| CSFD | 20 °C | 35.52 ± 3.37 a,b,c | 21.66 ± 0.40 b | 11.18 ± 0.78 b | 8.07 ± 3.14 b,c | 0.328 ± 0.013 b |
| 40 °C | 43.65 ± 6.50 c,d | 22.69 ± 0.58 b,c | 11.10 ± 0.51 b | 8.32 ± 3.52 b,c | 0.346 ± 0.008 bc | |
| 60 °C | 43.84 ± 1.47 d | 24.66 ± 1.41 c,d | 13.19 ± 0.63 b | 6.46 ± 2.11 a,b | 0.335 ± 0.005 b | |
| PSFD | 20 °C | 29.04 ± 1.24 a | 24.52 ± 1.06 c,d | 12.05 ± 1.36 b | 14.97 ± 2.27 d | 0.358 ± 0.002 c |
| 40 °C | 31.97 ± 1.03 a,b | 26.96 ± 0.79 d | 12.10 ± 0.46 b | 4.30 ± 1.27 a | 0.330 ± 0.003 b | |
| 60 °C | 39.29 ± 1.04 b,c,d | 34.85 ± 1.06 e | 15.59 ± 0.62 c | 11.44 ± 1.10 c,d | 0.304 ± 0.004 a | |
| MD * | DT | Antioxidant Activity | ||
|---|---|---|---|---|
| TPC [mg GAE/g DM] | ABTS [EC50; mg DM/mL] | DPPH [EC50; mg DM/mL] | ||
| FF | 23.02 ± 0.33 b ** | 7.37 ± 0.19 b | 11.78 ± 0.54 a,b | |
| CSFD | 20 °C | 20.74 ± 1.39 a,b | 6.64 ± 0.39 ab | 10.61 ± 0.42 a |
| 40 °C | 19.61 ± 2.25 a,b | 6.44 ± 0.73 a,b | 14.35 ± 1.01 b | |
| 60 °C | 22.04 ± 0.26 b | 5.86 ± 0.19 a | 12.70 ± 0.59 ab | |
| PSFD | 20 °C | 18.60 ± 1.12 a,b | 7.13 ± 0.17 b | 12.57 ± 0.60 a,b |
| 40 °C | 18.73 ± 0.77 a,b | 6.51 ± 0.08 a,b | 10.66 ± 0.98 a | |
| 60 °C | 18.54 ± 0.76 a | 6.43 ± 0.17 a,b | 13.70 ± 0.77 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biernacka, B.; Dziki, D.; Rudy, S.; Krzykowski, A.; Polak, R.; Dziki, L. Influence of Pretreatments and Freeze-Drying Conditions of Strawberries on Drying Kinetics and Physicochemical Properties. Processes 2022, 10, 1588. https://doi.org/10.3390/pr10081588
Biernacka B, Dziki D, Rudy S, Krzykowski A, Polak R, Dziki L. Influence of Pretreatments and Freeze-Drying Conditions of Strawberries on Drying Kinetics and Physicochemical Properties. Processes. 2022; 10(8):1588. https://doi.org/10.3390/pr10081588
Chicago/Turabian StyleBiernacka, Beata, Dariusz Dziki, Stanisław Rudy, Andrzej Krzykowski, Renata Polak, and Laura Dziki. 2022. "Influence of Pretreatments and Freeze-Drying Conditions of Strawberries on Drying Kinetics and Physicochemical Properties" Processes 10, no. 8: 1588. https://doi.org/10.3390/pr10081588







