Effect of Fulvic Acid in Landfill Leachate Membrane Concentrate on Evaporation Process
Abstract
:1. Introduction
| Phase | Age (Year) | pH [7] | CODcr (mg·L−1) | BOD5/COD [8] | TOC/COD [8] | Ammonia [9,10] (mg·L−1) |
|---|---|---|---|---|---|---|
| Young | <5 | <6.5 | 6000–60,000 | 0.5–1 | <0.3 | <400 |
| Intermediate | 5–10 | 6.5–7.5 | 4000–10,000 | 0.2–0.5 | 0.3–0.5 | 400–1500 |
| Old | >10 | >7.5 | 1000–6000 | <0.2 | >0.5 | >1500 |
2. Materials and Methods
2.1. Samples
2.2. Characterization
2.3. Evaporation Test
2.4. Softening Experiment
3. Results and Discussion
3.1. LLMC Characteristics
3.2. Effect of FA on Evaporation Parameters
3.2.1. Effect of FA on BP and Viscosity
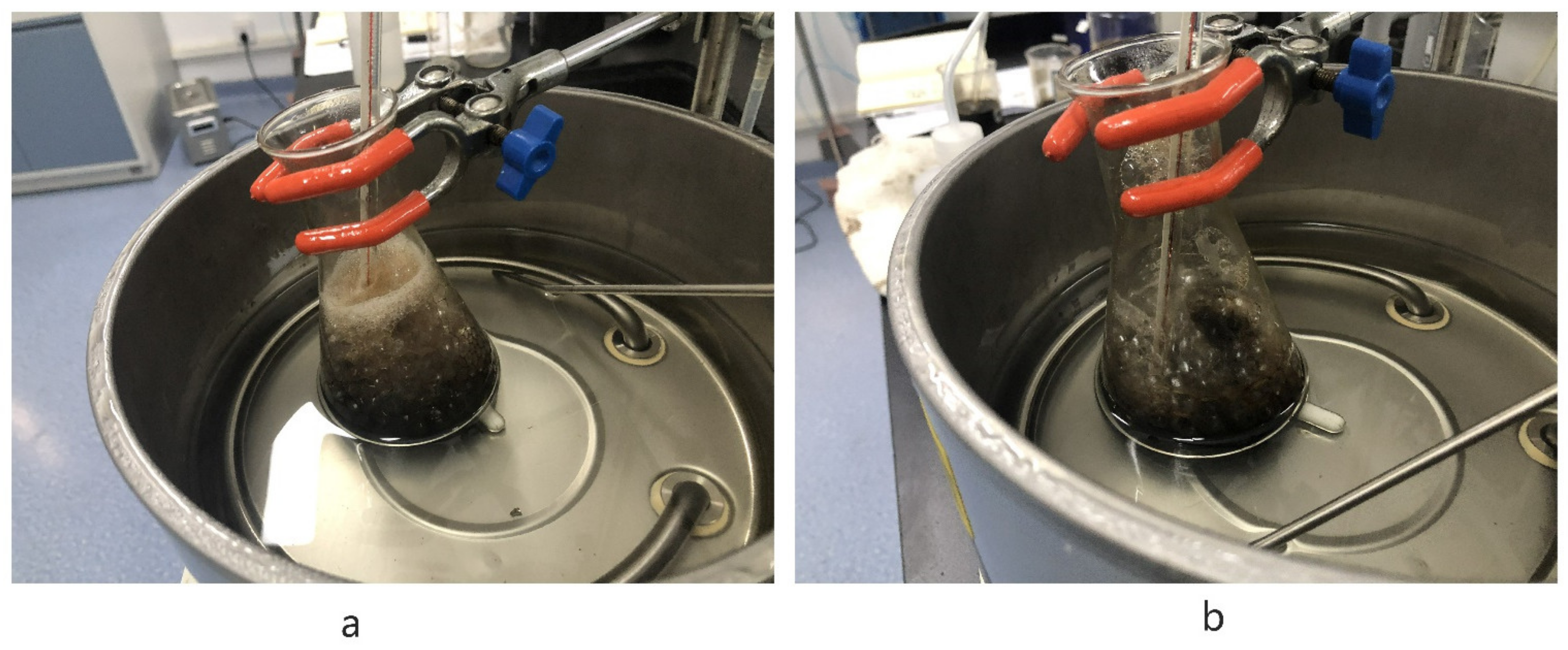
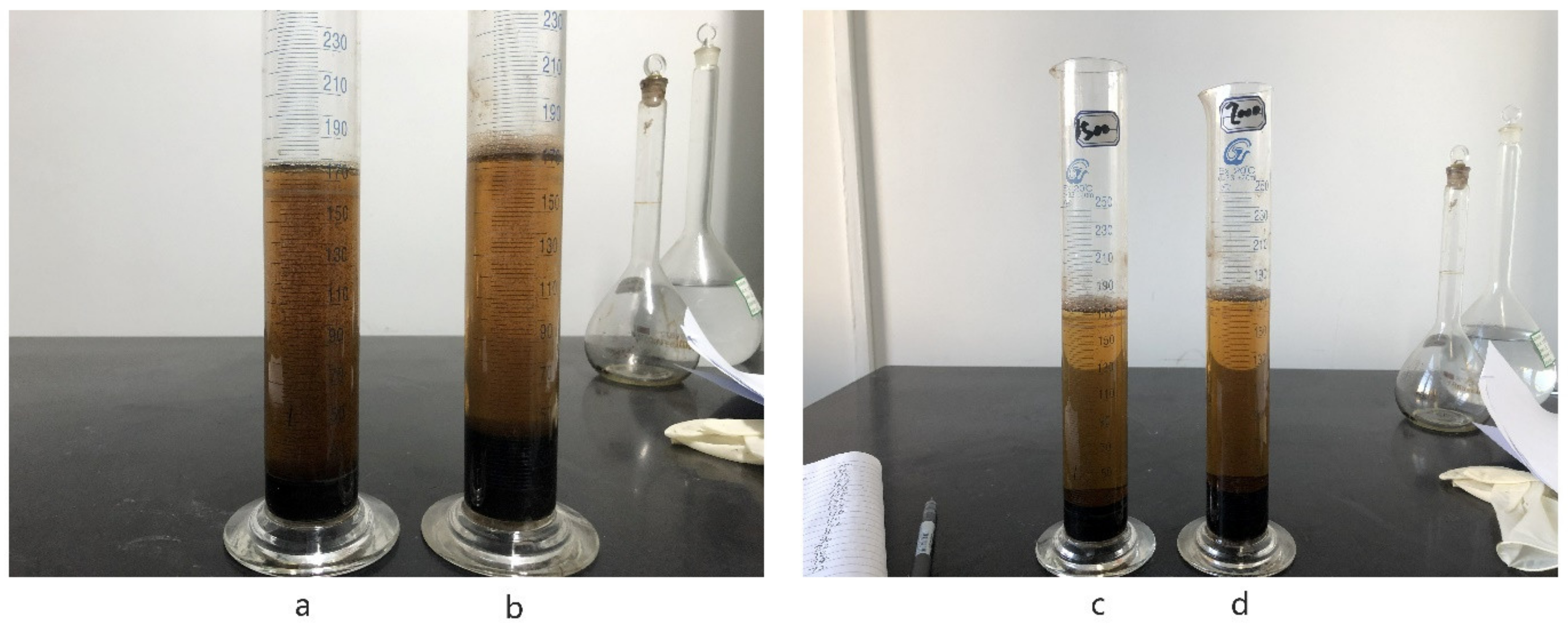
3.2.2. Effect of FA on Frothing Phenomenon
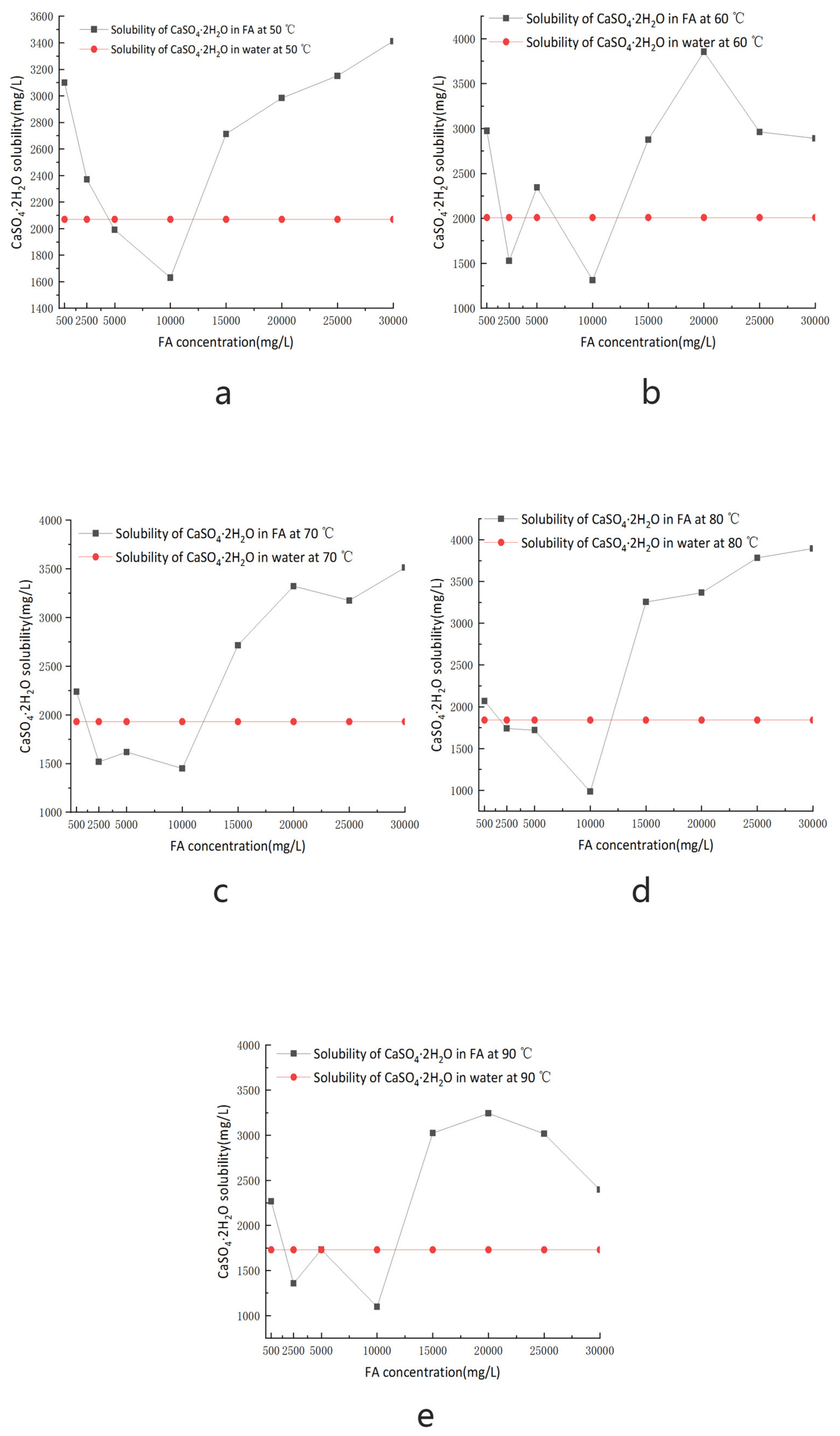
3.2.3. Effect of FA on the Solubility of Calcium Sulfate
3.3. Removal Efficiency of FA in the Softening Process
3.3.1. Removal Efficiency of FA of Configured Solutions
3.3.2. Removal Efficiency of FA of Actual LLMC Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Ministry of Ecology and Environment of the People’s Republic of China. The Bulletin on the State of China’s Ecological and Environment in 2020; State Council of China: Beijing, China, 2020. (In Chinese)
- Zhuang, Y. Current situation and management countermeasures of municipal solid waste treatment technology. Eng. Technol. Res. 2020, 13, 279–280. [Google Scholar]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Liu, J.; Cai, Z.; Yu, G.; Barcelo, D. Municipal solid waste landfills: An underestimated source of PPCPs in the water environment. Environ. Sci. Technol. 2020, 54, 9757–9768. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D.; Klimiuk, E. The effect of landfill age on municipal leachate composition. Bioresour. Technol. 2008, 99, 5981–5985. [Google Scholar] [CrossRef]
- Wijekoon, P.; Koliyabandara, P.A.; Cooray, A.T.; Su, S.L.; Athapattu, B.C.L.; Vithanage, M. Progress and prospects in mitigation of landfill leachate pollution: Risk, pollution potential, treatment and challenges. J. Hazard. Mater. 2022, 421, 126627. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukhopadhyay, S.; Hashim, M.A.; Sen Gupta, B. Contemporary environmental issues of landfill leachate: Assessment and remedies. Crit. Rev. Environ. Sci. Technol. 2014, 45, 472–590. [Google Scholar] [CrossRef]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments-A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. An overview of landfill leachate treatment via activated carbon adsorption process—ScienceDirect. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, C.; Pan, J.; Yang, G.; Sun, C.; Liu, Y.; Chen, X.; Zhao, Z. Leachate from municipal solid waste landfills in a global perspective: Characteristics, influential factors and environmental risks. J. Clean. Prod. 2021, 333, 130234. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the gironde estuary. Org. Geochem. 2008, 40, 706–719. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Qin, L.; Tian, X.; Wang, A.; Zhou, Y.; Meng, L.; Chen, Y. Removal of refractory organics in nanofiltration concentrates of municipal solid waste leachate treatment plants by combined Fenton oxidative-coagulation with photo-Fenton processes. Chemosphere 2016, 146, 442–449. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhen, L.; Zhang, H.; Zhang, Y.; Wang, C. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. J. Hazard. Mater. 2012, 229–230, 115–121. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Wu, Y.; Wang, C.; Zheng, J.; Xu, K.; Li, J. Recovery of potassium from landfill leachate concentrates using a combination of cation-exchange membrane electrolysis and magnesium potassium phosphate crystallization. Sep. Purif. Technol. 2015, 144, 1–7. [Google Scholar] [CrossRef]
- Qiao, M.; Zhao, X.; Wei, X. Characterization and treatment of landfill leachate membrane concentrate by Fe2+/NaClO combined with advanced oxidation processes. Sci. Rep. 2018, 8, 12525. [Google Scholar] [CrossRef]
- Labiadh, L.; Fernandes, A.; Ciriaco, L.; Pacheco, M.J.; Gadri, A.; Ammar, S.; Lopes, A. Electrochemical treatment of concentrate from reverse osmosis of sanitary landfill leachate. J. Environ. Manag. 2016, 181, 515–521. [Google Scholar] [CrossRef]
- Hendrych, J.; Hejralova, R.; Krouzek, J.; Spacek, P.; Sobek, J. Stabilisation/solidification of landfill leachate concentrate and its residue obtained by partial evaporation. Waste Manag. 2019, 95, 560–568. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Gentili, E.; Meoni, C.; Orsi, S.; Komilis, D. Effect of the recirculation of a reverse osmosis concentrate on leachate generation: A case study in an Italian landfill. Waste Manag. 2018, 76, 643–651. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Gu, Z.; Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment—ScienceDirect. Sci. Total Environ. 2020, 762, 148557. [Google Scholar]
- Shi, J.; Sun, D.; Dang, Y.; Qu, D. Characterizing the degradation of refractory organics from incineration leachate membrane concentrate by VUV/O3. Chem. Eng. J. 2022, 428, 132281. [Google Scholar] [CrossRef]
- Loh, W.; Cai, Q.; Li, R.; Jothinathan, L.; Hu, J. Reverse osmosis concentrate treatment by microbubble ozonation-biological activated carbon process: Organics removal performance and environmental impact assessment. Sci. Total Environ. 2021, 798, 149289. [Google Scholar] [CrossRef]
- Long, Y.; Xu, J.; Shen, D.; Du, Y.; Feng, H. Effective removal of contaminants in landfill leachate membrane concentrates by coagulation. Chemosphere 2017, 167, 512–519. [Google Scholar] [CrossRef]
- Ren, X.; Xu, X.; Xiao, Y.; Chen, W.; Song, K. Effective removal by coagulation of contaminants in concentrated leachate from municipal solid waste incineration power plants. Sci. Total Environ. 2019, 685, 392–400. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Jia, H.; Guo, J.; Meng, X.; Wang, J. Electrochemical methods for landfill leachate treatment: A review on electrocoagulation and electrooxidation. Sci. Total Environ. 2022, 806, 150529. [Google Scholar] [CrossRef]
- Reshadi, M.; Bazargan, A.; Mckay, G. A review of the application of adsorbents for landfill leachate treatment: Focus on magnetic adsorption. Sci. Total Environ. 2020, 731, 138863. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef]
- GB/T 31962-2015; Wastewater Quality Standards for Discharge to Municipal Sewers. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2015.
- Zhang, Q.-Q.; Tian, B.-H.; Zhang, X.; Ghulam, A.; Fang, C.-R.; He, R. Investigation on characteristics of leachate and concentrated leachate in three landfill leachate treatment plants. Waste Manag. 2013, 11, 2277–2286. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Li, X.; Sun, Y.; Wu, H.; Chen, D. Removal of humic substances from reverse osmosis (RO) and nanofiltration (NF) concentrated leachate using continuously ozone generation-reaction treatment equipment. Waste Manag. 2016, 56, 271–279. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Karatas, O.; Rezania, H.; Kobya, M.; Khataee, A. A review on treatment of membrane concentrates generated from landfill leachate treatment processes. Sep. Purif. Technol. 2021, 259, 118182. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Ye, Z.; Chen, Y.; Lin, P. Robust seawater desalination and sewage purification enabled by the solar-thermal conversion of the Janus-type graphene oxide evaporator. Desalination 2022, 522, 115406. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, X.; Zhang, K.; Shen, S. Investigation and optimization for multi-effect evaporation with thermal vapor compression (MEE-TVC) desalination system with various feed preheater arrangements. Desalination 2022, 521, 115379. [Google Scholar] [CrossRef]
- Shi, J.; Huang, W.; Han, H.; Xu, C. Review on treatment technology of salt wastewater in coal chemical industry of China. Desalination 2020, 493, 114640. [Google Scholar] [CrossRef]
- Ruan, G.; Wang, M.; An, Z.; Xu, G.; Ge, Y.; Zhao, H. Progress and perspectives of desalination in China. Membranes 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahali, M.; Ettouney, H. Developments in thermal desalination processes: Design, energy, and costing aspects. Desalination 2007, 214, 227–240. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Chen, H. Pretreatment of concentrated leachate by the combination of coagulation and catalytic ozo-nation with Ce/AC catalyst. Water Sci. Technol. 2016, 73, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Gao, T.; Shia, W.; Zhao, M.; Huang, Z.; Ren, H.; Ruan, W. Coagulation removal of dissolved organic matter (DOM) in nanofiltration concentrate of biologically treated landfill leachate by ZrCl4: Performance, mechanism and coagulant recycling. Chemosphere 2022, 301, 134768. [Google Scholar] [CrossRef] [PubMed]
- Beijing Petrochemical Engineering Company. Handbook of Physical and Chemical Constants for Chlor-Alkali Industry; Chemical Industry Press: Beijing, China, 1988; Volume 7, pp. 27–30. (In Chinese) [Google Scholar]
- Xue, X.; Lin, Z.W.; Fang, Y.M.; Zheng, F.L.; Chen, F.D.; Liu, Z.W.; Zhang, J.F. Research on the Application of “Chemical Softening + TUF + Plate-and-Frame Filtering” of Landfill Leachate in a Landfill Plant. Technol. Water Treat. 2022. in process. Available online: https://kns.cnki.net/kcms/detail/33.1127.P.20220630.1116.006.html (accessed on 10 August 2021). (In Chinese).
- Yue, D.; Xu, Y.; Bux, M.R.; Liu, F.; Nie, Y. Laboratory-scale experiments applied to the design of a two-stage submerged combustion evaporation system. Waste Manag. 2007, 27, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Mai, B.; Ding, H. Principles of Chemical Engineering, 2nd ed.; Chemical Industry Press: Beijing, China, 1998; Volume 318. (In Chinese) [Google Scholar]
- Liu, Y. Alkali Recovery of Pulping Black Liquor, 1st ed.; Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Liu, Y.; Pei, C.; Wang, J.; Xia, T.; Zhang, W.; Du, Y. Design and Analysis of an Evaporation System of Solutions with High Boiling Point Elevation. Chin. J. Process Eng. 2017, 17, 859–865. [Google Scholar]
- Jiang, Y.; Zhang, G. Glycerol Sweet Water Evaporation Calcium Sulfate Scale Cleaning and Prevention; Shandong Chemical Industry: Jinan, China, 2013. [Google Scholar]
- Zhang, J.; Ji, X.; Lu, W.; Chen, N. Chemical bond-parametric analysis of formation regularities of salt hydrates. J. Shanghai Univ. 2004, 10, 381–385. (In Chinese) [Google Scholar]
- Cai, Z.; Yang, H.; Cheng, R. Hydration number of carboxylic acid ions in water. Acta Chim. Sin. 2008, 66, 831–833. [Google Scholar]
- Li, X. Soil Chemistry; Higher Education Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- He, J.; Yan, L.; Yang, K.; Ma, M.; Liu, Y.; Cui, G. Study on the composition and properties of humic acids from different source. Chin. J. Soil Sci. 2003, 4, 343–345. (In Chinese) [Google Scholar]
- Fan, Q. Study on the Physicochemical Properties and Chemical Cohesive of Humic Acid after Radiation by UV light. Master’s Thesis, Xi’an University of Architecture & Technology, Xi’an, China, 2015. [Google Scholar]
- Jacob, B.; Waters, O.B. Calcium sulfate-sodium sulfate-sodium chloride-water system at 25° to 100°. J. Chem. Eng. Data 1968, 13, 336–344. [Google Scholar]
- Yeatts, L.B.; Marshall, W.L. Solubility of calcium sulfate dihydrate and association equilibriums in several aqueous mixed electrolyte salt systems at 25°. J. Chem. Eng. Data 1972, 17, 163–168. [Google Scholar] [CrossRef]
- Cameron, F.K.; Bell, J.M.; Robinson, W.O. The solubility of certain salts present in alkali soils. J. Phys. Chem. 1907, 11, 396–420. [Google Scholar] [CrossRef]
- Paolo, V.; Renato, G. Scaling of ammonia stripping towers in the treatment of groundwater polluted by municipal solid waste landfill leachate: Study of the causes of scaling and its effects on stripping performance. Rev. Ambiente Agua 2015, 10, 240–252. [Google Scholar]
- Luo, C.; Wen, S.; Lu, Y.; Dai, J.; Du, Y. Coprecipitation of humic acid and phosphate with Fe(III) enhances the sequestration of carbon and phosphorus in sediments. Chem. Geol. 2022, 588, 120645. [Google Scholar] [CrossRef]
- Liu, D.Y. Process Design Calculation and Application of Evaporator; Chemical Industry Press: Beijing, China, 2020; pp. 18–20. [Google Scholar]
- Zhang, L.L.; Wang, D.W.; Liu, Y.; Zhang, S.F. Calculation of coefficient of heat transfer in vapor-liquid-solid three-phase evaporator. J. Hebei Univ. Technol. 2011, 40, 41–45. [Google Scholar]
- Liu, J.H.; Liu, H.S.; Ding, H.D. Flow boiling heat transfer to highly viscous fluid in vertical flowing. J. Qingdao Inst. Chem. Technol. 1995, 16, 118–121. [Google Scholar]
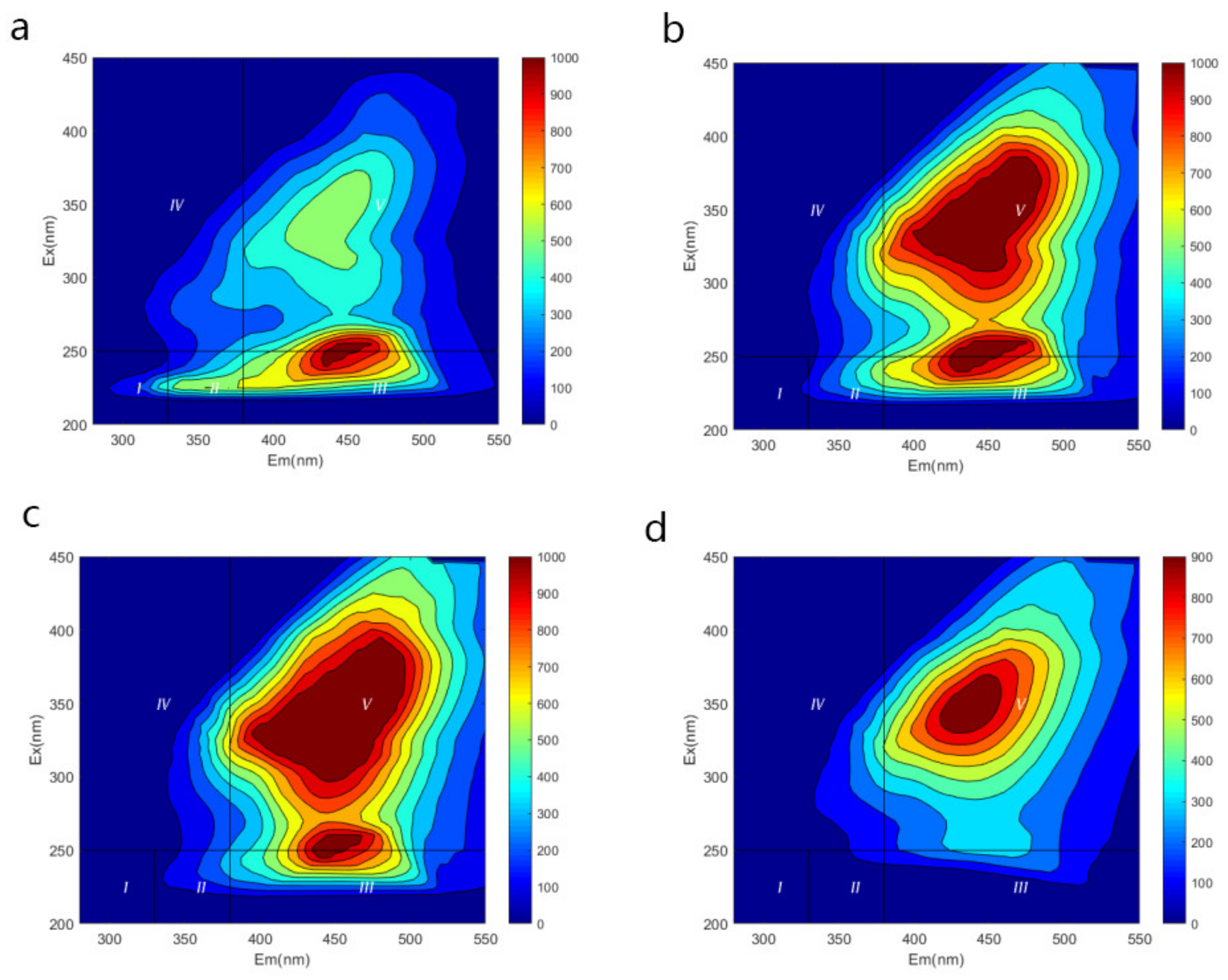


| Age | Region | Basic Info | Leachate Treatment a | Concentrate Treatment |
|---|---|---|---|---|
| 3 years | Xiangtan, Hunan | the third landfill area started in 2018 | two-stage biological treatment + UF + NF + RO | recirculation |
| 7 years | Shijiazhuang, Hebei | started in 2014 | pretreatment + two-stage DTRO | recirculation |
| 10 years | Beijing | started in 2012 | two-stage biological treatment + UF + NF + RO | built LLMC treatment system in 2018 |
| 10 years | Hengyang, Hunan | started in 2012 | two-stage biological treatment + UF + NF + RO | recirculation |
| Closure | Nanjing, Jiangsu | closed in 2014 | two-stage biological treatment + UF + NF + RO | recirculation |
| Indicators | Xiangtan, Hunan | Shijiazhuang, Hebei | Beijing | Hengyang, Hunan | Nanjing, Jiangsu |
|---|---|---|---|---|---|
| pH | 7.41 | 7.13 | 7.03 | 7.99 | 7.16 |
| Ammonia nitrogen, mg·L−1 | 2560 | 2780 | 1560 | 2420 | 2560 |
| CODcr, mg·L−1 | 5320 | 3400 | 1900 | 2770 | 5480 |
| BOD5/COD | 0.18 | 0.2 | 0.08 | 0.19 | 0.09 |
| TOC, mg·L−1 | 2365 | 1250 | 705 | 1946 | 1991 |
| FA, mg·L−1 | 1635 | 975 | 587 | 980 | 1378 |
| FA/TOC, wt.% | 69.13% | 78.00% | 83.26% | 50.31% | 69.21% |
| EC, ms/cm | 65.5 | 53.9 | 47.8 | 22.6 | 13.13 |
| Ca2+, mg·L−1 | 547.6 | 605 | 681.7 | 410.7 | 665.6 |
| Mg2+, mg·L−1 | 713.3 | 207 | 536.7 | 240.8 | 398.4 |
| TH, mg·L−1 | 4341.8 | 2375 | 3940.5 | 2030.08 | 3324 |
| Cl−, mg·L−1 | 8624 | 9765 | 9552 | 6153 | 2417 |
| SO42−, mg·L−1 | 954 | 658 | 468.8 | 490.3 | 227 |
| Initial Parameters | After Adding Ca(OH)2 | After Adding Na2CO3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH a | FA Concentration (mg·L−1) | Ca(OH)2 (mg·L−1) | FA Concentration (mg·L−1) | Ca2+ (mg·L−1) | Removal Efficiency of FA | pH a | FA Concentration (mg·L−1) | Ca2+ (mg·L−1) | Removal Efficiency of FA |
| 7.18 | 500 | 0.4661 | 23.04 | 503 | 91% | 12.44 | 10.52 | 17 | 96% |
| 7.23 | 1000 | 0.6544 | 38.77 | 738 | 92% | 12.46 | 17.39 | 58 | 97% |
| 7.42 | 1500 | 0.5931 | 57.01 | 922 | 93% | 12.22 | 30.67 | 76.5 | 96% |
| 7.53 | 2000 | 0.6213 | 68.98 | 1093 | 93% | 12.2 | 42.61 | 116 | 96% |
| Landfill | Indicator | Influent Concentration | Effluent Concentration | Removal Efficiency |
|---|---|---|---|---|
| Xiangtan | CODcr, mg·L−1 | 5320 | 3674 | 30.9% |
| FA, mg·L−1 | 1635 | 258.1 | 84% | |
| EC, μs/cm | 65,500 | 547 | 99.2% | |
| Ca2+, mg·L−1 | 547.6 | 98 | 82.1% | |
| Mg2+, mg·L−1 | 713.3 | 43 | 93.7% | |
| TH, mg·L−1 | 4341.08 | 424.17 | 90.2% | |
| Shijiazhuang | CODcr, mg·L−1 | 3400 | 2320 | 31.8% |
| FA, mg·L−1 | 975 | 187 | 80.8% | |
| EC, μs/cm | 53,900 | 495 | 99% | |
| Ca2+, mg·L−1 | 605 | 11 | 98.2% | |
| Mg2+, mg·L−1 | 207 | 6.12 | 97% | |
| TH, mg·L−1 | 2375 | 53 | 97.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wu, M.; Chen, Y.; Wang, H. Effect of Fulvic Acid in Landfill Leachate Membrane Concentrate on Evaporation Process. Processes 2022, 10, 1592. https://doi.org/10.3390/pr10081592
Liu L, Wu M, Chen Y, Wang H. Effect of Fulvic Acid in Landfill Leachate Membrane Concentrate on Evaporation Process. Processes. 2022; 10(8):1592. https://doi.org/10.3390/pr10081592
Chicago/Turabian StyleLiu, Lu, Mengyao Wu, Yuxiao Chen, and Heli Wang. 2022. "Effect of Fulvic Acid in Landfill Leachate Membrane Concentrate on Evaporation Process" Processes 10, no. 8: 1592. https://doi.org/10.3390/pr10081592





