In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Development of ZVI-Decorated Lignite Carbon through Carbothermal Reduction
2.3. Analytical Methods and Characterization
2.4. Batch Sorption Experiments
3. Results
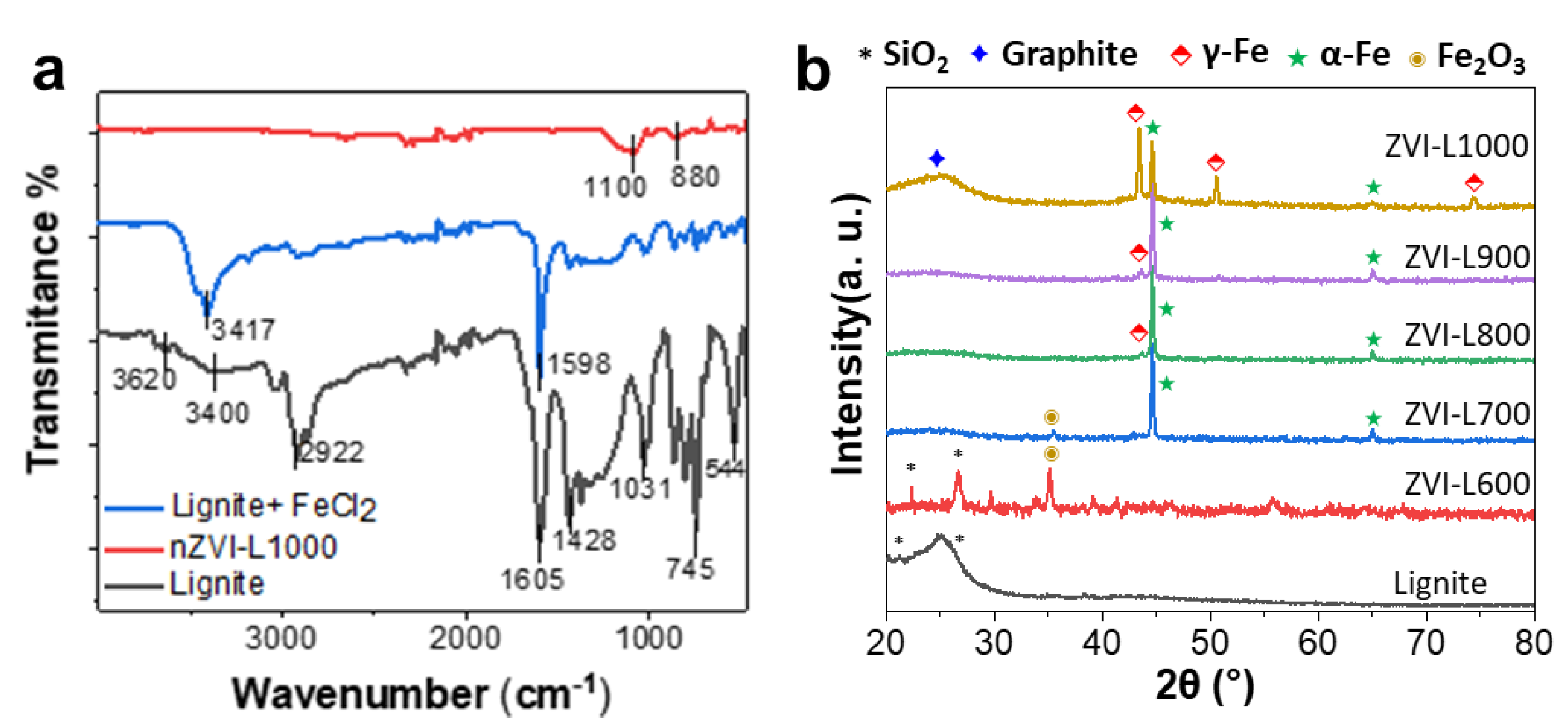
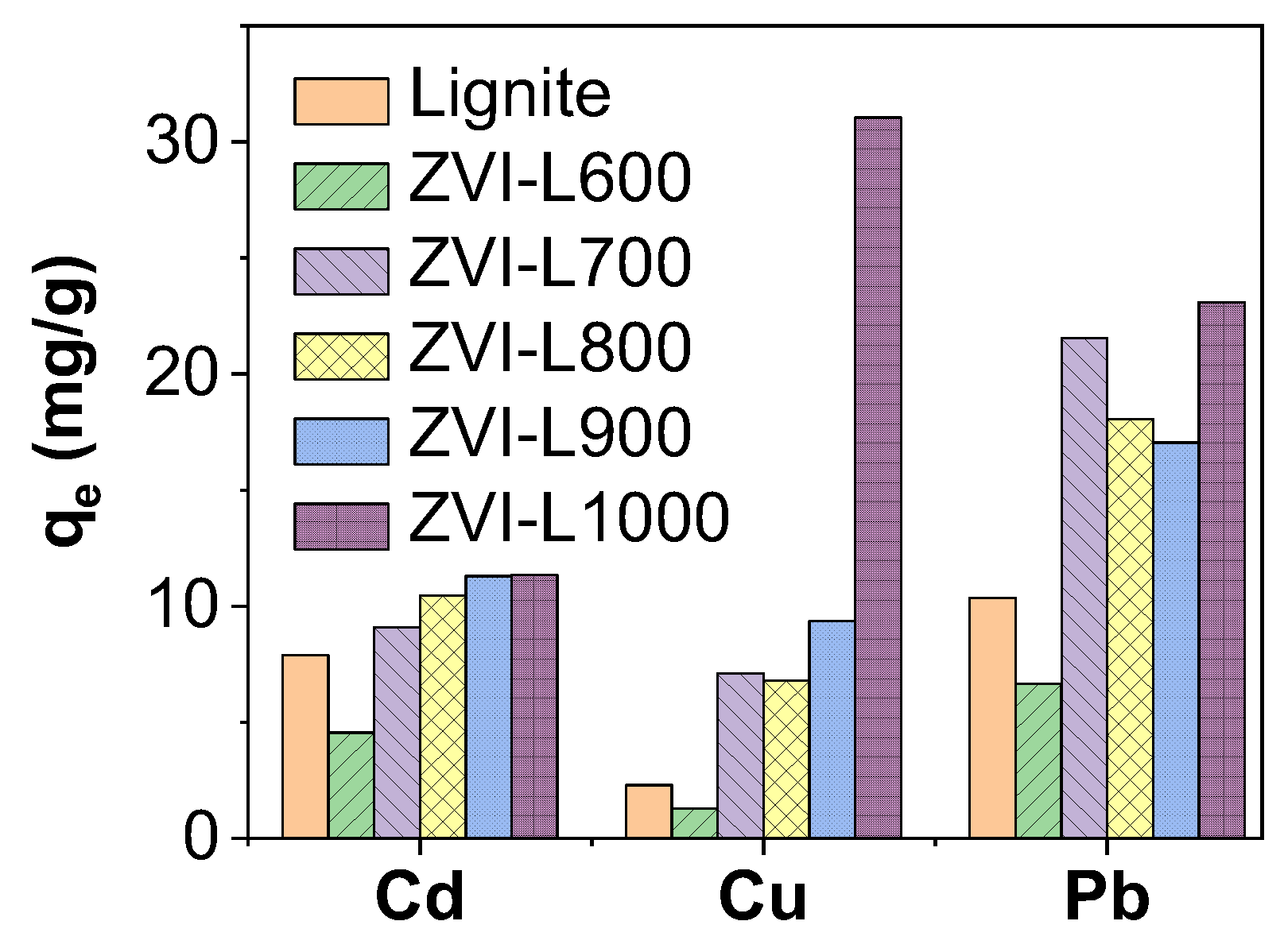
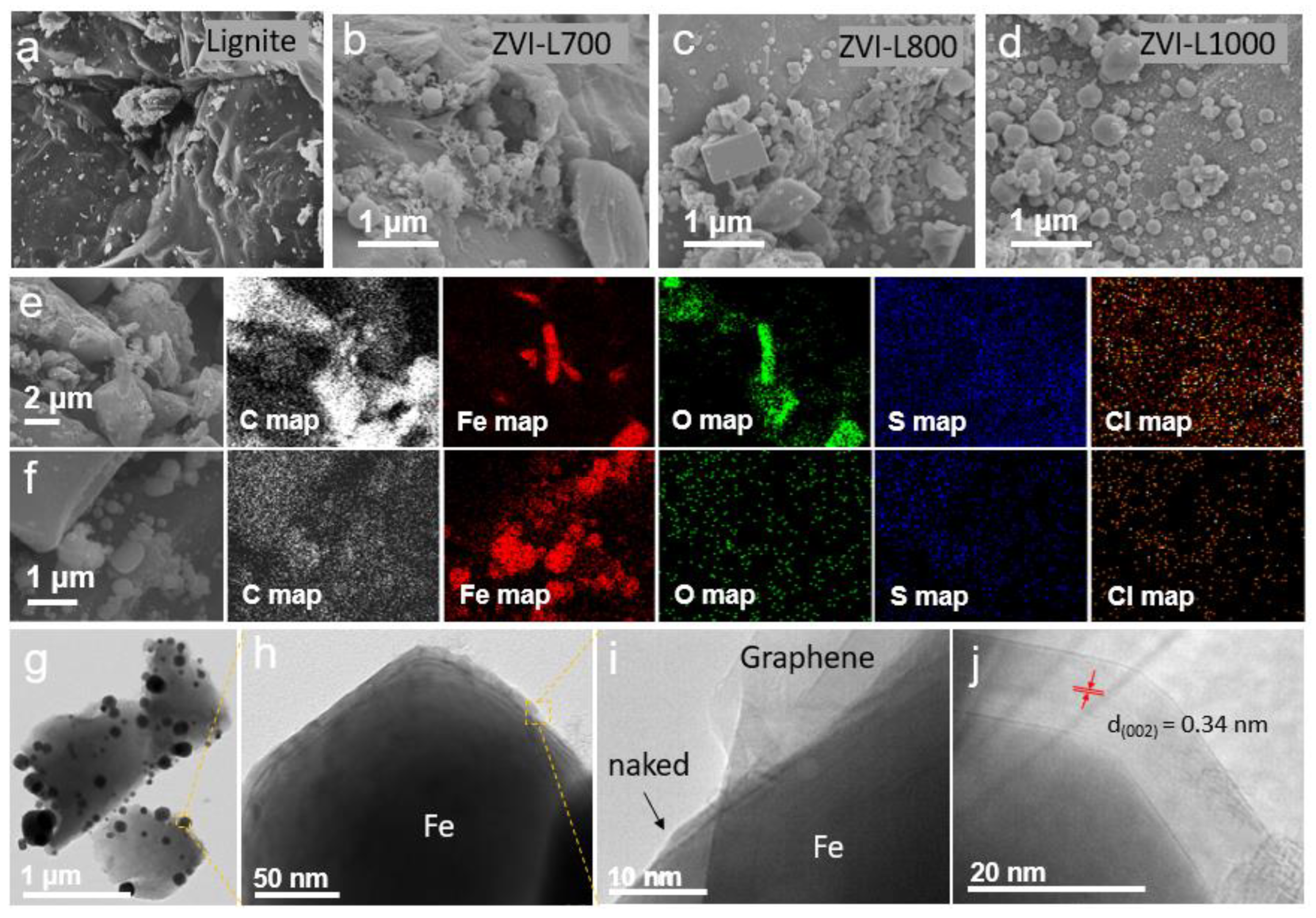
3.1. Structure, Morphology and Heavy Metal Uptake Performance of ZVI-LX Particles
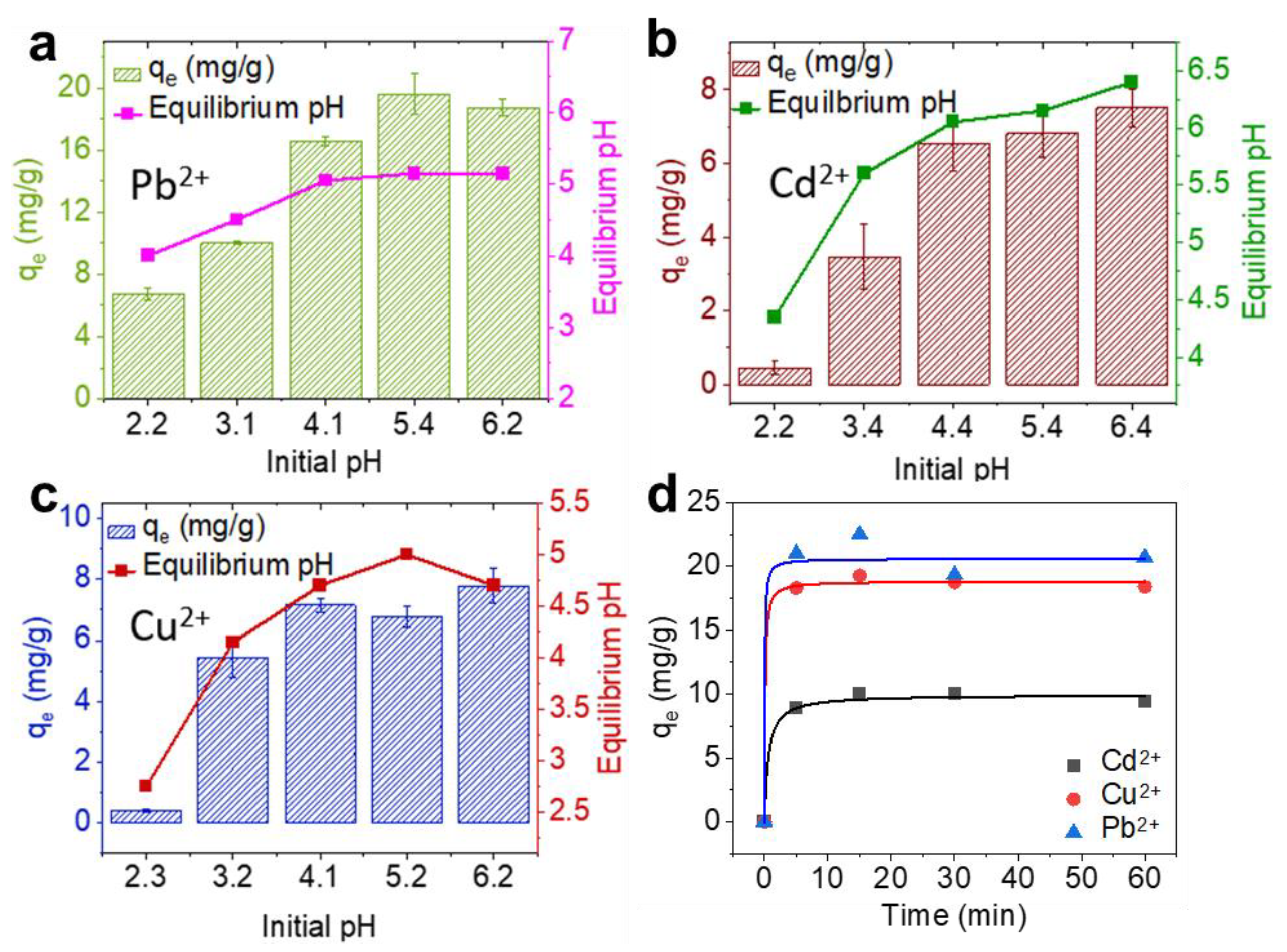
3.2. pH Dependence and the Kinetics of Heavy Metal Removal
3.3. Adsorption Isotherm
3.4. Simultaneous Cu2+, Pb2+ and Cd2+ Removal by Lignite vs. ZVI-L1000
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Treto-Suárez, M.A.; Prieto-García, J.O.; Mollineda-Trujillo, Á.; Lamazares, E.; Hidalgo-Rosa, Y.; Mena-Ulecia, K. Kinetic study of removal heavy metal from aqueous solution using the synthetic aluminum silicate. Sci. Rep. 2020, 10, 10836. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- US EPA. 2016. Available online: https://archive.epa.gov/epawaste/hazard/wastemin/web/html/priority.html (accessed on 3 March 2021).
- Chen, J.-Z.; Tao, X.-C.; Xu, J.; Zhang, T.; Liu, Z.-L. Biosorption of lead, cadmium and mercury by immobilized Microcystis aeruginosa in a column. Process Biochem. 2005, 40, 3675–3679. [Google Scholar] [CrossRef]
- Dublis, S.; Shah, S.; Nand, S.; Anderes, E. Chapter 67-Anemias excluding cobalamin and folate deficiencies. In Handbook of Clinical Neurology; Biller, J., Ferro, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1005–1014. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment; Environmental Remediation; Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-J.; Wang, J.-K.; Ferguson, S.; Wang, T.; Bao, Y.; Hao, H. Mechanism, synthesis and modification of nano zerovalent iron in water treatment. Nanoscale 2016, 8, 9962–9975. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Seipenbusch, M.; Rothenbacher, S.; Kirchhoff, M.; Schmid, H.-J.; Kasper, G.; Weber, A.P. Interparticle forces in silica nanoparticle agglomerates. J. Nanoparticle Res. 2010, 12, 2037–2044. [Google Scholar] [CrossRef]
- Zhang, X.; Karunaratne, T.; Navarathna, C.; Zhang, J.; Pittman, C.U. Nanoscale zero-valent iron-decorated biochar for aqueous contaminant removal. In Sustain. Biochar Water Wastewater Treat; Mohan, D., Pittman, C.U., Mlsna, T.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 611–641. [Google Scholar] [CrossRef]
- Chen, W.; Pan, L.; Chen, L.; Wang, Q.; Yan, C. Dechlorination of hexachlorobenzene by nano zero-valent iron/activated carbon composite: Iron loading, kinetics and pathway. RSC Adv. 2014, 4, 46689–46696. [Google Scholar] [CrossRef]
- Gil, A.; Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S. Adsorption/reduction of Hg(II) and Pb(II) from aqueous solutions by using bone ash/nZVI composite: Effects of aging time, Fe loading quantity and co-existing ions. Environ. Sci. Pollut. Res. 2018, 25, 2814–2829. [Google Scholar] [CrossRef]
- Zhang, X.; Navarathna, C.M.; Leng, W.; Karunaratne, T.; Thirumalai, R.V.K.G.; Kim, Y.; Pittman, C.U.; Mlsna, T.; Cai, Z.; Zhang, J. Lignin-based few-layered graphene-encapsulated iron nanoparticles for water remediation. Chem. Eng. J. 2021, 417, 129199. [Google Scholar] [CrossRef]
- Aivalioti, M.; Pothoulaki, D.; Papoulias, P.; Gidarakos, E. Removal of BTEX, MTBE and TAME from aqueous solutions by adsorption onto raw and thermally treated lignite. J. Hazard. Mater. 2012, 207–208, 136–146. [Google Scholar] [CrossRef]
- He, Q.; Ruan, P.; Miao, Z.; Wan, K.; Gao, M.; Li, X.; Huang, S. Adsorption of direct yellow brown D3G from aqueous solution using loaded modified low-cost lignite: Performance and mechanism. Environ. Technol. 2019, 42, 1642–1651. [Google Scholar] [CrossRef]
- Samaraweera, H.; Sharp, A.; Edwards, J.; Pittman, C.U., Jr.; Zhang, X.; Hassan, E.B.; Thirumalai, R.V.K.G.; Warren, S.; Reid, C.; Mlsna, T. Lignite, thermally-modified and Ca/Mg-modified lignite for phosphate remediation. Sci. Total Environ. 2021, 773, 145631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, B.; Ma, H. Studies on Chromium (VI) adsorption on sulfonated lignite. Desalination 2010, 255, 61–66. [Google Scholar] [CrossRef]
- Qi, Y.; Hoadley, A.F.A.; Chaffee, A.L.; Garnier, G. Characterisation of lignite as an industrial adsorbent. Fuel 2011, 90, 1567–1574. [Google Scholar] [CrossRef]
- Samaraweera, H.; Edwards, J.; Reid, C.; Perera, S.S.; Thirumalai, R.V.K.G.; Pittman, C.U., Jr.; Mlsna, T. Pyrolyzed Ca-impregnated lignite for aqueous phosphate removal: Batch and column studies. J. Environ. Chem. Eng. 2021, 9, 106077. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, S.; Wang, K.; Wang, L.; Xu, Y.; Wu, Z.; Shao, H.; Wang, Y.; Miao, M. Thermogravimetric Dynamics and FTIR Analysis on Oxidation Properties of Low-Rank Coal at Low and Moderate Temperatures. Int. J. Coal Prep. Util. 2015, 35, 39–50. [Google Scholar] [CrossRef]
- Milicevic, S.; Boljanac, T.; Martinovic, S.; Vlahovic, M.; Milosevic, V.; Babic, B. Removal of copper from aqueous solutions by low cost adsorbent-Kolubara lignite. Fuel Process. Technol. 2012, 95, 1–7. [Google Scholar] [CrossRef]
- Di, J.; Ruan, Z.; Zhang, S.; Dong, Y.; Fu, S.; Li, H.; Jiang, G. Adsorption behaviors and mechanisms of Cu2+, Zn2+ and Pb2+ by magnetically modified lignite. Sci. Rep. 2022, 12, 1394. [Google Scholar] [CrossRef]
- Mehrabi, N.; Masud, A.; Afolabi, M.; Hwang, J.; Calderon Ortiz, G.A.; Aich, N. Magnetic graphene oxide-nano zero valent iron (GO–nZVI) nanohybrids synthesized using biocompatible cross-linkers for methylene blue removal. RSC Adv. 2019, 9, 963–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, M.; Wang, J. Nanoscaled zero valent iron/graphene composite as an efficient adsorbent for Co(II) removal from aqueous solution. J. Colloid Interface Sci. 2016, 474, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S.; Jafari, A.; Zandipak, R.; Meidanchi, A. Removal of heavy metal (Hg(II) and Cr(VI)) ions from aqueous solutions using Fe2O3@SiO2 thin films as a novel adsorbent. Process. Saf. Environ. Prot. 2018, 120, 348–357. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Effects of Physical and Chemical States of Iron-Based Catalysts on Formation of Carbon-Encapsulated Iron Nanoparticles from Kraft Lignin. Materials 2018, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yan, Q.; Hassan, E.B.; Li, J.; Cai, Z.; Zhang, J. Temperature effects on formation of carbon-based nanomaterials from kraft lignin. Mater. Lett. 2017, 203, 42–45. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Q.; Li, J.; Chu, I.-W.; Toghiani, H.; Cai, Z.; Zhang, J. Carbon-Based Nanomaterials from Biopolymer Lignin via Catalytic Thermal Treatment at 700 to 1000 °C. Polymers 2018, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X. Synthesis and Characterization of Carbon-Based Nanomaterials from Lignin-ProQuest. 2016. Available online: https://www.proquest.com/docview/1858816366/abstract/9BE7891B9F3543B6PQ/1 (accessed on 17 January 2022).
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. Mass production of graphene materials from solid carbon sources using a molecular cracking and welding method. J. Mater. Chem. A 2019, 7, 13978–13985. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. In situ formation of graphene-encapsulated iron nanoparticles in carbon frames through catalytic graphitization of kraft lignin. Nanomater. Nanotechnol. 2018, 8, 1847980418818955. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. Synthetic Bio-Graphene Based Nanomaterials through Different Iron Catalysts. Nanomaterials 2018, 8, 840. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Chen, H.; Zhang, M.; Cao, X. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions. Bioresour. Technol. 2014, 152, 538–542. [Google Scholar] [CrossRef]
- Ling, L.; Huang, X.-Y.; Zhang, W.-X. Enrichment of Precious Metals from Wastewater with Core–Shell Nanoparticles of Iron. Adv. Mater. 2018, 30, 1705703. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ling, L.; Zhang, W. Pb(II) deposition-reduction-growth onto iron nanoparticles induced by graphitic carbon nitride. Chem. Eng. J. 2020, 387, 124088. [Google Scholar] [CrossRef]
- Samaraweera, H.; Pittman, C.U.; Thirumalai, R.V.K.G.; Hassan, E.B.; Perez, F.; Mlsna, T. Characterization of graphene/pine wood biochar hybrids: Potential to remove aqueous Cu2+. Environ. Res. 2021, 192, 110283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Q.; Huang, S.; Zhang, B.; Xu, C. High performance and simultaneous sequestration of Cr(VI) and Sb(III) by sulfidated zerovalent iron. J. Clean. Prod. 2018, 191, 436–444. [Google Scholar] [CrossRef]
- Du, Y.; Dai, M.; Cao, J.; Peng, C. Fabrication of a low-cost adsorbent supported zero-valent iron by using red mud for removing Pb(II) and Cr(VI) from aqueous solutions. RSC Adv. 2019, 9, 33486–33496. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, A.; Škerget, M. A review: A comparison of different adsorbents for removal of Cr (VI), Cd (II) and Ni (II), Turk. J. Chem. 2020, 44, 859–883. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Li, J.; Lv, Y. Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 2019, 219, 493–503. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, L.; Liu, J.; Zhang, Z.; Tan, C. Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility. Appl. Sci. 2019, 9, 4213. [Google Scholar] [CrossRef] [Green Version]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Li, J.; Cheng, K. Porous biochar-nanoscale zero-valent iron composites: Synthesis, characterization and application for lead ion removal. Sci. Total Environ. 2020, 746, 141037. [Google Scholar] [CrossRef]
- Panayotova, M.; Velikov, B. Influence of Zeolite Transformation in a Homoionic Form on the Removal of Some Heavy Metal Ions from Wastewater. J. Environ. Sci. Health Part A 2003, 38, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Nieva, A.; Doma, B.; Chao, H.; Leng, L.S. Biosorption of Pb2+ and Cu2+ in aqueous solutions using agricultural wastes. EPJ Web Conf. 2017, 162, 01082. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Liu, W.; Xiong, L.; Xu, N.; Ni, J. Influence of pH, ionic strength and humic acid on competitive adsorption of Pb(II), Cd(II) and Cr(III) onto titanate nanotubes. Chem. Eng. J. 2013, 215–216, 366–374. [Google Scholar] [CrossRef]
- Xiao, S.; Ma, H.; Shen, M.; Wang, S.; Huang, Q.; Shi, X. Excellent copper(II) removal using zero-valent iron nanoparticle-immobilized hybrid electrospun polymer nanofibrous mats. Colloids Surf. Physicochem. Eng. Asp. 2011, 381, 48–54. [Google Scholar] [CrossRef]
- Cheng, P.; Hu, Y.H. Dubinin-Astakhov model for acetylene adsorption on metal-organic frameworks. Appl. Surf. Sci. 2016, 377, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J. 2012, 187, 79–88. [Google Scholar] [CrossRef]
- Voogd, P.; Scholten, J.J.F.; van Bekkum, H. Use of the t-plot—De Boer method in pore volume determinations of ZSM-5 type zeolites. Colloids Surf. 1991, 55, 163–171. [Google Scholar] [CrossRef]
| Kinetic Model | Nonlinear Form | Linear Form |
|---|---|---|
| Pseudo-first-order | ||
| Pseudo-second-order | ||
| Langmuir isotherm | ||
| Freundlich isotherm |
| Isotherm Model Parameter | Cd2+ | Pb2+ | Cu2+ | |||
|---|---|---|---|---|---|---|
| Lignite | ZVI-L1000 | Lignite | ZVI-L1000 | Lignite | ZVI-L1000 | |
| Langmuir qe (mg/g) | 4.9 | 38.3 | 12.2 | 55.2 | 4.0 | 42.5 |
| KL (L/mg) | 1.0 | 0.01 | 0.07 | 0.05 | 1.0 | 0.07 |
| R2 | 0.99 | 0.99 | 0.95 | 0.99 | 0.99 | 0.97 |
| Freundlich | ||||||
| KF (L/mg) | 3.3 | 3.3 | 4.0 | 10.1 | 1.9 | 11.0 |
| n | 14.5 | 0.01 | 4.9 | 0.3 | 14.5 | 3.8 |
| R2 | 0.99 | 0.92 | 0.99 | 0.97 | 0.99 | 0.99 |
| Metal | Material | Fe wt% | Surface Area (m2/g) | Removal Conditions | Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Pb2+ | Ostric Bone Ash (OBA) | 1.45 | 67 | Pb2+ 5–1000 mg/L, 25 °C | 88 | [13] |
| Pb2+ | OBA-ZVI | 18.9 | 109 | 160 | ||
| Pb2+ | Acid ammonium persulfate oxidized corn stalk BC (HPB) | N/A | N/A | Pb2+ 50 mg/L, pH 6, 25 °C | 135.4 | [44] |
| Pb2+ | ZVI-HPB | N/A | N/A | Pb2+ 200 mg/L, pH 6, 25 °C | 480.9 | |
| Pb2+ | Red-mud-supported ZVI | N/A | 44.6 | Pb2+ 100–400 mg/L, pH = 6 | 149.4 | [39] |
| Cu2+ | MWCNT-reinforced ZVI- PAA/PVA polymer mats | N/A | N/A | Cu2+ 25–200 mg/L | 107.8 | [48] |
| Pb2+ | ZVI-L1000 | N/A | N/A | 25–400 mg/L, pH 6.4 (Cd2+), 6.2 (Cu2+), and 5.4 (Pb2+) 25 °C, 15 min, 25 °C | 55.2 | This study |
| Cd2+ | 38.3 | |||||
| Cu2+ | 42.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaraweera, H.; Nawalage, S.; Nayanathara, R.M.O.; Peiris, C.; Karunaratne, T.N.; Gunatilake, S.R.; Thirumalai, R.V.K.G.; Zhang, J.; Zhang, X.; Mlsna, T. In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation. Processes 2022, 10, 1659. https://doi.org/10.3390/pr10081659
Samaraweera H, Nawalage S, Nayanathara RMO, Peiris C, Karunaratne TN, Gunatilake SR, Thirumalai RVKG, Zhang J, Zhang X, Mlsna T. In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation. Processes. 2022; 10(8):1659. https://doi.org/10.3390/pr10081659
Chicago/Turabian StyleSamaraweera, Hasara, Samadhi Nawalage, R. M. Oshani Nayanathara, Chathuri Peiris, Tharindu N. Karunaratne, Sameera R. Gunatilake, Rooban V. K. G. Thirumalai, Jilei Zhang, Xuefeng Zhang, and Todd Mlsna. 2022. "In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation" Processes 10, no. 8: 1659. https://doi.org/10.3390/pr10081659
APA StyleSamaraweera, H., Nawalage, S., Nayanathara, R. M. O., Peiris, C., Karunaratne, T. N., Gunatilake, S. R., Thirumalai, R. V. K. G., Zhang, J., Zhang, X., & Mlsna, T. (2022). In Situ Synthesis of Zero-Valent Iron-Decorated Lignite Carbon for Aqueous Heavy Metal Remediation. Processes, 10(8), 1659. https://doi.org/10.3390/pr10081659







