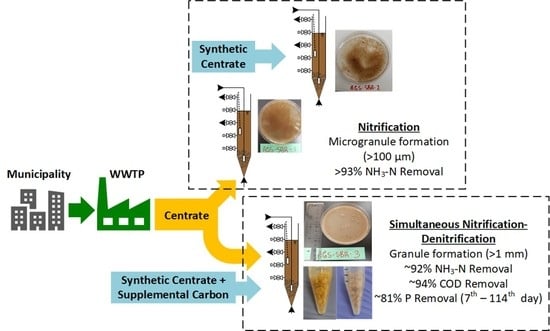
Cultivation of Nitrifying and Nitrifying-Denitrifying Aerobic Granular Sludge for Sidestream Treatment of Anaerobically Digested Sludge Centrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reaction Configuration and Operation
2.2. Seed Sludge
2.3. Wastewater Media Characteristics
- AGS-SBR-1: Centrate collected from ABTP was diluted to provide NH3-N feed concentrations between 80–300 mg/L. NaHCO3 was added as inorganic carbon source and pH control.
- AGS-SBR-2: Synthetic centrate with NaHCO3 as inorganic carbon source and pH control, NH4Cl as nitrogen source and K2HPO4 & KH2PO4 as phosphorus sources.
- AGS-SBR-3: Synthetic centrate with acetate as organic carbon source, NH4Cl as nitrogen source and K2HPO4 & KH2PO4 as phosphorus sources. The feed stream was switched to diluted centrate after day 79 with sodium acetate (CH3COONa) as supplemental carbon source.
2.4. Analytical Methods
2.4.1. Biomass (Solid Phase) Analysis
2.4.2. Wastewater (Liquid Phase) Analysis
2.4.3. Reactor Performance and Removal Analysis
2.4.4. Granule Structure and Morphology
3. Results and Discussion
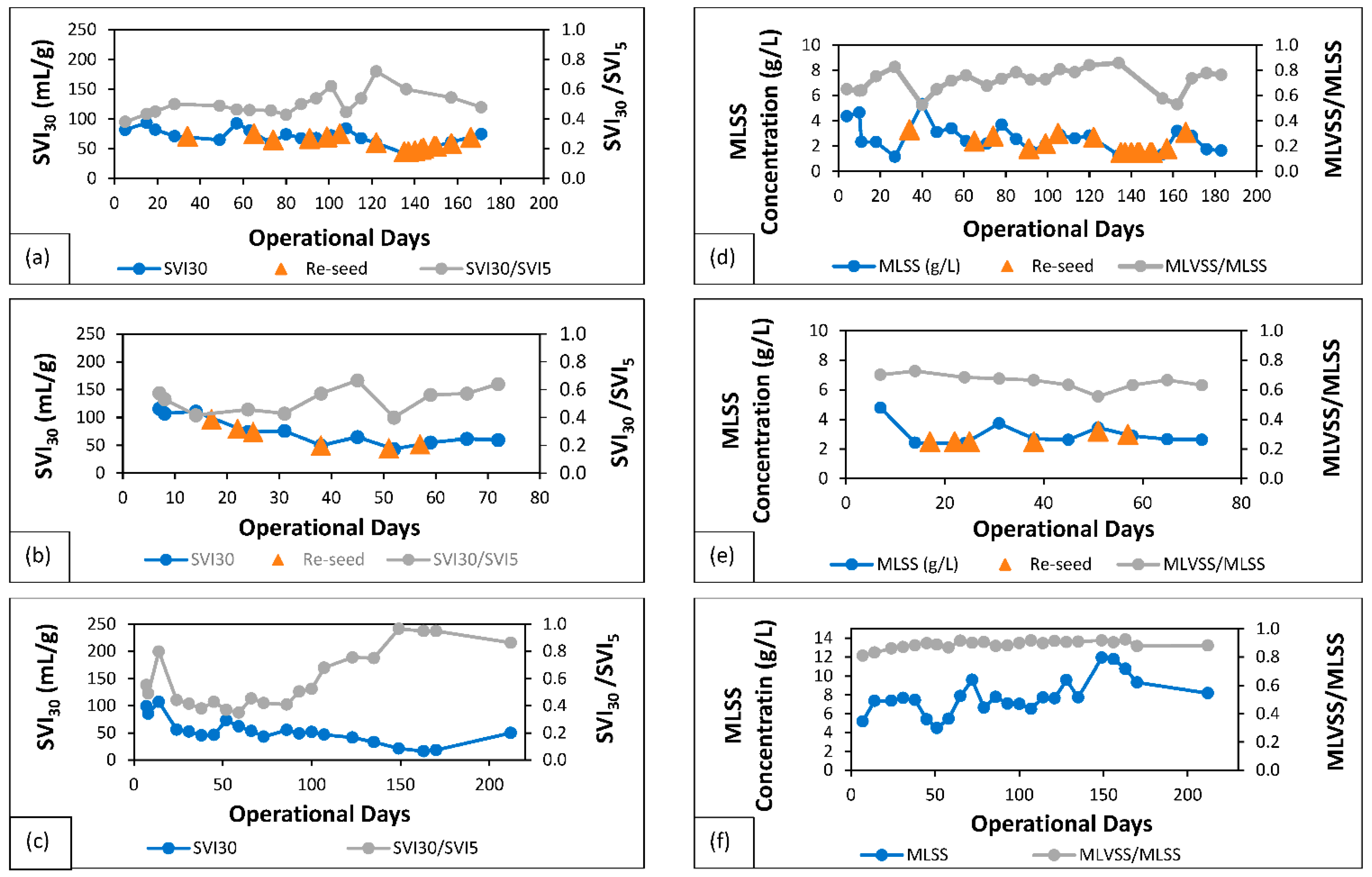
3.1. Characteristics of Granular Sludge
3.1.1. Centrate Nitrifying Sludge (AGS-SBR-1)
3.1.2. Synthetic Centrate Nitrifying Sludge (AGS-SBR-2)
3.1.3. Nitrifying-Denitrifying Sludge (AGS-SBR-3)
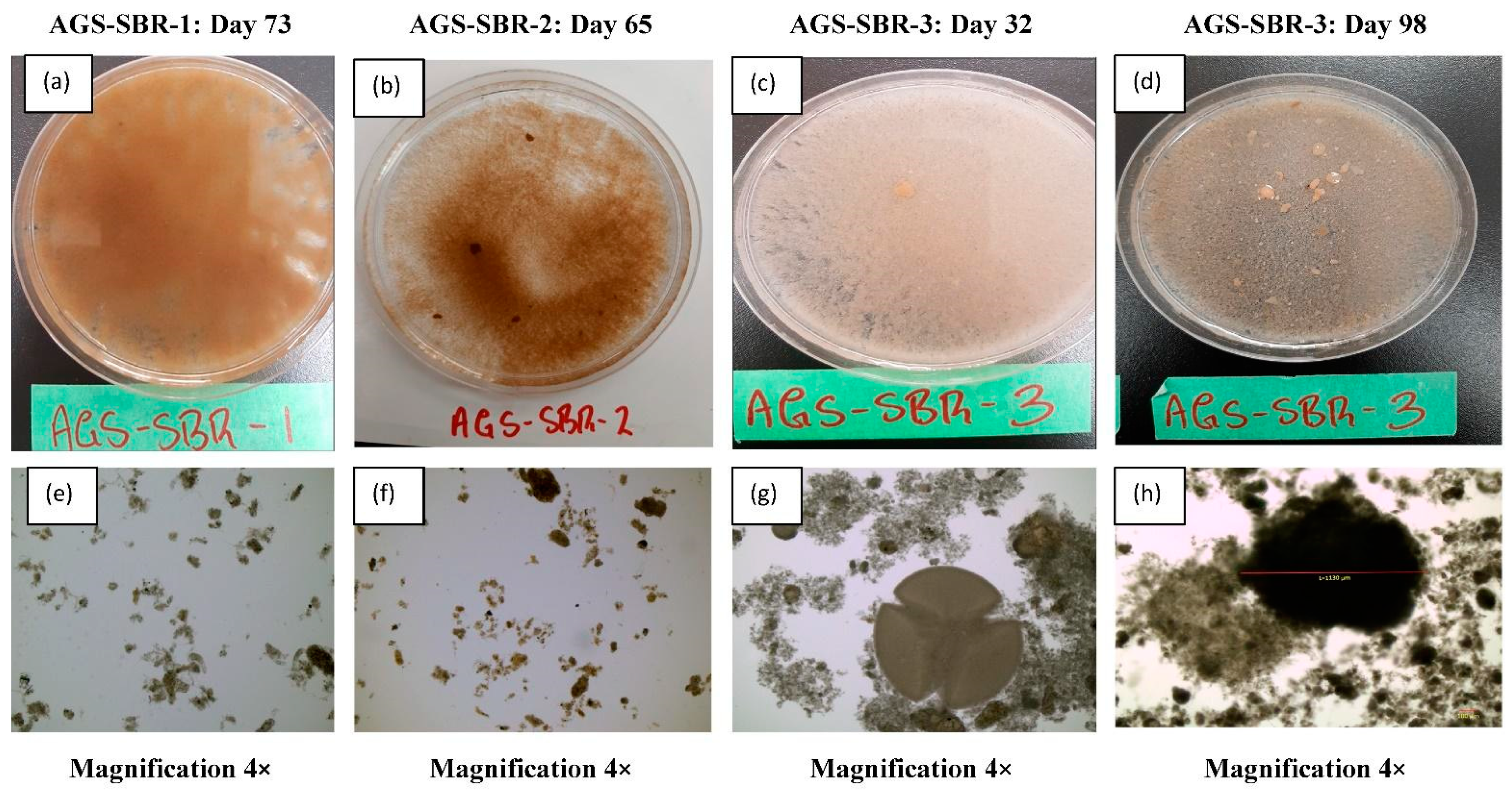
3.2. Morphology and Structure of Aerobic Granules
3.3. Pollutants Removal Efficiencies
3.3.1. Centrate Nitrifying SBR (AGS-SBR-1)
3.3.2. Synthetic Centrate Nitrifying SBR (AGS-SBR-2)
3.3.3. Nitrifying-Denitrifying SBR (AGS-SBR-3)
3.4. Pollutants Degradation in SBR Cycle
3.4.1. Cycle Test: AGS-SBR-1 (Day 120)
3.4.2. Cycle Test: AGS-SBR-2 (Day 72)
3.4.3. Cycle Test: AGS-SBR-3 (Day 128)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamatelatou, K. Novel biological processes for nutrient removal and energy recovery from wastewater. In Wastewater and Biosolids Management; IWA Publishing: London, UK, 2020; pp. 27–42. [Google Scholar]
- Mihelcic, J.R.; Ren, Z.J.; Cornejo, P.K.; Fisher, A.; Simon, A.J.; Snyder, S.W.; Zhang, Q.; Rosso, D.; Huggins, T.M.; Cooper, W.; et al. Accelerating Innovation that Enhances Resource Recovery in the Wastewater Sector: Advancing a National Testbed Network. Environ. Sci. Technol. 2017, 51, 7749–7758. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Saha, S.; Kurade, M.B.; Salama, E.S.; El-Dalatony, M.M.; Ha, G.-S.; Chang, S.W.; Jeon, B.-H. Perspective on anaerobic digestion for biomethanation in cold environments. Renew. Sustain. Energy Rev. 2019, 103, 85–95. [Google Scholar] [CrossRef]
- De Vrieze, J.; Smet, D.; Klok, J.; Colsen, J.; Angenent, L.T.; Vlaeminck, S.E. Thermophilic sludge digestion improves energy balance and nutrient recovery potential in full-scale municipal wastewater treatment plants. Bioresour. Technol. 2016, 218, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Ochs, P.; Martin, B.D.; Germain, E.; Stephenson, T.; van Loosdrecht, M.; Soares, A. Ammonia removal from thermal hydrolysis dewatering liquors via three different deammonification technologies. Sci. Total Environ. 2020, 755, 142684. [Google Scholar] [CrossRef]
- Stenström, F.; La Cour Jansen, J. Promotion of nitrifiers through side-stream bioaugmentation: A full-scale study. Water Sci. Technol. 2016, 74, 1736–1743. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Galvagno, G.; Cimon, C. Approaches and processes for ammonia removal from side-streams of municipal effluent treatment plants. Bioresour. Technol. 2018, 268, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Mentzer, C.; Pagilla, K.R. Full-scale N removal from centrate using a sidestream process with a mainstream carbon source. Water Environ. Res. 2020, 92, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.; Tsuchihashi, R.; Stensel, H.D. Technologies for Sidestream Nitrogen Removal; IWA Publishing: London, UK, 2016; Volume 15. [Google Scholar]
- Menkveld, H.W.H.; Broeders, E. Recovery of ammonia from digestate as fertilizer. Water Pract. Technol. 2018, 13, 382–387. [Google Scholar] [CrossRef]
- Lei, C.S.; Ma, J.F.; Li, D.L.; Zhang, W.Y.; Guang, X.H.; Ma, J. Composite denitrification reagent for high concentration ammonia removal by air stripping. Chin. Sci. Bull. 2010, 55, 2657–2661. [Google Scholar] [CrossRef]
- Aljerf, L. Advanced highly polluted rainwater treatment process. J. Urban Environ. Eng. 2018, 12, 50–58. [Google Scholar] [CrossRef]
- Thornton, A.; Pearce, P.; Parsons, S.A. Ammonium removal from digested sludge liquors using ion exchange. Water Res. 2007, 41, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Sun, Z.Y.; Chen, Y.T.; Xia, Z.Y.; Gou, M.; Tang, Y.Q. Performance and bacterial communities in conventional activated sludge and membrane bioreactor systems with Low C/N ratio wastewater for nitrogen removal. Environ. Eng. Sci. 2019, 36, 1112–1126. [Google Scholar] [CrossRef]
- Dold, P.; Du, W.; Burger, G.; Jimenez, J. Is Nitrite-Shunt Happening in the System? Are Nob Repressed? Proc. Water Environ. Fed. 2015, 2015, 1360–1374. [Google Scholar] [CrossRef]
- Kartal, B.; Kuenen, J.G.; Van Loodsdrecht, M.C.M. Sewage Treatment with Anammox. Am. Assoc. Adv. Sci. 2010, 328, 702–703. Available online: https://www.jstor.org/stable/40655872 (accessed on 14 March 2022). [CrossRef]
- Pedros, P.B.; Onnis-Hayden, A.; Tyler, C. Investigation of Nitrification and Nitrogen Removal from Centrate in a Submerged Attached-Growth Bioreactor. Water Environ. Res. 2008, 80, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, D.; Ficara, E.; Corbellini, V.; Tornotti, G.; Teli, A.; Canziani, R.; Malpei, F. Autotrophic nitrogen removal by a two-step SBR process applied to mixed agro-digestate. Bioresour. Technol. 2015, 176, 98–105. [Google Scholar] [CrossRef]
- López-Palau, S.; Dosta, J.; Pericas, A.; Mata-Álvarez, J. Partial nitrification of sludge reject water using suspended and granular biomass. J. Chem. Technol. Biotechnol. 2011, 86, 1480–1487. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, Y.; Wang, S.; Zheng, S.; Guo, J. Organic matter and concentrated nitrogen removal by shortcut nitrification and denitrification from mature municipal landfill leachate. J. Environ. Sci. 2007, 19, 647–651. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A. Treatment of Ammonium-Rich Digestate from Methane Fermentation Using Aerobic Granular Sludge. Water Air. Soil Pollut. 2018, 229, 1–12. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wu, W.W.; Tay, J.H.; Wang, J.L. Formation and long-term stability of nitrifying granules in a sequencing batch reactor. Bioresour. Technol. 2008, 99, 3919–3922. [Google Scholar] [CrossRef]
- Figdore, B.A.; Winkler, M.-K.H.; Stensel, H.D. Bioaugmentation with Nitrifying Granules in Low-SRT Flocculent Activated Sludge at Low Temperature. Water Environ. Res. 2017, 90, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic granular sludge process: A fast growing biological treatment for sustainable wastewater treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Wei, D.; Shi, L.; Yan, T.; Zhang, G.; Wang, Y.; Du, B. Aerobic granules formation and simultaneous nitrogen and phosphorus removal treating high strength ammonia wastewater in sequencing batch reactor. Bioresour. Technol. 2014, 171, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, I.; Erguder, T.H. Influent COD/TAN ratio affects the carbon and nitrogen removal efficiency and stability of aerobic granules. Ecol. Eng. 2016, 90, 12–24. [Google Scholar] [CrossRef]
- Figdore, B.A.; David Stensel, H.; Winkler, M.K.H. Bioaugmentation of sidestream nitrifying-denitrifying phosphorus-accumulating granules in a low-SRT activated sludge system at low temperature. Water Res. 2018, 135, 241–250. [Google Scholar] [CrossRef]
- Wilén, B.M.; Liébana, R.; Persson, F.; Modin, O.; Hermansson, M. The mechanisms of granulation of activated sludge in wastewater treatment, its optimization, and impact on effluent quality. Appl. Microbiol. Biotechnol. 2018, 102, 5005–5020. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Inc. Metcalf & Eddy; Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F.; Abu-Orf, M.; Bowden, G.; Pfrang, W. Wastewater Engineering: Treatment and Resource Recovery; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Ren, Y.; Ferraz, F.M.; Yuan, Q. Landfill Leachate Treatment Using Aerobic Granular Sludge. J. Environ. Eng. 2017, 143, 04017060. [Google Scholar] [CrossRef]
- Czarnota, J.; Tomaszek, J.A.; Masłoń, A.; Piech, A.; Łagód, G. Powdered Ceramsite and Powdered Limestone Use in Aerobic Granular Sludge Technology. Materials 2020, 13, 3894. [Google Scholar] [CrossRef]
- Fan, J.; Ji, F.; Xu, X.; Wang, Y.; Yan, D.; Xu, X.; Chen, Q.; Xiong, J.; He, Q. Prediction of the effect of fine grit on the MLVSS/MLSS ratio of activated sludge. Bioresour. Technol. 2015, 190, 51–56. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; zahra Ezzahraoui, F.; Morgan, G.; Iorhemen, O.T. A review of the state of development of aerobic granular sludge technology over the last 20 years: Full-scale applications and resource recovery. Case Stud. Chem. Environ. Eng. 2021, 5, 100173. [Google Scholar] [CrossRef]
- Law, Y.; Lant, P.; Yuan, Z. The effect of pH on N2O production under aerobic conditions in a partial nitritation system. Water Res. 2011, 45, 5934–5944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Long, B.; Cheng, Y.; Wu, J.; Zhang, L.; Zeng, Y.; Zeng, M.; Huang, S. Rapid domestication of autotrophic nitrifying granular sludge and its stability during long-term operation. Environ. Technol. 2021, 42, 2587–2598. [Google Scholar] [CrossRef]
- Shi, X.Y.; Sheng, G.P.; Li, X.Y.; Yu, H.Q. Operation of a sequencing batch reactor for cultivating autotrophic nitrifying granules. Bioresour. Technol. 2010, 101, 2960–2964. [Google Scholar] [CrossRef]
- Tsuneda, S.; Nagano, T.; Hoshino, T.; Ejiri, Y.; Noda, N.; Hirata, A. Characterization of nitrifying granules produced in an aerobic upflow fluidized bed reactor. Water Res. 2003, 37, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Figdore, B.A.; Stensel, H.D.; Winkler, M.K.H. Comparison of different aerobic granular sludge types for activated sludge nitrification bioaugmentation potential. Bioresour. Technol. 2018, 251, 189–196. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Chen, L.; Zhang, S.; Wang, L.; Liang, J.; Xia, W.; Wang, H.; Zhou, J. Simultaneous nitrification, denitrification and phosphorus removal in aerobic granular sequencing batch reactors with high aeration intensity: Impact of aeration time. Bioresour. Technol. 2018, 263, 214–222. [Google Scholar] [CrossRef]
- Klein, K.; Tenno, T. Estimating the impact of inhibitory substances on activated sludge denitrification process. Water Pract. Technol. 2019, 14, 863–871. [Google Scholar] [CrossRef]
- Luiz de Sousa Rollemberg, S.; Queiroz de Oliveira, L.; Nascimento de Barros, A.; Igor Milen Firmino, P.; Bezerra dos Santos, A. Pilot-scale aerobic granular sludge in the treatment of municipal wastewater: Optimizations in the start-up, methodology of sludge discharge, and evaluation of resource recovery. Bioresour. Technol. 2020, 311, 123467. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.J.; Lemaire, R.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification, and phosphorus removal in a lab-scale sequencing batch reactor. Biotechnol. Bioeng. 2003, 84, 170–178. [Google Scholar] [CrossRef]
- Kuba, T.; Smolders, G.; van Loosdrecht, M.C.M.; Heijnen, J.J. Biological phosphorus removal from wastewater by anaerobic-anoxic sequencing batch reactor. Water Sci. Technol. 1993, 27, 241–252. [Google Scholar] [CrossRef]
- Pronk, M.; de Kreuk, M.K.; de Bruin, B.; Kamminga, P.; Kleerebezem, R.; van Loosdrecht, M.C.M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, M.H. Alkalinity and pH. In The Microbiology of Anaerobic Digesters; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 99–103. [Google Scholar] [CrossRef]
- Sarvajith, M.; Nancharaiah, Y.V. Enhancing biological nitrogen and phosphorus removal performance in aerobic granular sludge sequencing batch reactors by activated carbon particles. J. Environ. Manag. 2022, 303, 114134. [Google Scholar] [CrossRef] [PubMed]
- Callado, N.H.; Foresti, E. Removal of organic carbon, nitrogen and phosphorus in sequential batch reactors integrating the aerobic/anaerobic processes. Water Sci. Technol. 2001, 44, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.-Y.; Stenstrom, M.K. Bioaugmentation to Improve Nitrification in Activated Sludge Treatment. Water Environ. Res. 2010, 82, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Mossakowska, A.; Reinius, L.-G.; Hultman, B. Nitrification reactions in treatment of supernatant from dewatering of digested sludge. Water Environ. Res. 1997, 69, 1128–1133. [Google Scholar] [CrossRef]
- Monti, A.; Hall, E.R. Comparison of Nitrification Rates in Conventional and Membrane-Assisted Biological Nutrient Removal Processes. Water Environ. Res. 2008, 80, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.A.; Erb, M.; Garel, C.; Audic, J.M. A Comparative Pilot-Scale Study of the Performance of Conventional Activated Sludge and Membrane Bioreactors under Limiting Operating Conditions. Water Environ. Res. 2003, 75, 225–231. [Google Scholar] [CrossRef]
- Choubert, J.M.; Racault, Y.; Grasmick, A.; Beck, C.; Heduit, A. Maximum nitrification rate in activated sludge processes at low temperature: Key parameters, optimal value. Eur. Water Manag. Online 2005, 1–13. [Google Scholar]
- Dytczak, M.A.; Londry, K.L.; Oleszkiewicz, J.A. Nitrifying Genera in Activated Sludge May Influence Nitrification Rates. Water Environ. Res. 2008, 80, 388–396. [Google Scholar] [CrossRef]
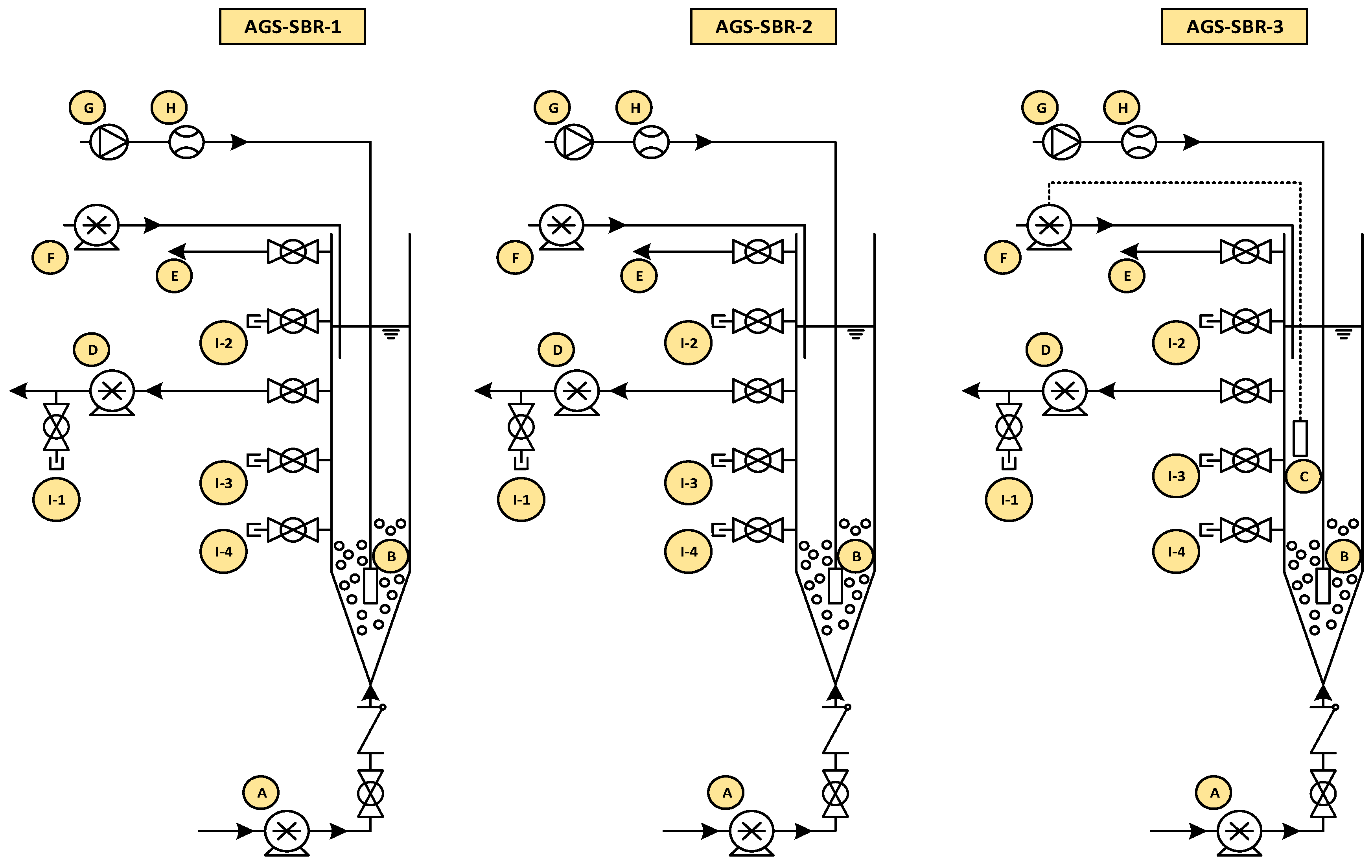


| AGS-SBR-1 | AGS-SBR-2 | AGS-SBR-3 | ||||
|---|---|---|---|---|---|---|
| Phase | 6-h | 4-h | 6-h | 4-h | 6-h | 4-h |
| Influent Filling (min) | 15 | 15 | 15 | 15 | 60 | 60 |
| Aeration (min) | 265–315 | 160–195 | 265–315 | 190–195 | 220–270 | 145–150 |
| Settling (min) | 10–60 | 10–45 | 10–60 | 10–15 | 10–60 | 10–15 |
| Effluent discharge (min) | 15 | 15 | 15 | 15 | 15 | 15 |
| Idle (min) | 5 | 5 | 5 | 5 | 5 | 5 |
| Parameter | Unit | AGS-SBR-1 | AGS-SBR-2 | AGS-SBR-3 |
|---|---|---|---|---|
| Operating Duration | days | 183 | 72 | 212 |
| pH | - | 7.8 ± 0.5 | 8 ± 0.5 | 7.8 ± 0.3 |
| Temperature | °C | 22–25 | 22–25 | 22–25 |
| VER | % | 50 | 50 | 50 |
| DO | mg/L | >2 (non-limiting) | >2 (non-limiting) | >2 (non-limiting) |
| Reactor | Period | Operation Days | Influent NH3-N Concentration (mg/L) | HRT (h) |
|---|---|---|---|---|
| AGS-SBR-1 | I | Days 1–61 | 123 ± 5 | 12 |
| II | Days 71–99 | 180 ± 17 | 12 | |
| III | Days 106–120 | 182 ± 9 | 8 | |
| IV | Days 134–155 | 255 ± 7 | 8 | |
| V | Days 162–183 | 292 ± 11 | 8 | |
| AGS-SBR-2 | I | Days 1–14 | 84 ± 2 | 12 |
| II | Days 24–51 | 187 ± 7 | 12 | |
| III | Days 58–72 | 192 ± 13 | 8 |
| Period | Operational Days | Influent NH3-N Concentration (mg/L) | Supplemental COD Concentration (g/L) | HRT (h) | COD:N:P | |
|---|---|---|---|---|---|---|
| AGS-SBR-3 | I | Days 1–38 | 77 ± 12 | 2.09 ± 0.15 | 12 | 105:4:1 |
| Days 38–72 | 88 ± 3 | 2.20 ± 0.07 | 8 | 110:4:1 | ||
| II | Days 79–93 | 84 ± 2 | 1.52 ± 0.01 | 8 | 76:4:1 | |
| III | Days 100–114 | 74 ± 11 | 0.95 ± 0.06 | 8 | 47:4:0.4 | |
| IV | Days 121–135 | 149 ± 7 | 0.72 ± 0.05 | 8 | 36:8:2 | |
| Days 149–156 | 131 ± 0 | 0.54 ± 0.06 | 8 | 27:7:1 | ||
| V | Days 163–212 | 121 ± 5 | 1.39 ± 0.05 | 8 | 70:6:2 |
| AGS-SBR-1 | AGS-SBR-2 | AGS-SBR-3 § | |||
|---|---|---|---|---|---|
| Parameter | Average Concentration (Per Litre) | Component | Per Litre | Component | Per Litre |
| NH3-N | 484.83 ± 46.96 mg | NaHCO3 | 1.2 g | CH3COONa | 2.56 g |
| NO2-N | 0.074 ± 0.05 mg | NH4Cl | 0.35 g | NH4Cl | 0.35 g |
| NO3-N | 0.91 ± 0.13 mg | K2HPO4 | 0.05 g | K2HPO4 | 0.03 g |
| TSS | 0.089 ± 0.01 mg | KH2PO4 | 0.045 g | KH2PO4 | 0.025 g |
| VSS | 0.075 ± 0.01 mg | CaCl2·2H2O | 0.03 g | CaCl2·2H2O | 0.03 g |
| tCOD | 674.90 ± 139.62 mg | MgSO4·7H2O | 0.025 g | MgSO4·7H2O | 0.025 g |
| sCOD | 547.68 ± 136.34 mg | FeSO4·7H2O | 0.02 g | FeSO4·7H2O | 0.02 g |
| CaCO3 | 1922.58 ± 263.84 mg | Micronutrients * | 1 mL | Micronutrients * | 1 mL |
| TN | 524.75 ± 29.29 mg | ||||
| PO43− | 103.10 ± 11.27 mg | ||||
| Reactor Type | Parameter | References |
|---|---|---|
| Aerobic Granular Sludge Sequencing Batch Reactor (AGS-SBR) | Maximum specific ammonia oxidizing rate = 29.8 mg N/g VSS/h | [23] |
| Aerobic Granular Sludge Sequencing Batch Reactor (AGS-SBR) | Maximum specific ammonia oxidizing rate NIT granules = 31.25 mg N/g VSS/h NDN-PAO granules = 5.83 mg N/g VSS/h | [39] |
| Activated Sludge Sequencing batch reactor (AS-SBR) | Specific Nitrification rate = 30–45 g NH4-N/kg MLVSS/h | [49,50] |
| CAS | Specific nitrification rates in the membrane and conventional mixed liquor: between 3 and 5 mg N/g VSS/h | [51] |
| CAS | Specific nitrification rates: between 4 and 7 mg N/g VSS/h were measured in both membrane and conventional systems. | [51,52] |
| Activated Sludge (AS) | Maximum Nitrification rate = 1.0–4.5 mg N/g MLVSS/h | [53] |
| SBR | Ammonia utilization rate (AUR) = 2.95 +/− 0.26 mg NH4+-N/g VSS/h Ammonia utilization rate (AUR) was faster = 6.16 +/− 0.34 mg NH4+-N/g VSS/h | [54] |
| AGS-SBRs | Nitrifying AGS-SBR with diluted centrate: Nitrification rate = 45.30 mg N/L·h Specific nitrification rate = 19.19 mg N/g VSS·h Nitrifying AGS-SBR with synthetic centrate: Nitrification rate = 40.36 mg N/L·h Specific nitrification rate = 27.36 mg N/g VSS·h Nitrifying/Denitrifying AGS-SBR: Nitrification rate = 54.48 NH3-N/L·h Specific nitrification rate = 6.86 mg NH3-N/g VSS·h. SND efficiency during periods with negligible NOx-N accumulation was 87 ± 11%. | (This Study) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, G.; Hamza, R.A. Cultivation of Nitrifying and Nitrifying-Denitrifying Aerobic Granular Sludge for Sidestream Treatment of Anaerobically Digested Sludge Centrate. Processes 2022, 10, 1687. https://doi.org/10.3390/pr10091687
Morgan G, Hamza RA. Cultivation of Nitrifying and Nitrifying-Denitrifying Aerobic Granular Sludge for Sidestream Treatment of Anaerobically Digested Sludge Centrate. Processes. 2022; 10(9):1687. https://doi.org/10.3390/pr10091687
Chicago/Turabian StyleMorgan, Guillian, and Rania Ahmed Hamza. 2022. "Cultivation of Nitrifying and Nitrifying-Denitrifying Aerobic Granular Sludge for Sidestream Treatment of Anaerobically Digested Sludge Centrate" Processes 10, no. 9: 1687. https://doi.org/10.3390/pr10091687
APA StyleMorgan, G., & Hamza, R. A. (2022). Cultivation of Nitrifying and Nitrifying-Denitrifying Aerobic Granular Sludge for Sidestream Treatment of Anaerobically Digested Sludge Centrate. Processes, 10(9), 1687. https://doi.org/10.3390/pr10091687