Influencing Factors on Synthesis and Properties of MXene: A Review
Abstract
:1. Introduction
- (1)
- Electrical and optical properties
- (2)
- Heat endurance
- (3)
- Mechanical properties
- (4)
- Magnetic performance
2. Influencing Factors on MXene Synthesis
2.1. Selection of Materials
2.2. Parameters during Process
- (1)
- Impact factors of the in situ etching method
- A higher concentration of HF makes a more thorough dissection, which provides thinner MXene two-dimension material [11].
- With short etching time and low temperature, the MXenes prepared are in a wide frequency range with high impedance matching and a large attenuation coefficient [12].
- (2)
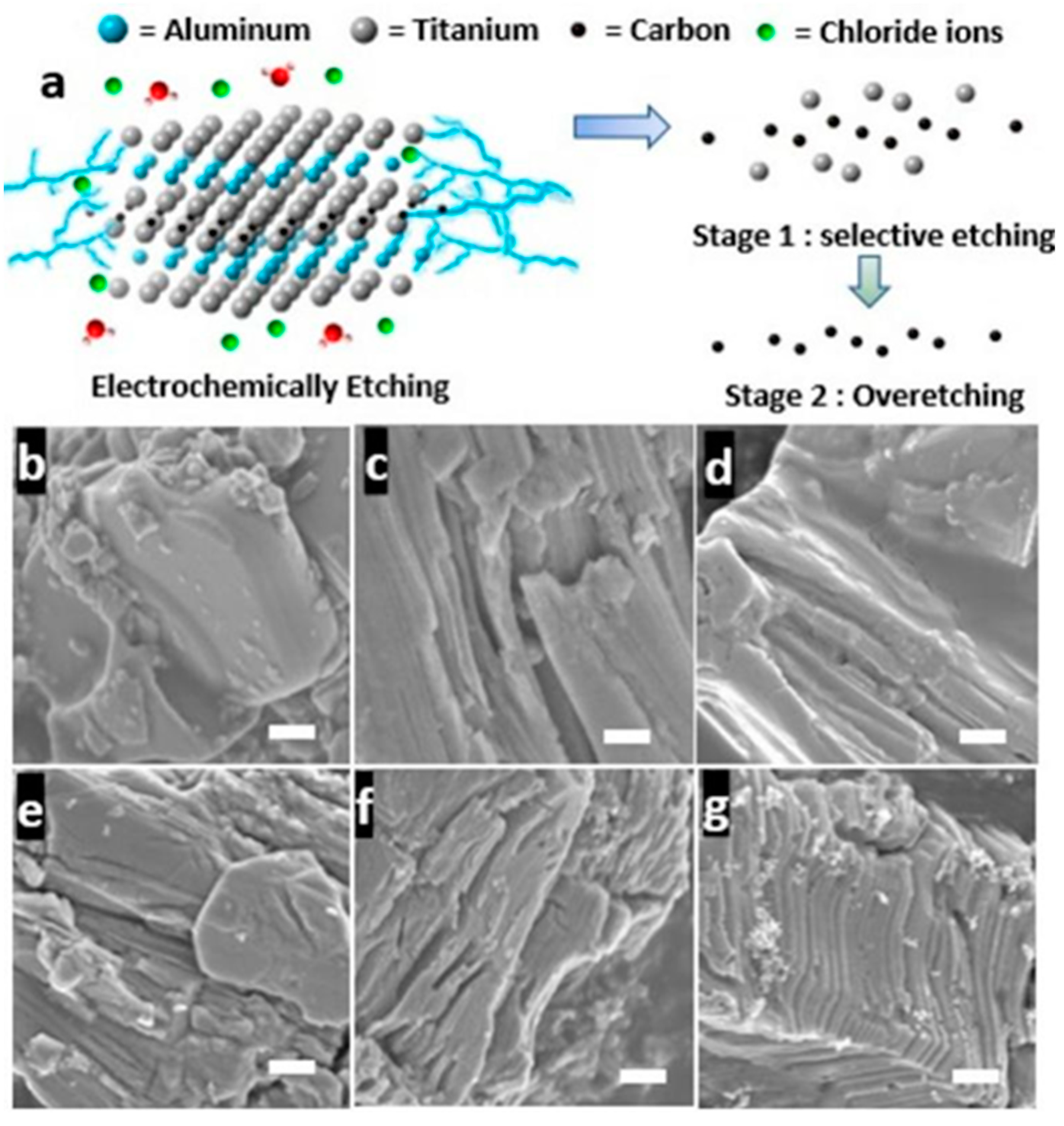
- Impact factors of the electrochemical-etching method
- Selection of etcher—Use the mixture of NH4Cl and TMAOH as an electrolyte, apply a voltage of +5 V, use tetramethyloxic ammonium hydroxide for separation, and a few layers Ti3C2Tx can be achieved [14];
- Voltage significantly affects Ti3C2 formation—Increased voltage and ultrasound can promote the stratification of Ti3C2, but the Ti3C2 formed still adheres to the working electrode, inducing excessive corrosion and CDC formation [16].
- (3)
- Impact factors of the molten fluorinated salt method
- (4)
- Impact factors of chemical vapor deposition process
- Low concentration of methane is crucial to obtaining ultrathin Mo2C crystals instead of graphene [21];
- Thickness depends on the behavior of the high-quality, ultrathin α-Mo2C crystals;
- The logarithmic temperature impacts significantly on the thin crystals that are exhibited.
3. Impacts of MXene Modification on Its Properties
3.1. Effects of Surface Modification on MXene Properties
- Characteristics of the MXene surface are associated with friction and adhesion properties. With negative friction factors, MXene has better hydrophilicity and more adhesivity [26]. The interaction of strength and friction may increase with the hydrophilicity of the MXene surface.
- The changes in electrostatic potential around the surface of the material affect the electronic structure and work function of MXene. The surface groups can strongly affect the density of state and the work function [27].
- The influence of N-doping mode and doping process on the electrical properties of MXene structure has been explored [28]. There are three possible routines for N-doping: lattice substitution, functional group substitution, and surface adsorption.
- In the acidic aqueous phase, alkyl phosphoric acid ligands were applied via interfacial nucleophilic addition and sequential condensation reactions to form a Ti-O-P bond, grafting to the MXene surface. Relying on the spatial stability of long alkyl chains and the solvation effects of strong nonpolar effects, the solubility of MXene in nonpolar organic solvents is enhanced [29].
3.2. Impact Factors on the Catalytic Performance of MXene
- Plasma treatment was performed to enhance the surface functional groups on the Ti3C2. The enhanced surface functional groups on MXenes benefit in providing abundant active sites on the surface for photocatalytic reactions [30].
- Oxygen vacancies embedded in the Ti3C2O2 MXenes can favor a highly selective photocatalytic CO2 reduction. Based on calcination, TiO2 nanoparticles (NPs) were grown in situ on highly conductive MXenes Ti3C2 [31]. A unique rice husk-like structure was obtained by evenly distributing NPs over Ti3C2. By producing CH4, the optimized TiO2/Ti3C2 composite exhibited 3.7-times higher photocatalytic CO2 reduction performance compared to the commercial TiO2.
- Double heterojunction (S-type heterojunction at the TiO2/C3N4 interface and Schoteryl heterojunction at the C3N4/TCQD interface) play a major role in improving photocatalytic activity and jointly accelerate the electron-hole pair separation, migration, and utilization of photogenerated charge carriers with strong redox capacity [32].
- Ti3C2 serves as a co-catalyst that enhances the performance of semiconductors in reduction of CO2. By constructing composite catalysts with other semiconductors in the shape of QDs, it effectively optimizes the capabilities of CO2 reduction and conversion. Compared with 2D ultrathin nanosheets, 0D QDs can be more uniformly dispersed in the liquid and have abundant active edge sites. In the process of CO2 reduction, based on the photocatalysis by Ti3C2 QDs/Cu2O NWs/Cu, the yield of methanol reached 153.38 ppm·cm−2, which is an improvement of 8.25 fold from Cu2O NWs/Cu [34].
3.3. Impact Factors on the Energy Storage and Conversion Properties
- Metal oxide can provide a sufficient Li+ or Na+ ion reservoir. The ion-intercalation sites make conductive MXene serve as effective pathways for electron transfer, which can also enlarge the interlayer spacing of layered MXene.
- Surface-exposed MXene can be used as a high-capacity anode material for non-lithium-ion batteries. In particular, for the Mg2+ and Al3+ batteries, the capacitance and ion mobility of the exposed MXene are higher than that of the oxygen-sealed MXene. Mg and Al can form a stable metal layer on the surface of the exposed MXene, with a high theoretical capacity [38].
- Metallic conductivity, unique 2D structure, and surface defects are important properties of MXene, which make it a prominent electrocatalyst (introduced in Section 3.1).
3.4. Impact Factors on Microwave Absorbing Properties
- Ti3CNTx exhibits exceptionally excellent microwave absorption properties after annealing, owing to the significant increase in the electrical conductivity, voids, and dipolar polarization capability of Ti3CNTx after annealing [41].
- Building a special morphology, such as three-dimensional porous structure, multilayer wave absorption structure, shell structure, flower structure, etc., can improve the electromagnetic wave absorption and multiple reflection capability.
- Construction of a single material structure, such as designing hollow, porous, or shell structure magnetic particles instead of solid magnetic particles, can meet the requirements for multiple reflections and low weight.
3.5. Impact Factors on Adsorption Properties
- Modifications of MXene may yield more effective results on heavy metal removal. Alkalization-grafting with sodium ions and a silane coupling agent (APTES) is the most effective modification method so far; it achieved better adsorption capacity (385 mg/g) by using a lower amount of adsorbent (0.1 g/L) and equilibrium time (30 min).
- One of the most important mechanisms of the adsorption is ion exchange, which replaces heavy metal ions by attaching to the surface of MXene functional groups (Table 3). In the case of alkylated Ti3C2(OH/ONa)xF2−x adsorbing Pb2+, the Pb2+ adsorbed is a function of pH with more preferential sorption at higher levels within the range 5–7 [42]. At low pH, the low affinity of MXenes toward Pb2+ may regenerate Pb2+ from used flakes in the solutions [43].
4. Conclusions
- Databases and computational models are needed to address the parameters of MXene synthesis, modification, and the positive/negative factors for application in various fields.
- Extend the advantages of MXene in engineering practice, e.g., make significant contributions to carbon reduction programs.
- MXenes as adsorbents are applied to remove heavy metals from synthetic wastewater. Thus, MXene is also expected to be investigated in removing pollutants (e.g., heavy metals, salts, and organic pollutants) in multiple media (air pollution, solid waste, etc.), and applied to the actual complex and complicated environment.
- Chemicals are introduced in the process of MXene synthesis and modification, hence, occupational hygiene, environmental issues, and laboratory safety should be considered.
Author Contributions
Funding
Conflicts of Interest
References
- Peng, J.; Chen, X.; Ong, W.J. Surface and heterointerface engineering of 2D MXenes and their nanocomposites: Insights into electro- and photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhou, A. Hydrothermal synthesis of TiO2/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater. Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Mathis, T.S. Selective etching of silicon from Ti3SiC2 (MAX) to obtain 2D titanium carbide (MXene). Angew. Chem. Int. Ed. 2018, 130, 5442–5446. [Google Scholar] [CrossRef]
- Michael, G.; Lukatskaya, M.R.; Zhao, M.Q. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar]
- Mashtalir, O.; Naguib, M.; Dyatkin, B. Kinetics of aluminum extraction from Ti3AlC2 in hydrofluoric acid. Mater. Chem. Phys. 2013, 139, 147–152. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M. Transparent conductive two-dimensional titanium carbide epitaxial thin films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 216–219. [Google Scholar] [CrossRef]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, Q. Amine-assisted delamination of Nb2C MXene for Li-ion energy storage devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L. Large-scaledelamination of multi-layers transition metal carbides and carbonitrides “Mxenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
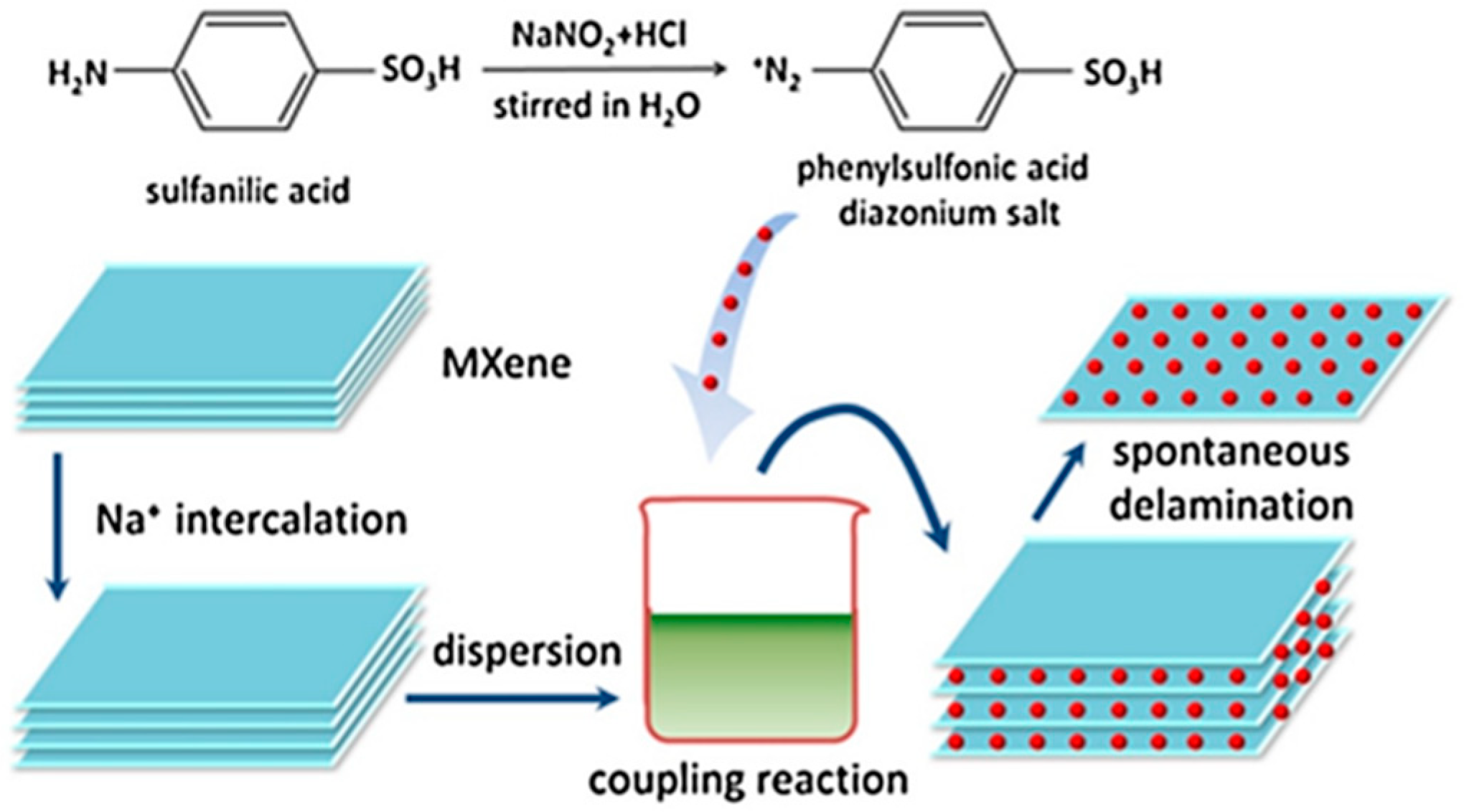
- Wang, H.; Zhang, J.; Wu, Y. Surface modified MXene Ti3C2 multilayers by aryl diazonium salts leading to large-scale delamination. Appl. Surf. Sci. 2016, 384, 287–293. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Shi, H.; Su, X.; Liu, Y. Preparation of two-dimensional layered Ti3C2 and its enhanced microwave absorption properties. Basic Sci. J. Text. Univ. 2020, 33, 51–58. [Google Scholar]
- Sun, W.; Shah, S.A.; Chen, Y. Electrochemical etching of Ti2AlC to Ti2CTx (MXene) in low-concentration hydrochloric acid solution. J. Mater. Chem. 2017, 5, 21663–21668. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, P.P.; Wang, F.X. Fluoride-Free Synthesis of Two-Dimensional Titanium Carbide (MXene) Using A Binary Aqueous System. Angew. Chem. Int. Ed. 2018, 130, 15717–15721. [Google Scholar] [CrossRef]
- Pang, Y.; Wong, Y.T.; Yuan, S. Universal strategy for HF-free facile and rapid synthesis of two-dimensional MXenes as multifunctional energy materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.; Guo, C.S.; Zou, Y. Rapid synthesis of MXenes at room temperature. Mater. Sci. Technol. 2019, 35, 1904–1907. [Google Scholar] [CrossRef]
- Shen, J. The Study on the Preparation of Transition Metal Oxides by Improved Molten Salt Method with Liquid Phase and Its Control. Ph.D. Thesis, Huaqiao University, Quanzhou, China, 2018. [Google Scholar]
- Yan, M.; Yang, L.; Li, C. Preparation of Two-dimensional Ti2CTx by Molten Fluorinated Salt Method. J. Wuhan Univ. Technol. (Mater. Sci.) 2019, 34, 299–302. [Google Scholar] [CrossRef]
- Li, C.; Yang, L.; Zou, Y. Preparation of Ti2CTx with NaF-KF Molten-salt System. J. Ceram. 2019, 40, 800–804. [Google Scholar]
- Larmour, I.A.; Bell, S.E.; Saunders, G.C. Remerakably simple fabrication of superhydrophobic surfaces using electroless galvanic deposition. Angew. Chem. 2007, 46, 1710–1712. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef]
- Li, T.F.; Yao, L.L.; Liu, Q.L. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T = OH, O) via Alkali Treatment. Angew. Chem. 2018, 57, 6115–6119. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Luo, S.J.; Xie, L.Y. Boosting the Yield of MXene 2D Sheets via a Facile Hydrothermal-Assisted Intercalation. ACS Appl. Mater. Interfaces 2019, 11, 8443–8452. [Google Scholar] [CrossRef] [PubMed]
- Ba, Z.; Liang, D.; Xie, Y. Progress of MXenes Composites:Interface Modification and Structure Design. Chem. J. Chin. Univ. 2021, 42, 1225–1240. [Google Scholar]
- Li, J.; Yuan, X.T.; Lin, C.; Yang, Y.Q.; Xu, L.; Du, X.; Xie, J.L.; Lin, J.H.; Sun, J.L. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Guan, Y.X.; Zhang, M.M.; Qin, J.; MaX, X.; Li, C.; Tang, J.L. Hydrophilicity-Dependent Distinct Frictional Behaviors of Different Modified MXene Nanosheets. J. Phys. Chem. C 2020, 124, 13664–13671. [Google Scholar] [CrossRef]
- Agresti, A.; Pazniak, A.; Pescetelli, S.; Di, V.A.; Rossi, D.; Pecchia, A.; Auf, M.M.; Lied, A.; Larciprete, R.; Kuznetsov, D.V.; et al. Titanium-carbide MXenes for work function and interface engineering in perovskite solar cells. Nat. Mater. 2019, 18, 1228–1234. [Google Scholar] [CrossRef]
- Lu, C.J.; Yang, L.; Yan, B.Z.; Sun, L.B.; Zhang, P.G.; Zhang, W.; Sun, Z.M. Nitrogen-Doped Ti3C2 MXene: Mechanism Investigation and Electrochemical Analysis. Adv. Funct. Mater. 2020, 30, 2000852. [Google Scholar] [CrossRef]
- Kim, D.; Kot, Y.; Kim, H.; Lee, G.H.; Cho, S.; Koo, C.M. Nonpolar Organic Dispersion of 2D Ti3C2Tx MXene Flakes via Simultaneous Interfacial Chemical Grafting and Phase Transfer Method. ACS Nano 2019, 13, 13818–13828. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, D.; Xiang, Q. Plasma-modified Ti3C2Tx/CdS hybrids with oxygen-containing groups for high-efficiency photocatalytic hydrogen production. Nanoscale 2019, 11, 18787–18805. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reductivity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene embellished g-C3N4 nanosheets for improving photocatalytic redox capacity. J. Alloys Compd. 2021, 877, 0925–8388. [Google Scholar] [CrossRef]
- Cao, S.W.; Shen, B.J.; Tong, T.; Fu, J.W.; Yu, J.G. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Graphene transforms wide band gap ZnS to a visible light photocatalyst. The new role of graphene as a macromolecular photosensitizer. ACS Nano 2012, 27, 9777–9789. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Li, Q.; Zhou, J.; Fan, J.; Li, B.; Sun, J.; Lv, K. 2D/2D Ti3C2 MXene/g-C3N4 nanosheets heterojunction for high efficient CO2 reduction photocatalyst: Dual effects of urea. Appl. Catal. B Environ. 2020, 268, 118738. [Google Scholar] [CrossRef]
- Zeng, Z.; Yan, Y.; Chen, J.; Zan, P.; Tian, Q.; Chen, P. Boosting the Photocatalytic Ability of Cu2O Nanowires for CO2 Conversion by MXene Quantum Dots. Adv. Funct. Mater. 2018, 29, 1806500. [Google Scholar] [CrossRef]
- Xie, Y.; Dall’Agnese, Y.; Naguib, M. Prediction and char⁃acterization of MXene nanosheet anodes for non-lithium-ion batteries. ACS Nano 2014, 8, 9606–9615. [Google Scholar] [CrossRef]
- Meng, W.U.; Lei, R.; Zhang, J.F.; Li, Y.X.; Ji, Z.Y.; Ying, G.B. Research progress in preparation and performance of MXene and its composite absorbing materials. Acta Mater. Compos. Sin. 2022, 39, 942–955. [Google Scholar]
- Zhang, Z.; Cai, Z.; Zhang, Y. The recent progress of MXene-based microwave absorption materials. Carbon 2021, 174, 484–499. [Google Scholar] [CrossRef]
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 2020, 369, 446–450. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q. Unique Lead Adsorption Behavior of Activated Hydroxyl Group in Two-dimensional Titanium Carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Kassab, A.; Eid, K.; Abdullah, A.M.; Ozoemena, K.I.; Elzatahry, A. Unveiling fabrication and environmental remediation of Mxene-based nanoarchitectures in toxic metals removal from wastewater: Strategy and mechanism. Nanomaterials 2020, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Du, S.; Li, X.; Yu, Q.; Wei, H.; Yang, Y. Removal of radioactive palladium based on novel 2d titanium carbides. Chem. Eng. J. 2019, 358, 283–290. [Google Scholar] [CrossRef]
- Jun, B.M.; Her, N.; Chang, M.P.; Yoon, Y. Effective removal of Pb(II) from synthetic wastewater using Ti3C2Tx Mxene. Environ. Sci. Water Res. Technol. 2019, 6, 173–180. [Google Scholar] [CrossRef]
- Dong, Y.; Sang, D.; He, C.; Sheng, X.; Lei, L. Anhui Provincial Laboratory of Optoelectronic and Magnetism Functional Materials. Coll. Chem. Chem. Eng. 2019, 9, 29015–29022. [Google Scholar]
- Du, Y.; Yu, B.; Wei, L.; Wang, Y.; Ye, S. Efficient removal of Pb(II) by Ti3C2Tx powder modified with a silane coupling agent. J. Mater. Sci. 2019, 54, 13283–13297. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lü, Q.F.; Zhuang, H. Facile preparation of biosurfactant-functionalized Ti2CTx Mxene nanosheets with an enhanced adsorption performance for Pb(II) ions. J. Mol. Liq. 2019, 297, 111810. [Google Scholar] [CrossRef]

| Molten Salt System | Reaction Temperature |
|---|---|
| LiF–NaF–KF [18] | 600 °C |
| NaF–KF [19] | 850 °C |
| Materials | Synthesis | Production | Improvement | Production Rate (µmol·L−1·h−1) | Reaction Condition | Mechanism |
|---|---|---|---|---|---|---|
| Ti3C2/C3N4 [33] | Sonochemical method | CO/CH4 | 3.64 times | CO: 10.67 CH4: 4.19 | UV–vis irradiation for 4 h | Schottky heterojunction |
| Ti3C2/Bi2WO6 [34] | In situ grown on Ti3C2 | CH4/CH3OH | 4.6 times | CH4: 1.78 CH3OH: 0.44 | Anaerobic/ solar irradiation | 2D/2D heterojunction |
| Ti3C2/g-C3N4 [35] | Calcining the mixture of multilayered Ti3C2 particles and urea | CO/CH4 | 8.1 times | CO: 5.19 CH4: 0.044 | Visible light irradiation (λ ≥ 420 nm) | Interface contact between 2D g-C3N4 and Ti3C2 |
| Ti3C2/Cu2O [36] | CH3OH | 8.25 times 2.15 times | CH3OH: 78.50 | 300 W Xe lamp irradiation | Ti3C2 QDs promote charge transfer | |
| TiO2/C3N4/Ti3C2 [37] | Interfacial self-assembly | CO/CH4 | 3 times 8 times | CO: 4.39 CH4: 1.20 | Anaerobic/xenon lamp irradiation | Heterojunction (lower carrier recombination rate) |
| Adsorbent | Applied MXene Modification | N2 Surface Area (A) (m2/g), Point of Zero Charge (PZC) | Adsorbent Dose D (g/L), Optimum pH, Equilibrium Time T (min) | Maximum Adsorption Capacity Qm (mg/g) |
|---|---|---|---|---|
| Multilayer Ti3C2Tx − 45 [43] | Drying the hydrofluoric acid-etched Ti3AlC2 powder | A: 76.4 | D: 0.5; T: 600 | 185 |
| Multilayer Ti3C2Tx − 35 [43] | Drying the hydrofluoric acid-etched Ti3AlC2 powder | A: 65.4 | D: 0.5; T: 600 | 164 |
| Multilayer Ti3C2Tx − 25 [43] | Drying the hydrofluoric acid-etched Ti3AlC2 powder | A: 19.8 | D: 0.5; T: 600 | 119 |
| Multilayer Ti3C2Tx [44] | Drying the hydrofluoric acid-etched Ti3AlC2 powder | A: 10.0 | D: 0.05; pH: 6 T: 120 | ~36 |
| Multilayer Ti3C2Tx/alginate [45] | Mixing of sodium alginate with Ti3C2Tx | D: 1; pH: 6 T: 15 | 383 | |
| Multilayer Ti3C2Tx − KH570 [46] | Mixing of silane coupling agent (KH570) with Ti3C2Tx at 70C | A: 75.4 PZC: 2.6 | D: 3.2; pH: 5 T: 30 | 147 |
| Ti2CTx − EHL [47] | Biosurfactant enzymatic hydrolysis lignin mixed with Ti2CTx | A: 22.5 PZC: 3.2 | D: 1.6; pH: 5 T: 1440 | 233 |
| Ti2CTx − CS [47] | Biosurfactant chitosan mixed with Ti2CTx | - | 93.5 | |
| Ti2CTx − LS [47] | Biosurfactant lignosulfonatemixed with Ti2CTx | - | 104 | |
| Delaminated alk − Ti3C2Tx [48] | Alkalization intercalation modification with sodium ions | A: 72.0 PZC: 3.8 | D: 0.1; pH: 6.3 T: 30 | 188 |
| Delaminated alk − Ti3C2Tx − NH2 [48] | Alkalization-grafting with sodium ions and silane coupling agent (APTES) | A: 129.2 PZC: 4.1 | D: 0.1; pH: 6.3 T: 30 | 385 |
| Delaminated alk − Ti3C2Tx NH2 [48] | Alkalization-grafting with sodium ions and silane coupling agent (APTES) | A: 129.2 PZC: 4.1 | D: 0.1; pH: 6.3 T: 30 | 118 |
| Multilayer Ti3C2(OH/ONa)xF2 − x [37] | Alkalization–intercalation method | D: 0.5; pH: 6.8 T: 120 | ~140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Song, W.; Liu, H.; Ding, H.; Yan, Y.; Chen, R. Influencing Factors on Synthesis and Properties of MXene: A Review. Processes 2022, 10, 1744. https://doi.org/10.3390/pr10091744
Zhang L, Song W, Liu H, Ding H, Yan Y, Chen R. Influencing Factors on Synthesis and Properties of MXene: A Review. Processes. 2022; 10(9):1744. https://doi.org/10.3390/pr10091744
Chicago/Turabian StyleZhang, Lin, Weiwei Song, Hongshi Liu, Hong Ding, Yibo Yan, and Ruihan Chen. 2022. "Influencing Factors on Synthesis and Properties of MXene: A Review" Processes 10, no. 9: 1744. https://doi.org/10.3390/pr10091744




