Obtention of New Edible Biofilms from Water Kefir Grains in Comparison with Conventional Biofilms from Taro (Colocasia esculenta) and Cassava (Manihot esculenta) Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Kefir Grains and Culture Conditions
2.2. Starch Extraction from Taro (Colocasia esculenta) and Cassava (Manihot esculenta)
2.3. Preparation of Kefir Grains, Taro and Cassava Edible Films
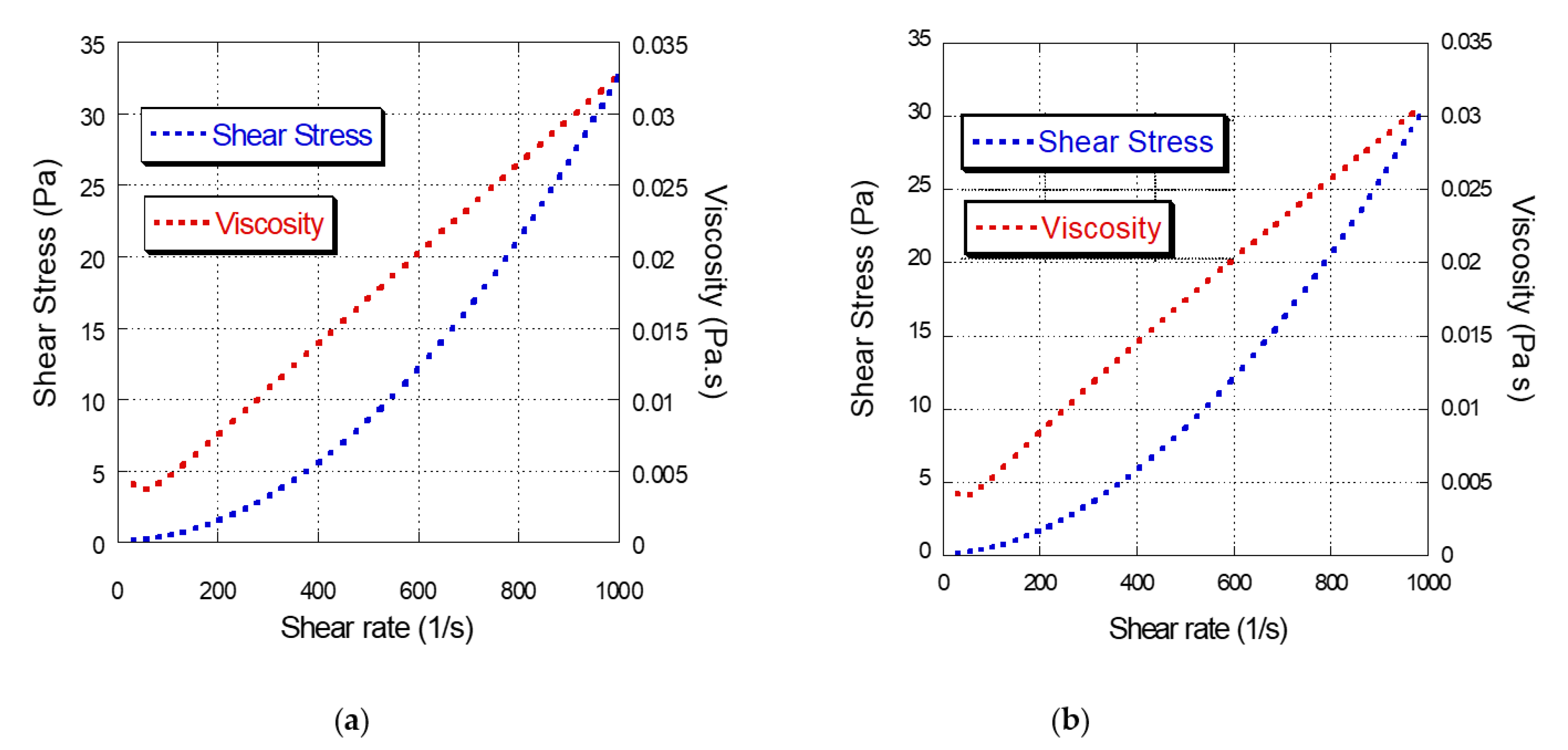
2.4. Steady-State Rheological Determination of Film-Forming Suspensions of Kefir, Taro and Cassava
2.5. Thickness Measurements, Visual Aspects and Evaluation of the Quality of Edible Films
2.6. Microstructural Characterization by Scanning Electron Microscope (SEM)
2.7. Water Activity (aw)
2.8. Mechanical Properties of the Edible Films Obtained
2.9. Statistical Analysis
3. Results and Discussions
3.1. Biomass Growth of Water Kefir Grains and pH Kinetics
3.2. Steady State Rheological Determination of the SFP Film-Forming Suspensions
3.3. The Visual Aspect, the Evaluation of the Quality and the Thickness of the Films
3.4. Film Microstructure by Scanning Electron Microscopy (SEM)
3.5. Aw of Kefir, Taro and Cassava Edible Films
3.6. Mechanical Properties of Kefir, Taro and Cassava Edible Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiralt, A.; Menzel, C.; Hernandez-García, E.; Collazo, S.; Gonzalez-Martinez, C. Use of By-Products in Edible Coatings and Biodegradable Packaging Materials for Food Preservation. In Sustainability of the Food System; Academic Press: Cambridge, MA, USA, 2020; pp. 101–127. [Google Scholar]
- Gontard, N.; Guilbert, S. Bio-Packaging: Technology and Properties of Edible and/or Biodegradable Material of Agricultural Origin. In Food Packaging and Preservation; Springer: Boston, MA, USA, 1994; pp. 159–181. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Valorization of Food-Grade Industrial Waste in the Obtaining Active Biodegradable Films for Packaging. Ind. Crop. Prod. 2016, 87, 218–228. [Google Scholar] [CrossRef]
- de Paiva, I.M.; Steinberg, R.D.S.; Lula, I.S.; de Souza-Fagundes, E.M.; Mendes, T.D.O.; Bell, M.J.V.; Nicoli, J.R.; Nunes, C.; Neumann, E. Lactobacillus Kefiranofaciens and Lactobacillus Satsumensis Isolated from Brazilian Kefir Grains Produce Alpha-Glucans That Are Potentially Suitable for Food Applications. LWT-Food Sci. Technol. 2016, 72, 390–398. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Peltzer, K.; Phaswana-Mafuya, N.; Pengpid, S. Use of Edible Films and Coatings for Functional Foods Developments: A Review. In Functional Foods Sources, Health Effects and Future Perspectives; Nelson, D.L., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 1–26. [Google Scholar]
- Briassoulis, D.; Giannoulis, A. Evaluation of the Functionality of Bio-Based Food Packaging Films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible Films and Coating: Characteristics and Properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable Polymers for Food Packaging: A Review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Stadie, J.; Gulitz, A.; Ehrmann, M.A.; Vogel, R.F. Metabolic activity and Symbiotic Interactions of Lactic Acid Bacteria and Yeasts Isolated from Water Kefir. Food Microbiol. 2013, 35, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Felton, L.A. Mechanisms of Polymeric Film Formation. Int. J. Pharm. 2013, 457, 423–427. [Google Scholar] [CrossRef]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Bourtoom, T. Plasticizer Effect on the Properties of Biodegradable Blend Film from Rice Starch-Chitosan. Songklanakarin J. Sci. Technol. 2008, 30, 149–165. [Google Scholar]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, Mechanical and Optical Properties of Plasticized Yam Starch Films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for Chitosan Based Antimicrobial Films in Food Applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Delgado, J.F.; Sceni, P.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Development of Innovative Biodegradable Films Based on Biomass of Saccharomyces Cerevisiae. Innov. Food Sci. Emerg. Technol. 2016, 36, 83–91. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. β-Glucan, a Promising Polysaccharide for Bio-based Films Developments for Food Contact Materials and Medical Applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and Characterisation of a New Biodegradable Edible Film Made from Kefiran, an Exopolysaccharide Obtained from Kefir Grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Fels, L.; Jakob, F.; Vogel, R.F.; Wefers, D. Structural Characterization of The Exopolysaccharides from Water Kefir. Carbohydr. Polym. 2018, 189, 296–303. [Google Scholar] [CrossRef]
- Farnworth, E.R. Kefir? a Complex Probiotic. Food Sci. Technol. Bull. Funct. Foods 2005, 2, 1–17. [Google Scholar] [CrossRef]
- Pidoux, M. The Microbial Flora of Sugary Kefir Grain (The Gingerbeer Plant): Biosynthesis of the Grain Fromlactobacillus Hilgardii Producing a Polysaccharide Gel. World J. Microbiol. Biotechnol. 1989, 5, 223–238. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; DE Antoni, G.L. Chemical and Microbiological Characterisation of Kefir Grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef]
- Velez, C.A.C.; Peláez, M.L. Capacidad Antifúngica de Sobrenadantes Libres de Células Obtenidos de la Fermentación de un Sustrato de “Panela” Con Gránulos de Kefir de Agua. Rev. Colomb. Biotecnol. 2015, 17, 22–32. [Google Scholar] [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Starch films loaded with do-nut-shaped starch-quercetin microparticles: Characterization and release kinetics. Int. J. Biol. Macromol. 2018, 118, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Novelo-Cen, L.; Betancur-Ancona, D. Chemical and Functional Properties of Phaseolus Lunatus and Manihot Esculenta Starch Blends. Starch-Stärke 2005, 57, 431–441. [Google Scholar] [CrossRef]
- Simsek, S.; El, S.N. Production of Resistant Starch from Taro (Colocasia Esculenta L. Schott) Corm and Determination of Its Effects on Health By In Vitro Methods. Carbohydr. Polym. 2012, 90, 1204–1209. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of Edible Films Based on Nanoreinforced Gelatinized Starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Oropeza-De la Rosa, E.; López-Ávila, L.G.; Luna-Solano, G.; Cantú-Lozano, D. Bioethanol Production Process Rheology. Ind. Crops Prod. 2017, 106, 59–64. [Google Scholar] [CrossRef]
- Liu, J.B.; Yu, D.; Zhang, J.; Yang, M.; Wang, Y.; Wei, Y.; Tong, J. Rheological Properties of Sewage Sludge During Enhanced Anaerobic Digestion with Microwave-H2O2 Pretreatment. Water Res. 2016, 98, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; P.E. Freeman Press: East Lancing, MI, USA, 1996. [Google Scholar]
- Ahmed, S.; Ikram, S. Chitosan and Gelatin Based Biodegradable Packaging Films With UV-Light Protection. J. Photochem. Photobiol. B Biol. 2016, 163, 115–124. [Google Scholar] [CrossRef]
- de Moraes, J.O.; Scheibe, A.S.; Sereno, A.; Laurindo, J.B. Scale-Up of the Production of Cassava Starch Based Films Using Tape-Casting. J. Food Eng. 2013, 119, 800–808. [Google Scholar] [CrossRef]
- ASTM D2240-E96; Standard Test Methods for Water Vapor Transmission of Material. ASTM International: West Conshohocken, PA, USA, 2016.
- AOAC (Association of Official Analytical Chemists). Method 978.18. In Official Methods of Analysis, 10th ed.; Horwitz, H., Ed.; AOAC International: Washington, DC, USA, 1980. [Google Scholar]
- Fama, L.; Flores, S.; Gerschenson, L.; Goyanes, S. Physical Characterization of Cassava Starch Biofilms with Special Reference to Dynamic Mechanical Properties at Low Temperatures. Carbohydr. Polym. 2006, 66, 8–15. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; González, G. Effects of Exposure to Pulsed Light on Surface and Structural Properties of Edible Films Made from Cassava and Taro Starch. Food Bioprocess Technol. 2016, 9, 1812–1824. [Google Scholar] [CrossRef]
- Horvat, M.; Pannuri, A.; Romeo, T.; Dogsa, I.; Stopar, D. Viscoelastic Response of Escherichia Coli Biofilms to Genetically Altered Expression of Extracellular Matrix Components. Soft Matter 2019, 15, 5042–5051. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.; Pinotti, A. Correlations Between Structural, Barrier, Thermal and Mechanical Properties of Plasticized Gelatin Films. Innov. Food Sci. Emerg. Technol. 2010, 11, 369–375. [Google Scholar] [CrossRef]
- Shashikumar, C.; Mitra, S.; Singha, S. Microbial Polysaccharides (MPs) in Food Packaging. Biopolym. Based Food Packag. Innov. Technol. Appl. 2022, 1, 225–263. [Google Scholar]
- Zanirati, D.F.; Abatemarco, M.; Sandes, S.H.D.C.; Nicoli, J.R.; Nunes, C.; Neumann, E. Selection of Lactic Acid Bacteria from Brazilian Kefir Grains for Potential Use as Starter or Probiotic Cultures. Anaerobe 2015, 32, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Sarbon, N.M. Chicken Gelatin Films: Rheological Properties of Film Forming Solutions and Film Characterisation as Influenced by Starch Incorporation. Int. Food Res. J. 2020, 27, 6. [Google Scholar]
- Silva, K.R.; Rodrigues, S.A.; Filho, L.X.; Lima, S. Antimicrobial Activity of Broth Fermented with Kefir Grains. Appl. Biochem. Biotechnol. 2008, 152, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Physical, Mechanical, Barrier, And Thermal Properties of Polyol-Plasticized Biodegradable Edible Film Made from Kefiran. Carbohydr. Polym. 2011, 84, 477–483. [Google Scholar] [CrossRef]
- Gontard, N.; Guilbert, S.; Cuq, J.-L. Water and Glycerol as Plasticizers Affect Mechanical and Water Vapor Barrier Properties of an Edible Wheat Gluten Film. J. Food Sci. 1993, 58, 206–211. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A Comparison of Milk Kefir and Water Kefir: Physical, Chemical, Microbiological and Functional Properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Laureys, D.; Van Jean, A.; Dumont, J.; De Vuyst, L. Investigation of the Instability and Low Water Kefir Grain Growth During an Industrial Water Kefir Fermentation Process. Appl. Microbiol. Biotechnol. 2017, 101, 2811–2819. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water Content, Water Activity, Water Structure and the Stability of Foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Labuza, T.P.; McNally, L.; Gallagher, D.; Hawkes, J.; Hurtado, F. Stability of Intermediate Moisture Foods. 1. Lipid Oxidation. J. Food Sci. 1972, 37, 154–159. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic Edible Films: Modified Procedure for Water Vapor Permea-Bility and Explanation of Thickness Effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. The Water Kefir Grain Inoculum Determines the Characteristics of the Resulting Water Kefir Fer-Mentation Process. J. Appl. Microbiol. 2017, 122, 719–732. [Google Scholar] [CrossRef]
- Famá, L.; Gerschenson, L.; Goyanes, S. Starch-vegetable fibre composites to protect food products. Carbohydr. Polym. 2009, 75, 230–235. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Chen, K.-N.; Lo, Y.-M.; Chiang, M.-L.; Chen, H.-C.; Liu, J.-R.; Chen, M.-J. Investigation of Microorganisms Involved in Biosynthesis of the Kefir Grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef]
- Qi, H.J.; Joyce, K.; Boyce, M.C. Durometer Hardness and the Stress-Strain Behavior of Elastomeric Materials. Rubber Chem. Technol. 2003, 76, 419–435. [Google Scholar] [CrossRef]











| Temperature °C | 25 °C | 35 °C | |||
|---|---|---|---|---|---|
| Substrate Concentration (°Brix) | 25 °Brix | 17 °Brix | 25 °Brix | 17 °Brix | |
| Source of the substrate | Muscovado sugar (P) | Ya = P25 | Ya = P17 | Yb = P25 | Yb = P17 |
| Refined sugar (A) | Ya = A25 | Ya = A17 | Yb = A25 | Yb = A17 | |
| Muscovado sugar with pineapple skin (PP) | Ya = PP25 | Ya = PP17 | Yb = PP25 | Yb = PP17 | |
| Refined sugar with pineapple skin (AP) | Ya = AP25 | Ya = AP17 | Yb = AP25 | Yb = AP17 | |
| Water kefir grains 3% wt/wt |
| Taro 4.5 and 5.5 g |
| Casava 4.5 and 5.5 g |
| Solution | Name | aw |
|---|---|---|
| 25 °C | ||
| P2O5 | Phosphorus pentoxide | 0.000 |
| LiCI | Lithium chloride | 0.115 |
| CH3 COOK | Potassium acetate | 0.226 |
| MgCl2 | Magnesium chloride | 0.327 |
| K2CO3 | Potassium carbonate | 0.438 |
| Mg (NO3)2 | Magnesium nitrate | 0.528 |
| NaNO2 | Nitrate of sodium | 0.577 |
| NaCI | Sodium chloride | 0.765 |
| Wk-SFP Samples | Temperature [°C] | Experimental Model | R2 |
|---|---|---|---|
| P25 | 25 | 0.991 | |
| 35 | 0.991 | ||
| A25 | 25 | 0.990 | |
| 35 | 0.990 | ||
| P17 | 25 | 0.991 | |
| 35 | 0.992 | ||
| A17 | 25 | 0.991 | |
| 35 | 0.992 | ||
| PP25 | 25 | 0.992 | |
| 35 | 0.992 | ||
| AP25 | 25 | 0.991 | |
| 35 | 0.991 | ||
| PP17 | 25 | 0.992 | |
| 35 | 0.992 | ||
| AP17 | 25 | 0.989 | |
| 35 | 0.993 |
| SFP-Taro samples | Experimental Model | R2 |
|---|---|---|
| (a) SFP at 25 °C and 4.5 g | 0.9914 | |
| (b) SFP at 25 °C and 5.5 g | 0.9916 |
| SFP-Cassava Samples | Experimental Model | R2 |
|---|---|---|
| (a) SFP at 25 °C and 4.5 g | 0.9873 | |
| (b) SFP at 25 °C and 5.5 g | 0.9922 |
| Water Kefir Biofilms | Average Thickness (mm) |
|---|---|
| P25 | 0.9990 ±0.0016a |
| A25 | 0.9981 ±0.0011a |
| P17 | 0.9985 ±0.0016a |
| A17 | 0.9974 ±0.0012a |
| PP25 | 0.9985 ±0.0011a |
| AP25 | 0.9983 ±0.0014a |
| PP17 | 0.9984 ±0.0015a |
| AP17 | 0.9983 ±0.0012a |
| Edible Films of TPS | Average Thickness (mm) |
|---|---|
| Taro 5.5 g | 0.9982 ±0.0003a |
| Taro 4.5 g | 0.9984 ±0.0006a |
| Cassava 5.5 g | 0.9975 ±0.0007a |
| Cassava 4.5 g | 0.9981 ±0.0007a |
| Edible Films | Average Hardness |
|---|---|
| Kefir 3% wt/wt | 57.94 ±3.94b |
| Taro 5.5 g | 90.77 ±0.84a |
| Taro 4.5 g | 91.71 ±0.70a |
| Cassava 5.5 g | 91.48 ±1.09a |
| Cassava 4.5 g | 89.82 ±0.96a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares-Bravo, P.; Cabo-Araoz, S.D.; Luna-Solano, G.; Urrea-Garcia, G.R.; Cantú-Lozano, D. Obtention of New Edible Biofilms from Water Kefir Grains in Comparison with Conventional Biofilms from Taro (Colocasia esculenta) and Cassava (Manihot esculenta) Starch. Processes 2022, 10, 1804. https://doi.org/10.3390/pr10091804
Linares-Bravo P, Cabo-Araoz SD, Luna-Solano G, Urrea-Garcia GR, Cantú-Lozano D. Obtention of New Edible Biofilms from Water Kefir Grains in Comparison with Conventional Biofilms from Taro (Colocasia esculenta) and Cassava (Manihot esculenta) Starch. Processes. 2022; 10(9):1804. https://doi.org/10.3390/pr10091804
Chicago/Turabian StyleLinares-Bravo, Paul, Samantha D. Cabo-Araoz, Guadalupe Luna-Solano, Galo R. Urrea-Garcia, and Denis Cantú-Lozano. 2022. "Obtention of New Edible Biofilms from Water Kefir Grains in Comparison with Conventional Biofilms from Taro (Colocasia esculenta) and Cassava (Manihot esculenta) Starch" Processes 10, no. 9: 1804. https://doi.org/10.3390/pr10091804
APA StyleLinares-Bravo, P., Cabo-Araoz, S. D., Luna-Solano, G., Urrea-Garcia, G. R., & Cantú-Lozano, D. (2022). Obtention of New Edible Biofilms from Water Kefir Grains in Comparison with Conventional Biofilms from Taro (Colocasia esculenta) and Cassava (Manihot esculenta) Starch. Processes, 10(9), 1804. https://doi.org/10.3390/pr10091804








