Synthesis, Modification, and Characterization of Fe3O4@SiO2-PEI-Dextranase Nanoparticles for Enzymatic Degradation of Dextran in Fermented Mash
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials and Reagents
2.2. Sugarcane Mash Obtainment
2.3. Characterization of Fermented Sugarcane Mash
2.4. Obtaining the Enzyme
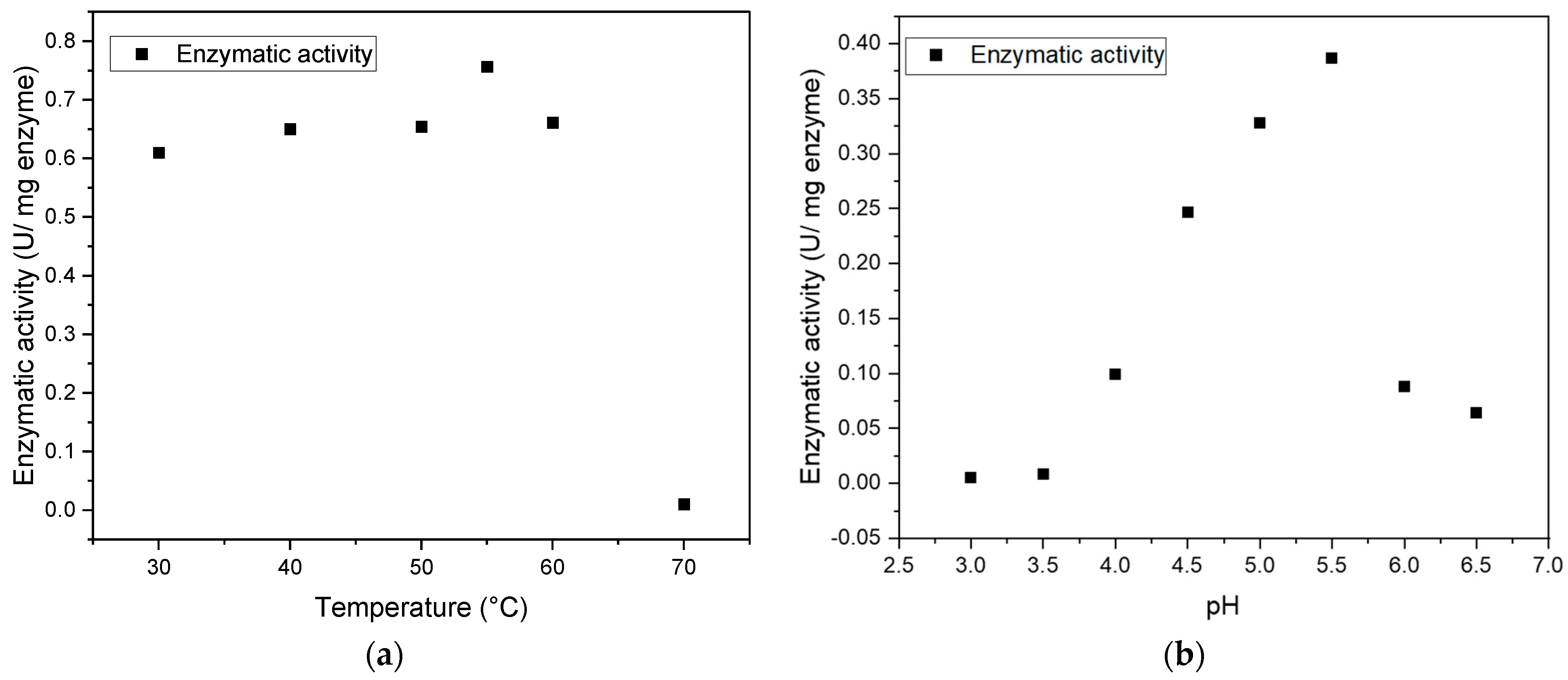
2.5. Characterization of the Enzyme
2.5.1. Rheological Characterization of Commercial Enzyme
2.5.2. Determination of Dextranase Activity
2.5.3. Determination of pH Stability Determination
2.5.4. Determination of pH Stability Determination
2.6. Synthesis of Magnetite Nanoparticles (Fe-NPs) and Modification with TEOS and PEI (Fe3O4@SiO2-PEI)
2.7. Characterization of Bare Fe-NPs and Modified Fe-NPs
2.8. Immobilization of Modified Fe3O4 NPs (Fe3O4@SiO2-PEI-Dextranase)
2.9. Enzymatic Degradation of Dextran in Fermented Mash
3. Results and Discussion
3.1. Physicochemical and Rheological Characterization of Fermented Sugarcane Mash
3.2. Characterization of the Enzyme
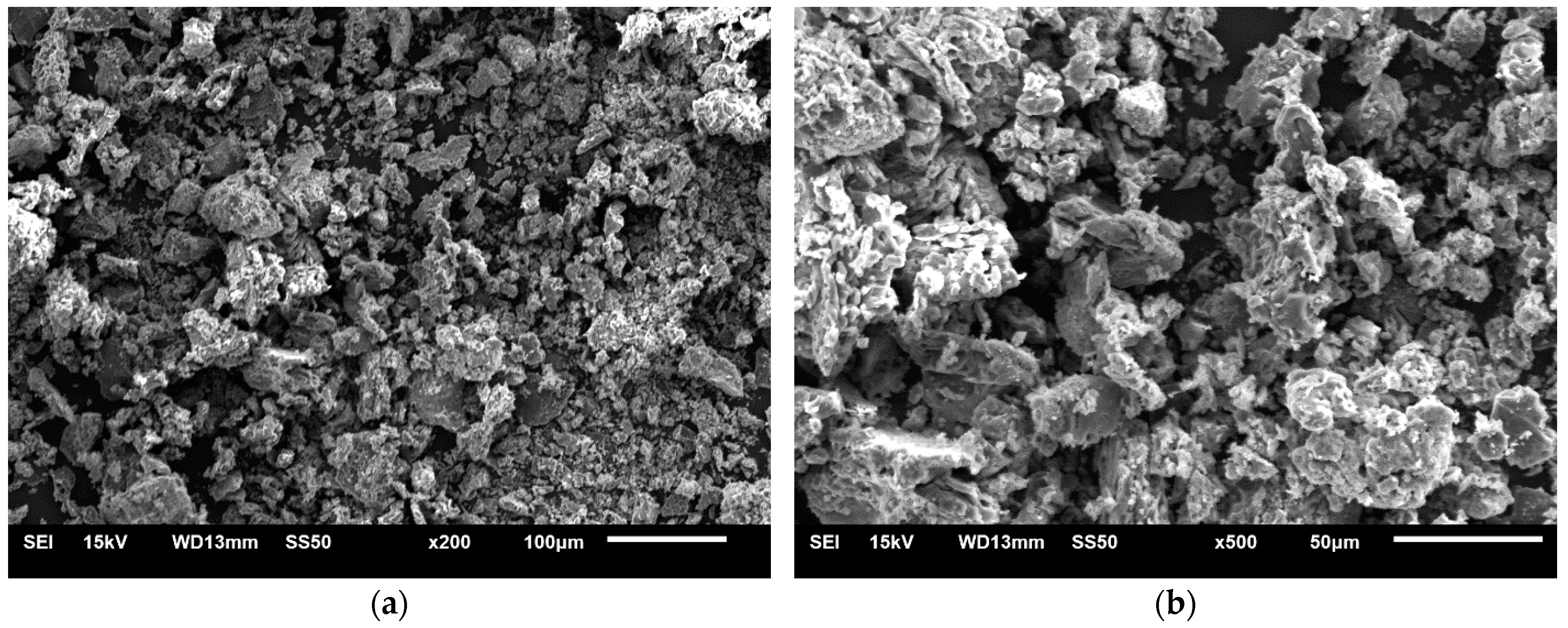
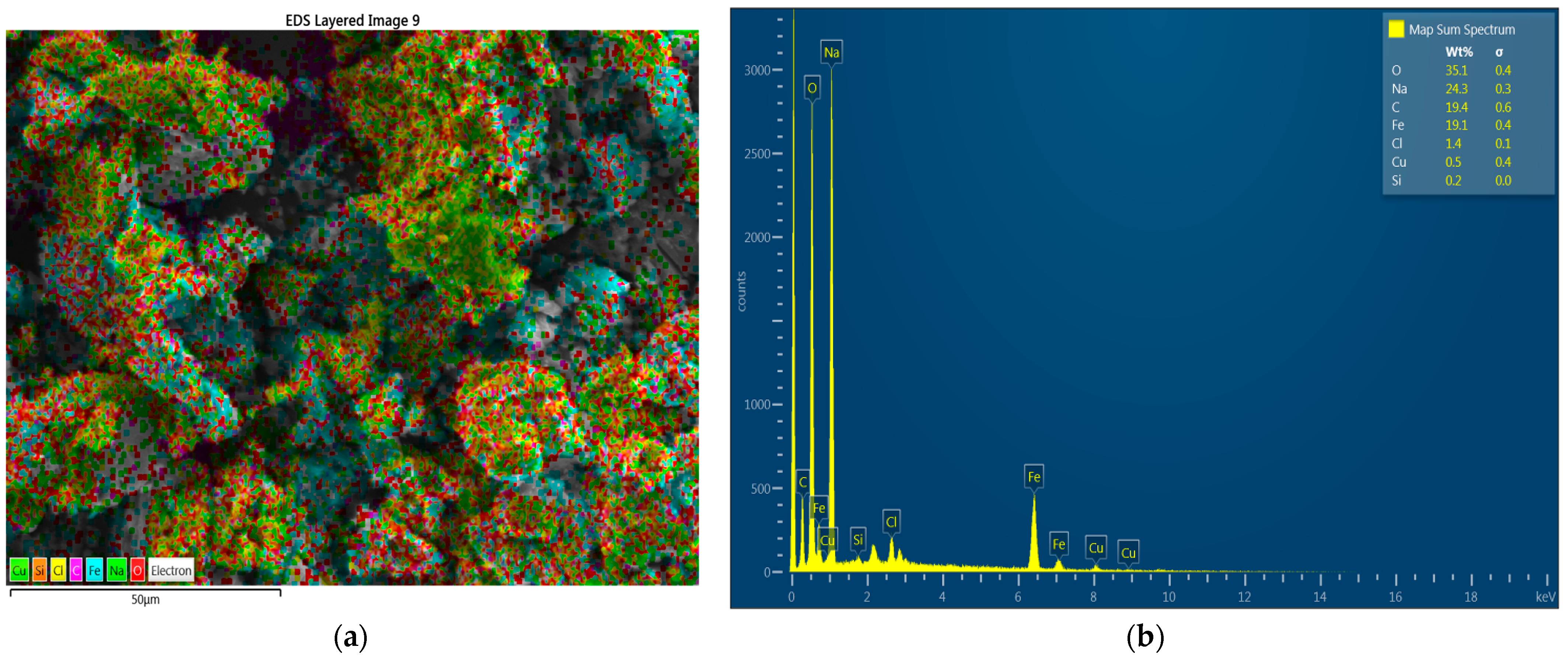
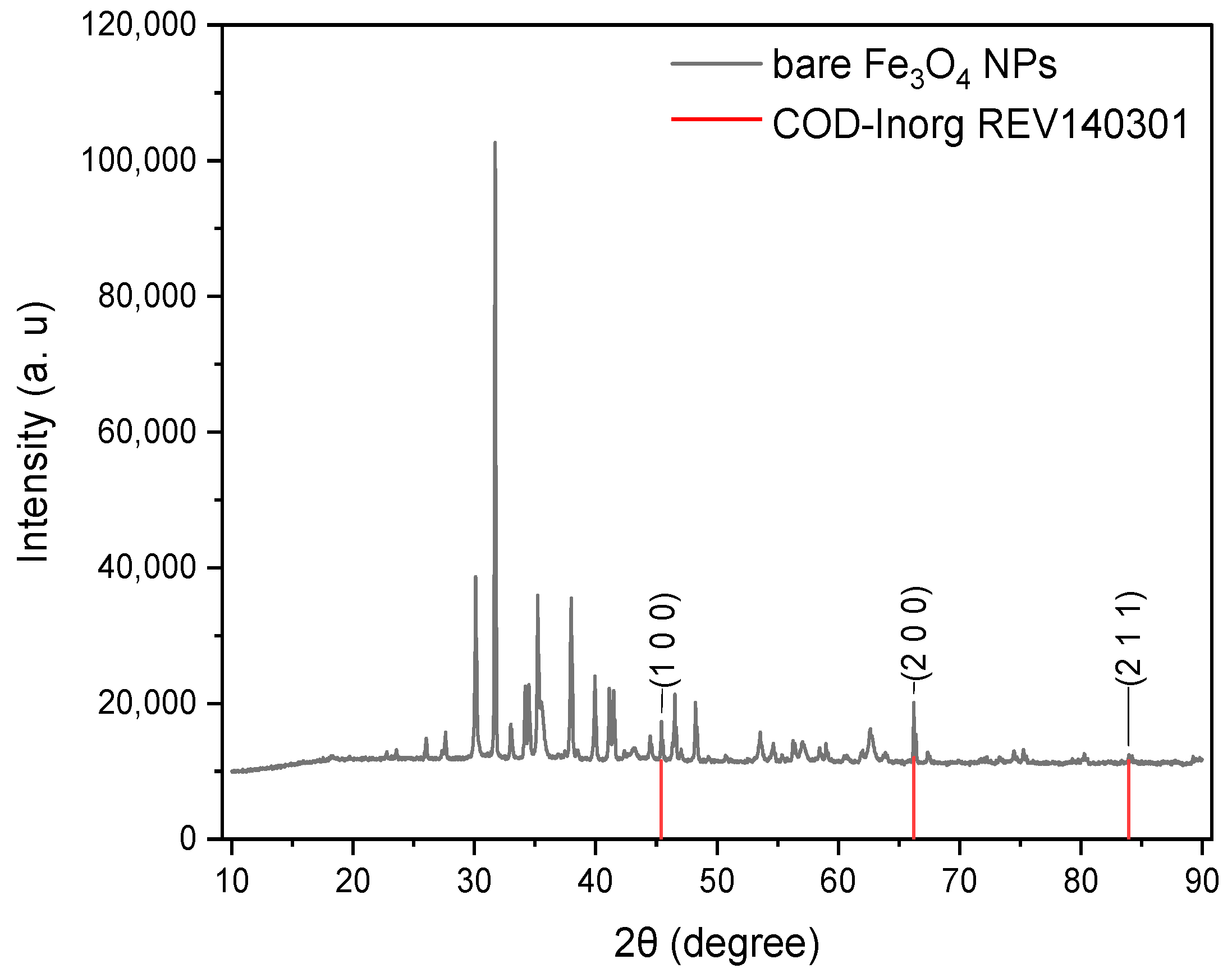
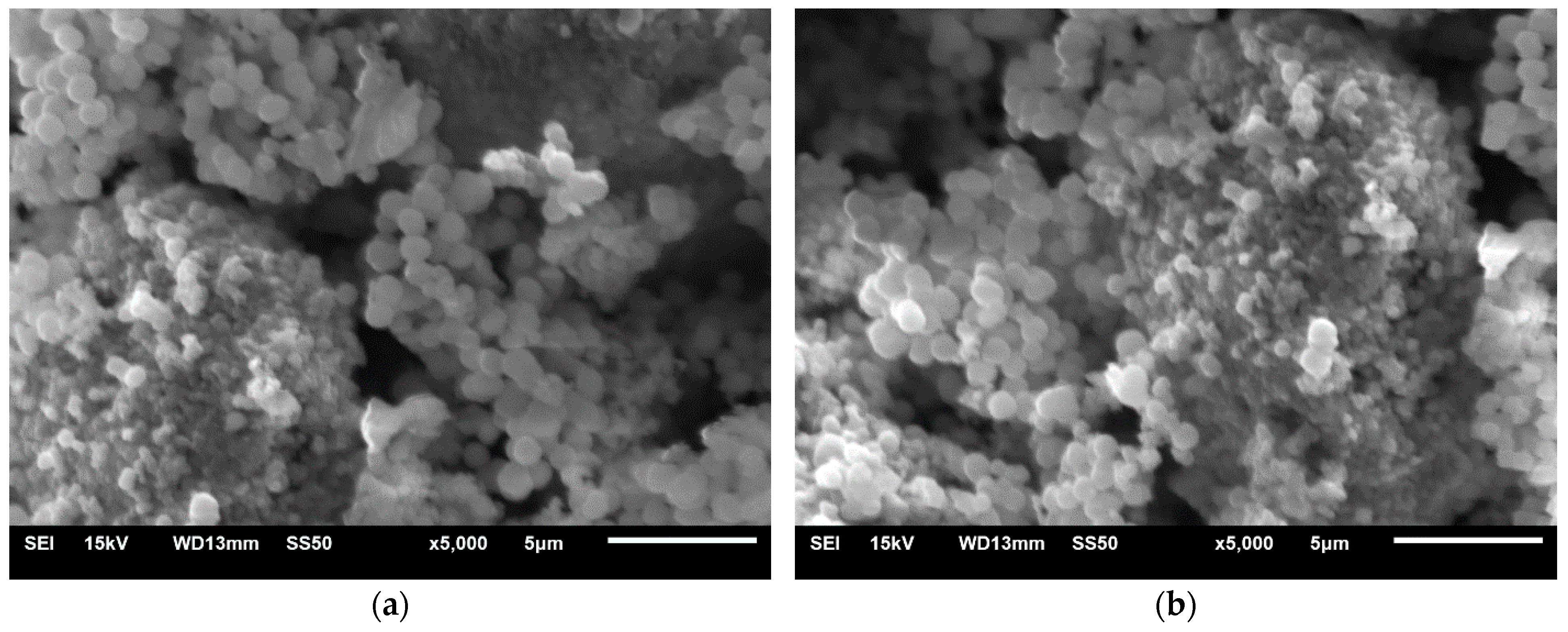
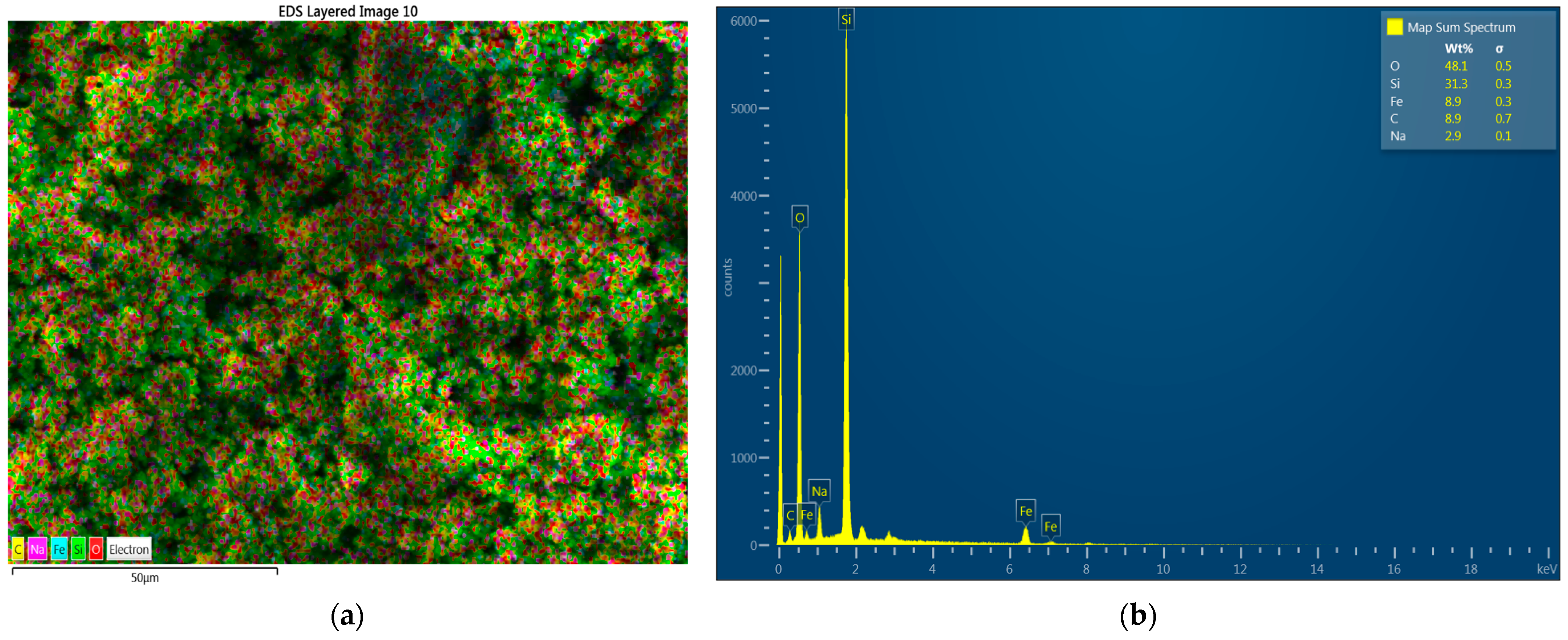
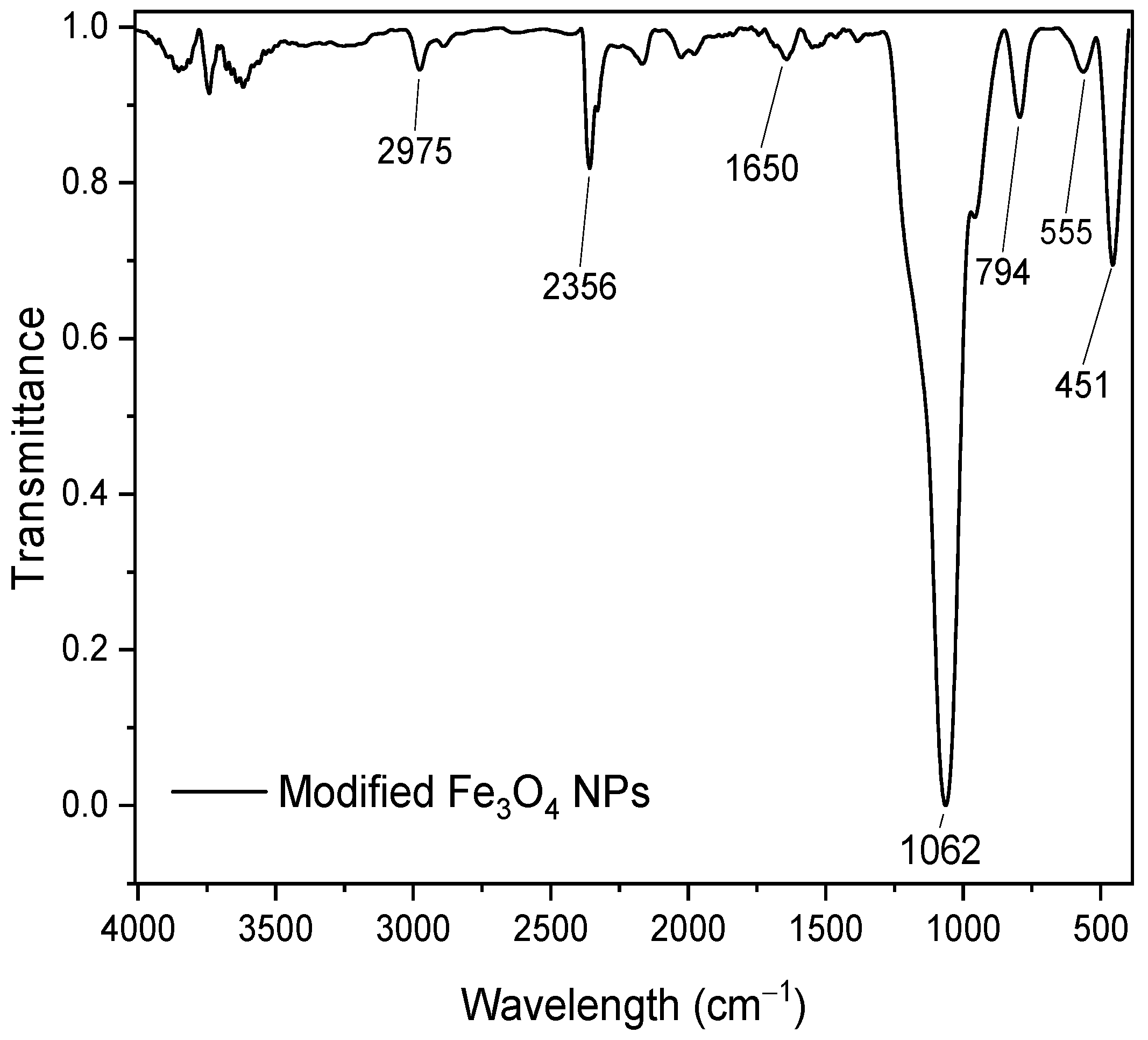
3.3. Characterization of Magnetite Nanoparticles(SEM, EDS, XRD y FTIR)
3.3.1. Characterization of Bare Fe3O4 NPs
3.3.2. Characterization of Modified Fe3O4 NPs
3.4. Immobilization of Modified Fe3O4 NPs (Fe3O4@SiO2-PEI-Dextranase)
3.5. Enzymatic Degradation of Dextran in Fermented Mash
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, K.; Hagen, S.; Schlumbach, K.; Rohde, A.; Flöter, E. Dextranase application in sucrose solutions-towards a better understanding. Int. Sugar J. 2016, 118, 582–588. [Google Scholar]
- Bashari, M.; Tounkara, F.; Abdelhai, M.H.; Lagnika, C.; Xu, X.; Jin, Z. Impact of Dextranase on Sugar Manufacturing and its Kinetic on the Molecular Weights of Remaining Dextran. Sugar Tech 2013, 15, 84–93. [Google Scholar] [CrossRef]
- Oropeza-De la Rosa, E.; López-ávila, L.G.; Luna-Solano, G.; Urrea-García, G.R.; Cantú-Lozano, D. Dextran hydrolysis and its rheology in mashes from bioethanol production process. Rev. Mex. Ing. Quim. 2019, 18, 543–554. [Google Scholar] [CrossRef]
- Iqbal, S.; Marchetti, R.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A. Enzymatic and acidic degradation of high molecular weight dextran into low molecular weight and its characterizations using novel Diffusion-ordered NMR spectroscopy. Int. J. Biol. Macromol. 2017, 103, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, H.; Wang, X.; Zu, H.; Wang, C.; Dong, D.; Lyu, M.; Wang, S. Immobilization of Dextranase on Nano-Hydroxyapatite as a Recyclable Catalyst. Materials 2021, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Dilute solution properties and degree of chain branching for dextran. Carbohydr. Polym. 2012, 88, 373–381. [Google Scholar] [CrossRef]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Rúas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; López, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657. [Google Scholar] [CrossRef]
- Ninchan, B.; Vanichsriratana, W.; Sriroth, K. Investigation of the optimized dextran-degrading enzyme conditions on the decomposition of different molecular weights of pure dextran using response surface methodology. Arch. Ind. Biotechnol. 2016, 1, 8–19. [Google Scholar]
- Bashari, M.; Jin, Z.; Wang, J.; Zhan, X. A novel technique to improve the biodegradation efficiency of dextranase enzyme using the synergistic effects of ultrasound combined with microwave shock. Innov. Food Sci. Emerg. Technol. 2016, 35, 125–132. [Google Scholar] [CrossRef]
- Arabacı, N.; Karaytuğ, T.; Demirbas, A.; Ocsoy, I.; Katı, A. Nanomaterials for Enzyme Immobilization. In Green Synthesis of Nanomaterials for Bioenergy Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 165–190. [Google Scholar] [CrossRef]
- De Simone, A.; Naldi, M.; Bartolini, M.; Davani, L.; Andrisano, V. Immobilized Enzyme Reactors: An Overview of Applications in Drug Discovery from 2008 to 2018. Chromatographia 2019, 82, 425–441. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC—Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ishfaq, N.; Salman, S.; Bashir, M.H.; Ashfaq, B. Enzyme Immobilization and Applications of Magnetic Nanoparticles in Smart Enzyme Immobilization. Int. J. Res. Sci. Technol. 2017, 3, 387–396. [Google Scholar]
- Anwer, M.K.; Ali, E.A.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Saqr, A.A.; Ansari, M.N.; Aboudzadeh, M.A. Development of sustained release baricitinib loaded lipid-polymer hybrid nanoparticles with improved oral bioavailability. Molecules 2022, 27, 168. [Google Scholar] [CrossRef] [PubMed]
- Lapitan, L.D.S.; Zhou, D. A Simple Magnetic Nanoparticle-Poly-Enzyme Nanobead Sandwich Assay for Direct, Ultrasensitive DNA Detection, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 630, ISBN 9780128201435. [Google Scholar]
- Gutarra, M.L.E.; Miranda, L.S.M.; De Souza, R.O.M.A. Enzyme Immobilization for Organic Synthesis. In Organic Synthesis Using Biocatalysis; Academic Press: Cambridge, MA, USA, 2016; pp. 99–126. [Google Scholar] [CrossRef]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the Central Nervous System. Materials. 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Ubaidullah, M.; Mane, R.S.; Al-Enizi, A.M. Types, Synthesis Methods and Applications of Ferrites; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128192375. [Google Scholar]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel Approaches for Preparation of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323461481. [Google Scholar]
- Alterary, S.S.; Alkhamees, A. Synthesis, surface modification, and characterization of Fe3O4@SiO2core@shell nanostructure. Green Process. Synth. 2021, 10, 384–391. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Song, X.; Cai, J. Improving the stability and reusability of dextranase by immobilization on polyethylenimine modified magnetic particles. New J. Chem. 2018, 42, 8391–8399. [Google Scholar] [CrossRef]
- Prosheva, M.; Aboudzadeh, M.A.; Leal, G.P.; Gilev, J.B.; Tomovska, R. High-Performance UV Protective Waterborne Polymer Coatings Based on Hybrid Graphene/Carbon Nanotube Radicals Scavenging Filler. Part. Part. Syst. Charact. 2019, 36, 1800555. [Google Scholar] [CrossRef]
- Bertrand, E.; Pierre, G.; Delattre, C.; Gardarin, C.; Bridiau, N.; Maugard, T.; Štrancar, A.; Michaud, P. Dextranase immobilization on epoxy CIM® disk for the production of isomaltooligosaccharides from dextran. Carbohydr. Polym. 2014, 111, 707–713. [Google Scholar] [CrossRef]
- Gines-Palestino, R.S.; Oropeza-De la Rosa, E.; Montalvo-Romero, C. Cantú-Lozano Rheokinetic and effectiveness during the phenol removal in mescal vinasses with a rotary disk photocatalytic reactor (RDPR). Rev. Mex. Ing. Química 2020, 19, 653–665. [Google Scholar]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, B.; Murali, K.R.; Rita, J. Optical, Morphological and Microestructural Investigation of TiO2 nanoparticles for Photocatalytic application. Iran. J. Catal. 2021, 3, 1–11. [Google Scholar]
- Oropeza-De la Rosa, E.; López-Ávila, L.G.; Luna-Solano, G.; Cantú-Lozano, D. Bioethanol production process rheology. Ind. Crops Prod. 2017, 106, 59–64. [Google Scholar] [CrossRef]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Short communication: Characterization of molasses chemical composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef]
- Ebaya, M.M.A.; El-Mowafy, M.; Adel El-Sokkary, M.M.; Hassan, R. Purification, Characterization, and Biocatalytic and Antibiofilm Activity of a Novel Dextranase from Talaromyces sp. Int. J. Microbiol. 2020, 2020, 9198048. [Google Scholar] [CrossRef]
- Gibriel, A.Y.; Amin, A.A.; Nessrien Yassien, N.M.; El Banna, H.A.; Khaled, F.M. Characterization of Free and Immobilized P. aculeatum NRRL-896 Dextranase. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1135–1146. [Google Scholar]
- Karimi Pasandideh, E.; Kakavandi, B.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Esrafili, A.; Rezaei Kalantary, R. Silica-coated magnetite nanoparticles core-shell spheres (Fe3O4@SiO2) for natural organic matter removal. J. Environ. Health Sci. Eng. 2016, 14, 21. [Google Scholar] [CrossRef]
- Mohammadi, H.; Nekobahr, E.; Akhtari, J.; Saeedi, M.; Akbari, J.; Fathi, F. Synthesis and characterization of magnetite nanoparticles by co-precipitation method coated with biocompatible compounds and evaluation of in-vitro cytotoxicity. Toxicol. Rep. 2021, 8, 331–336. [Google Scholar] [CrossRef]
- Xu, J.; Ju, C.; Sheng, J.; Wang, F.; Zhang, Q.; Sun, G.; Sun, M. Synthesis and characterization of magnetic nanoparticles and its application in lipase immobilization. Bull. Korean Chem. Soc. 2013, 34, 2408–2412. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface Study of Fe3O4 Nanoparticles Functionalized With Biocompatible Adsorbed Molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Nanditha, A.; Manokaran, J.; Balasubramanian, N. Fabrication of Lys-PVA-Fe3O4 modified electrode for the electrochemical determination of uric acid. Res. J. Chem. Environ. 2014, 18, 54–61. [Google Scholar]
- Yang, L.; Tian, J.; Meng, J.; Zhao, R.; Li, C.; Ma, J.; Jin, T. Modification and characterization of Fe3O4 nanoparticles for use in adsorption of alkaloids. Molecules 2018, 23, 562. [Google Scholar] [CrossRef]
- Lo, B.; Gorczyca, E.; Kasapis, S.; Zisu, B. Effect of low-frequency ultrasound on the particle size, solubility and surface charge of reconstituted sodium caseinate. Ultrason. Sonochem. 2019, 58, 104525. [Google Scholar] [CrossRef]
- Aquino, P.; Osorio, A.M.; Ninán, E.; Torres, F. Characterization of ZnO nanoparticles synthesized by precipitation method and its evaluation in the incorporation in enamel paints. Rev Soc Quím Perú. 2018, 84, 17. [Google Scholar]
- López-Cuenca, S.; Aguilar-Martínez, J.; Rabelero-Velasco, M.; Hernández-Ibarra, F.J.; López-Ureta, L.C.; Pedroza-Toscano, M.A. Spheroidal zinc oxide nanoparticles synthesized by semicontinuous precipitation method at low temperatures. Rev. Mex. Ing. Química 2019, 18, 1179–1187. [Google Scholar] [CrossRef]
- Allafchian, A.R.; Jalali, S.A.H.; Amiri, R.; Shahabadi, S. Synthesis and characterization of the NiFe2O4 @TEOS-TPS@Ag nanocomposite and investigation of its antibacterial activity. Appl. Surf. Sci. 2016, 385, 506–514. [Google Scholar] [CrossRef]
- Wencel, D.; Dolan, C.; Barczak, M.; Keyes, T.E.; McDonagh, C. Synthesis, tailoring and characterization of silica nanoparticles containing a highly stable ruthenium complex. Nanotechnology 2013, 24, 365705. [Google Scholar] [CrossRef]

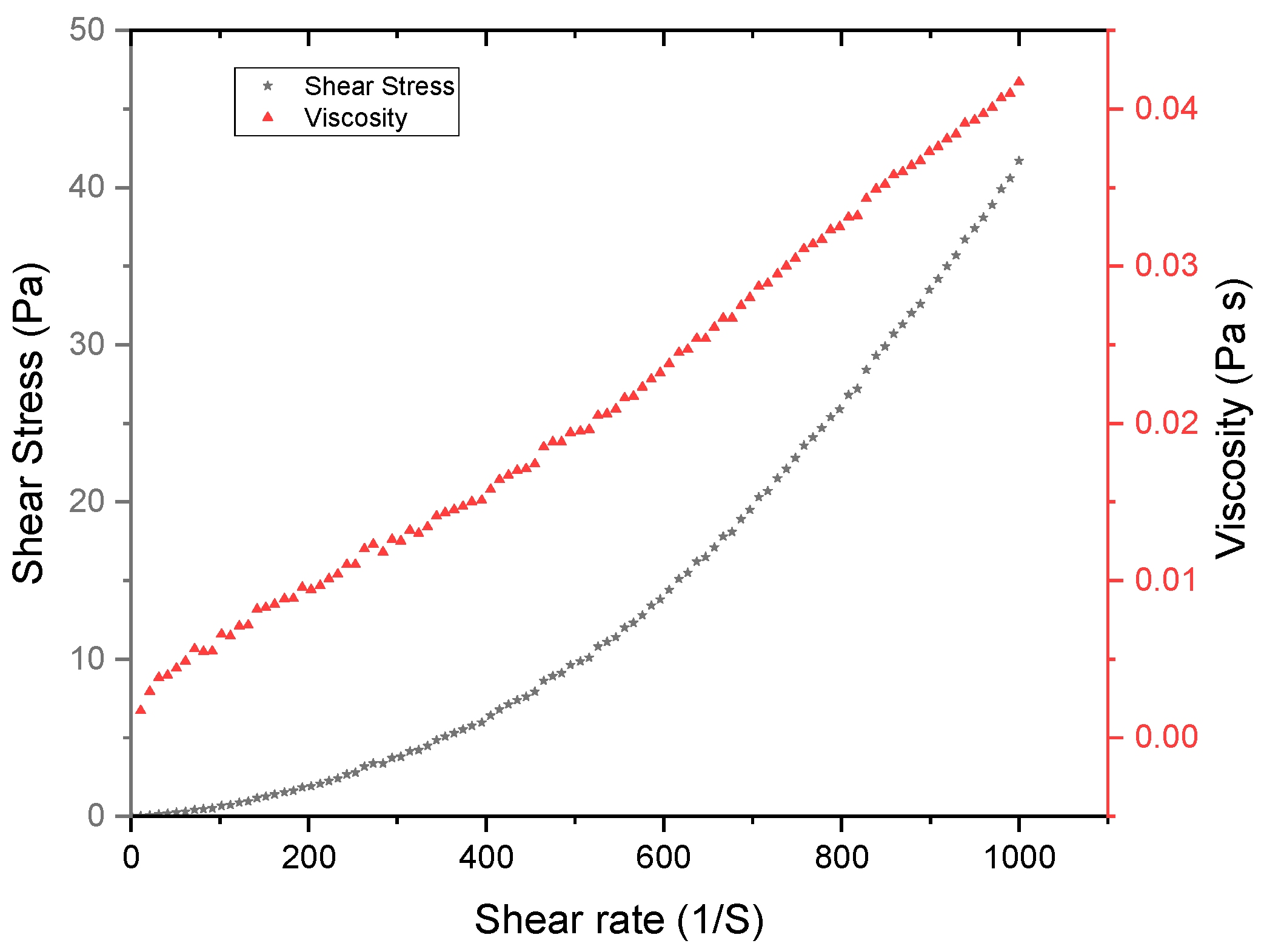


| Model | Experimental Model | R2 |
|---|---|---|
| Herschel–Bulkley | + 8.381 × 10−5 | 0.9974 |
| Determination | Value | Technique |
|---|---|---|
| pH | 5.21 ± 0.11 | Potentiometry |
| Density (g/mL) | 1.02 ± 0.03 | Pycnometry |
| Viscosity (Pa s) | 0.02 ± 0.004 | Rheometry |
| Moisture (%) | 91.50 ± 1.8 | Gravimetry |
| Brix (°) | 13 ± 0.2 | Refractometry |
| Reducing sugars (g/L) | 22.23 ± 1.12 | Miller DNS |
| Carbohydrates (g/L) | 29.58 ± 2.46 | Anthrone method |
| Dextran (ppm) | 1899.20 ± 17.10 | AOAC official method Dextran in raw cane sugar; Robert’s copper method |
| Protein (mg/L) | 0.40 ± 0.08 | Lowry’s method |
| Total suspended solids (TSS) (g/L) | 16.40 ± 1.75 | 2540 D Standard methods |
| Total volatile solids (TVS) (g/L) | 15.20 ± 2.54 | 2540 E Standard methods |
| Total chemical oxygen demand (TCOD) (g/L) | 184.67 ± 12.43 | 5220 D Standard methods |
| Soluble chemical oxygen demand (SCOD) (g/L) | 182.50 ± 8.85 | 5220 D Standard methods |
| Model | Experimental Model | R2 |
|---|---|---|
| Herschel–Bulkley | σ = 0.0978 + 6.570 × 10−5 | 0.9947 |
| 2θ (Degree) | (hkl) | θ (Radian) | FWHM (Degree) | FWHM β(Radian) | Crystal Size D (nm) | Dislocation Density δ | Stress Ɛ | Average Crystal Size D (nm) | Crystal Size Deviation D (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 45.41 | (1 0 0) | 0.3963 | 0.105 | 0.0018 | 82.0158 | 0.000149 | 0.00109 | 91.3666 | 9.9147 |
| 66.21 | (2 0 0) | 0.5778 | 0.105 | 0.0018 | 90.3217 | 0.000123 | 0.00070 | ||
| 83.94 | (2 1 1) | 0.7325 | 0.105 | 0.0018 | 101.7625 | 0.000097 | 0.00051 |
| 2θ (Degree) | (Hkl) | θ (Radian) | FWHM (Degree) | FWHM Β (Radian) | Crystal Size D (nm) | Dislocation Density δ | Stress Ɛ | Average Crystal Size D(nm) | Crystal Size Deviation D (nm) |
|---|---|---|---|---|---|---|---|---|---|
| 18.43 | (1 1 1) | 0.1608 | 1.6281 | 0.0284 | 4.9433 | 0.0409 | 0.0438 | 13.5007 | 4.5466 |
| 30.19 | (2 0 2) | 0.2635 | 0.6828 | 0.0119 | 12.0507 | 0.0069 | 0.0110 | ||
| 35.55 | (3 1 1) | 0.3102 | 0.5252 | 0.0092 | 15.8845 | 0.0040 | 0.0071 | ||
| 43.22 | (4 0 0) | 0.3772 | 0.5777 | 0.0101 | 14.7912 | 0.0046 | 0.0064 | ||
| 57.16 | (5 1 1) | 0.4988 | 0.5252 | 0.0092 | 17.2251 | 0.0034 | 0.0042 | ||
| 62.78 | (4 0 4) | 0.5479 | 0.5777 | 0.0101 | 16.1093 | 0.0039 | 0.0041 |
| Experiment | Enzyme | Temperature (°C) | Time (h) | DNS(g/L) | Carbohydrates (g/L) | Dextran (ppm) | Removal % |
|---|---|---|---|---|---|---|---|
| 1 | Free | 55 | 1 | 11.91 ± 0.11 | 14.89 ± 0.17 | 1647.60 ± 15.28 | 14.89 |
| 2 | Free | 55 | 2 | 9.38 ± 0.06 | 14.25 ± 0.09 | 752.25 ± 18.47 | 61.14 |
| 3 | Nano | 55 | 1 | 11.54 ± 0.18 | 14.43 ± 0.13 | 1329.30 ± 26.85 | 31.33 |
| 4 | Nano | 55 | 2 | 11.03 ± 0.09 | 15.59 ± 0.06 | 929.55 ± 20.74 | 51.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amador-Gómez, L.P.; Luna Solano, G.; Urrea-García, G.R.; Gines-Palestino, R.S.; Cantú-Lozano, D. Synthesis, Modification, and Characterization of Fe3O4@SiO2-PEI-Dextranase Nanoparticles for Enzymatic Degradation of Dextran in Fermented Mash. Processes 2023, 11, 70. https://doi.org/10.3390/pr11010070
Amador-Gómez LP, Luna Solano G, Urrea-García GR, Gines-Palestino RS, Cantú-Lozano D. Synthesis, Modification, and Characterization of Fe3O4@SiO2-PEI-Dextranase Nanoparticles for Enzymatic Degradation of Dextran in Fermented Mash. Processes. 2023; 11(1):70. https://doi.org/10.3390/pr11010070
Chicago/Turabian StyleAmador-Gómez, Luis Pablo, Guadalupe Luna Solano, Galo Rafael Urrea-García, Ruby Sheila Gines-Palestino, and Denis Cantú-Lozano. 2023. "Synthesis, Modification, and Characterization of Fe3O4@SiO2-PEI-Dextranase Nanoparticles for Enzymatic Degradation of Dextran in Fermented Mash" Processes 11, no. 1: 70. https://doi.org/10.3390/pr11010070
APA StyleAmador-Gómez, L. P., Luna Solano, G., Urrea-García, G. R., Gines-Palestino, R. S., & Cantú-Lozano, D. (2023). Synthesis, Modification, and Characterization of Fe3O4@SiO2-PEI-Dextranase Nanoparticles for Enzymatic Degradation of Dextran in Fermented Mash. Processes, 11(1), 70. https://doi.org/10.3390/pr11010070







