Processes and Interactions Impacting the Stability and Compatibility of Vitamin K and Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- 0.1 mol·L−1 NaX, further named as NaX, where X = F−, Cl−, Br−, I−);
- 0.1 mol·L−1 NaX, 6.65 × 10−5 mol·L−1 VK, noted further as NaX–VK;
- 0.1 mol·L−1 NaX, 75 mg·L−1 AuNPs hereinafter referred to as NaX–AuNPs;
- 0.1 mol·L−1 NaX, 6.65 × 10−5 mol·L−1 VK, 75 mg·L−1 AuNPs referred to below as NaX–VK–AuNPs.
2.2. Methods and Procedures
2.2.1. Electrochemical Measurements
2.2.2. UV-Vis Spectrophotometry
3. Results and Discussion
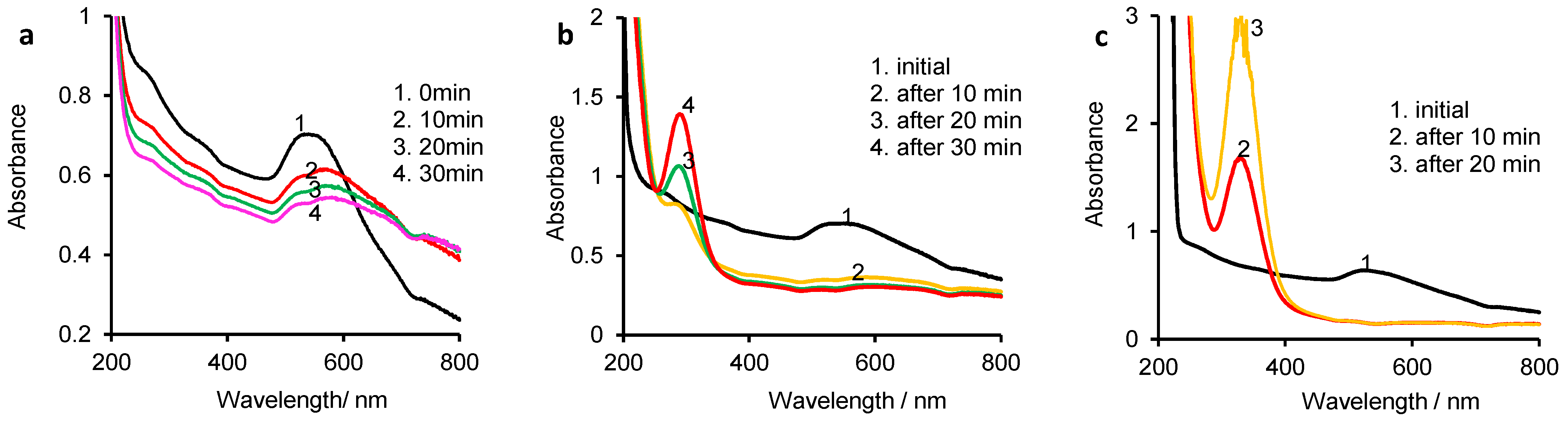
3.1. UV-Vis Spectrophotometry of Initial Media
3.2. Evolution of Vitamin K and Gold Nanoparticles in NaX Solutions Studied by Cyclic Voltammetry (CV) Assisted by UV-Vis Spectrophotometry
3.2.1. Vitamin K/Gold Nanoparticle Behavior in the Presence of F− Ions
3.2.2. Vitamin K/Gold Nanoparticle Behavior in the Presence of Cl− Ions
3.2.3. Vitamin K/Gold Nanoparticle Behavior in the Presence of Br− Ions
3.2.4. The Calculation of the Net Amount of Electric Charge Passed on Electrode Active Area
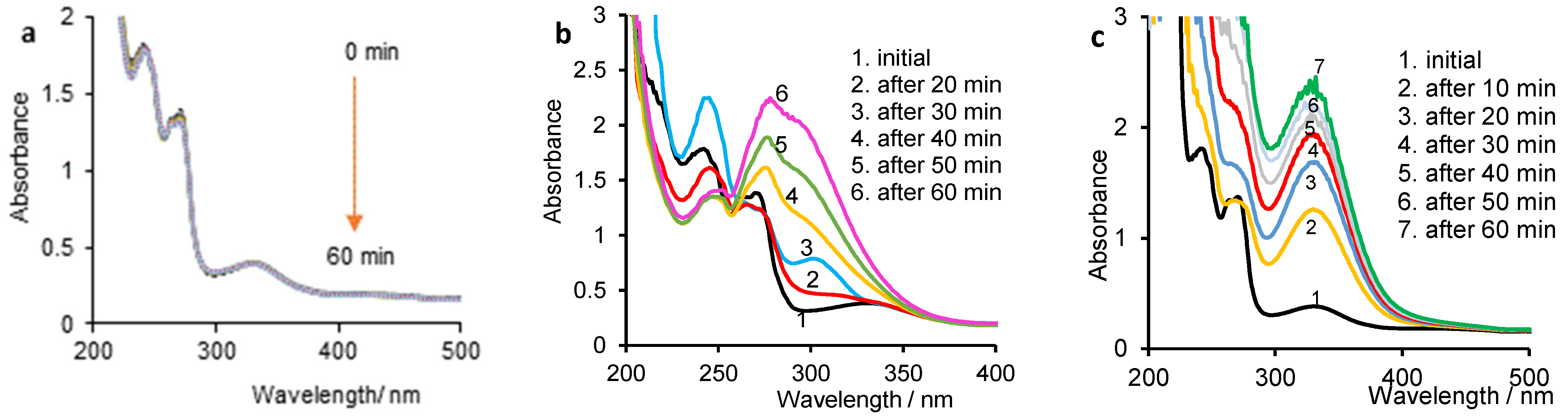
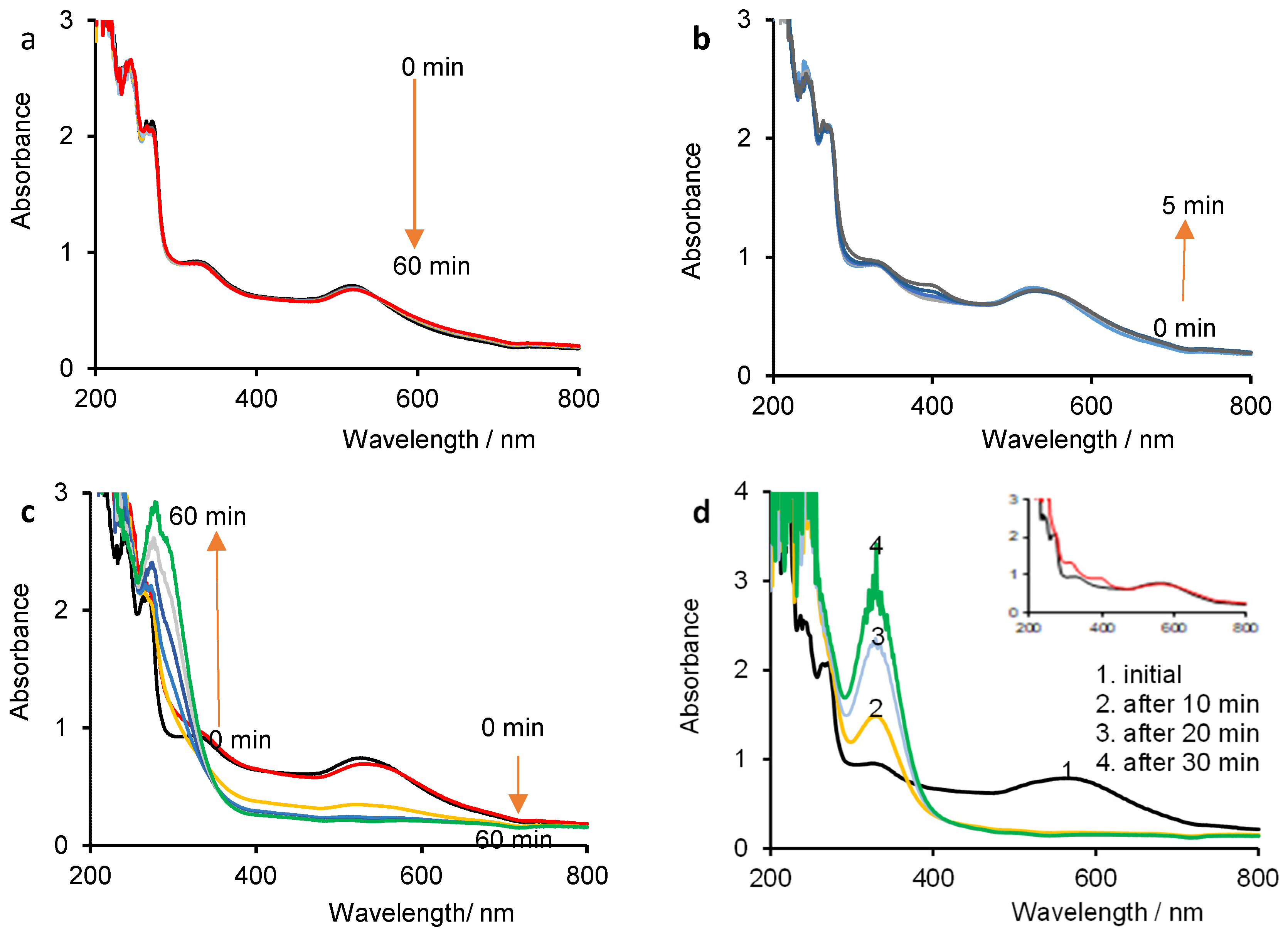
3.3. Evolution of Vitamin K and Gold Nanoparticles over Time in NaX Solutions Studied by Constant Current Density Electrolysis Assisted by UV-Vis Spectrophotometry
3.3.1. Degradation of VK over Time in NaX Solutions
3.3.2. Spectroelectrochemical Behavior of AuNPs over Time in NaX Solutions
3.3.3. Spectroelectrochemical Behavior of VK/AuNP System over Time in NaX Solutions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malhotra, R.; Nicholson, C.J.; Wang, D.; Bhambhani, V.; Paniagua, S.; Slocum, C.; Sigurslid, H.H.; Cardenas, C.L.; Li, R.; Boerboom, S.L.; et al. Matrix Gla protein levels are associated with arterial stiffness and incident heart failure with preserved ejection fraction. Arterioscler. Thromb. Vasc. 2022, 42, e61–e73. [Google Scholar] [CrossRef] [PubMed]
- Tarento, T.D.C.; McClure, D.D.; Vasiljevski, E.; Schindeler, A.; Dehghani, F.; Kavanagh, J.M. Microalgae as a source of vitamin K1. Algal Res. 2018, 36, 77–87. [Google Scholar] [CrossRef]
- Wei, X.; Hang, Y.; Li, X.; Hua, X.; Cong, X.; Yi, W.; Guo, X. Effects of dietary vitamin K3 levels on growth, coagulation, calcium content, and antioxidant capacity in largemouth bass, Micropterus salmoides. Aquac. Fish. 2021, in press. [Google Scholar] [CrossRef]
- Giarma, E.; Mpampali, Z.; Lialiaris, T.; Mourelatos, D. Cytoprotective and genotoxic effects of vitamins K1 and B1 on irinotecan in vitro. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2019, 837, 60–64. [Google Scholar] [CrossRef]
- Chetot, T.; Mouette-Bonnet, M.; Taufana, S.; Fourel, I.; Lefebvre, S.; Benoit, E.; Lattard, V. Differences in teratogenicity of some vitamin K antagonist substances used as human therapeutic or rodenticide are due to major differences in their fate after an oral administration. Toxicol. Lett. 2020, 333, 71–79. [Google Scholar] [CrossRef]
- Bar, A.; Kus, K.; Manterys, A.; Proniewski, B.; Sternak, M.; Przyborowski, K.; Moorlag, M.; Sitek, B.; Marczyk, B.; Jasztal, A.; et al. Vitamin K2-MK-7 improves nitric oxide-dependent endothelial function in ApoE/LDLR−/− mice. Vasc. Pharmacol. 2019, 122–123, 106581. [Google Scholar] [CrossRef] [PubMed]
- Apalset, E.M.; Gjesdal, C.G.; Eide, G.E.; Tell, G.S. Intake of vitamin K1 and K2 and risk of hip fractures: The Hordaland Health Study. Bone 2011, 49, 990–995. [Google Scholar] [CrossRef]
- Moore, A.E.; Kim, E.J.; Dulnoan, D.; Dolan, A.L.; Voong, K.; Ahmad, I.; Gorska, R.; Harrington, D.J.; Hampson, G. Serum vitamin K1 (phylloquinone) is associated with fracture risk and hip strength in post-menopausal osteoporosis: A cross-sectional study. Bone 2020, 141, 115630. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.-J.; Kim, Y.-R. Improving solubility and stability of fat-soluble vitamins (A, D, E, and K) using large-ring cycloamylose. LWT Food Sci. Technol. 2022, 153, 112502. [Google Scholar] [CrossRef]
- Campani, V.; Biondi, M.; Mayol, L.; Cilurzo, F.; Pitaro, M.; De Rosa, G. Development of nanoemulsions for topical delivery of vitamin K1. Int. J. Pharm. 2016, 511, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Setoguchi, S.; Nagata-Akaho, N.; Terada, K.; Watase, D.; Yamakawa, H.; Toki, E.; Koga, M.; Matsunaga, K.; Karube, Y.; et al. Ester derivatives of phyllohydroquinone effectively deliver the active form of vitamin K1 topically, owing to their non-photosensitivity. Eur. J. Pharm. Sci. 2020, 155, 105519. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Azar, P.A.; Husain, S.W.; Maibach, H.I.; Nafisi, S. Experimental design in formulation optimization of vitamin K1 oxide-loaded nanoliposomes for skin delivery. Int. J. Pharm. 2020, 579, 119136. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Gupta, B.D. Simultaneous estimation of vitamin K1 and heparin with low limit of detection using cascaded channels fiber optic surface plasmon resonance. Biosens. Bioelectron. 2016, 86, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Cholerton, S.; Park, B.K. Quantitative analysis of pharmacological concentrations of vitamin K1 and vitamin K1 2,3-epoxide in rat liver by high-performance hquid chromatography. J. Chromatogr. Biomed. Appl. 1986, 375, 147–153. [Google Scholar] [CrossRef]
- Gentili, A.; Cafolla, A.; Gasperi, T.; Bellante, S.; Caretti, F.; Curini, R.; Pérez Fernández, V. Rapid, high performance method for the determination of vitamin K1, menaquinone-4 and vitamin K1 2,3-epoxide in human serum andplasma using liquid chromatography-hybrid quadrupole linear iontrap mass spectrometry. J. Chromatogr. A 2014, 1338, 102–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Chhonker, Y.S.; Bala, V.; Hagg, A.; Snetselaar, L.G.; Wahls, T.L.; Murry, D.J. Reversed phase UPLC/APCI-MS determination of vitamin K1 and menaquinone-4 in human plasma: Application to a clinical study. J. Pharmaceut. Biomed. 2020, 183, 113147. [Google Scholar] [CrossRef] [PubMed]
- Japelt, R.B.; Jakobsen, J. Analysis of vitamin K1 in fruits and vegetables using accelerated solvent extraction and liquid chromatography tandem mass spectrometry with atmospheric pressure chemical ionization. Food Chem. 2016, 192, 402–408. [Google Scholar] [CrossRef]
- Konieczna, L.; Kazmierska, K.; Roszkowska, A.; Szlagatys-Sidorkiewicz, A.; Baczek, T. The LC–MS method for the simultaneous analysis of selected fat-soluble vitamins and their metabolites in serum samples obtained from pediatric patients with cystic fibrosis. J. Pharmaceut. Biomed. 2016, 124, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Barkhan, P. Studies on the metabolites of phyleoquinone (vitamin K1) in the urine of man. Biochim. Biophys. Acta 1973, 297, 300–312. [Google Scholar] [CrossRef]
- Haque, J.A.; McDonald, M.G.; Kulman, J.D.; Rettie, A.E. A cellular system for quantitation of vitamin K cycle activity: Structure-activity effects on vitamin K antagonism by warfarin metabolites. Blood 2014, 123, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Rostami-Javanroudi, S.; Babakhanian, A. New electrochemical sensor for direct quantification of vitamin K in human blood serum. Microchem. J. 2021, 163, 105716. [Google Scholar] [CrossRef]
- Sýs, M.; Jashari, G.; Švecová, B.; Arbneshi, T.; Metelka, R. Determination of vitamin K1 using square wave adsorptive stripping voltammetry at solid glassy carbon electrode. J. Electroanal. Chem. 2018, 821, 10–15. [Google Scholar] [CrossRef]
- Torkashvand, M.; Gholivand, M.B.; (Arman) Taherpour, A.; Boochani, A.; Akhtar, A. Introduction of a carbon paste electrode based on nickel carbide for investigation of interaction between warfarin and vitamin K1. J. Pharmaceut. Biomed. 2017, 139, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Jedlinska, K.; Strus, M.; Bas, B. A new electrochemical sensor with the refreshable silver liquid amalgam film multi-electrode for sensitive voltammetric determination of vitamin K2 (menaquinone). Electrochim. Acta 2018, 265, 355–363. [Google Scholar] [CrossRef]
- Aljarba, N.H.; Imtiaz, S.; Anwar, N.; Alanazi, I.S.; Alkahtani, S. Anticancer and microbial activities of gold nanoparticles: A mechanistic review. J. King Saud Univ. Sci. 2022, 34, 101907. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens Bioelectron. 2018, 121, 125–136. [Google Scholar]
- Tutunaru, B.; Samide, A.; Neamtu, C.; Tigae, C. Spectroelectrochemical studies of interactions between vitamin A and nanocolloidal silver. Int. J. Electrochem. Sci. 2018, 13, 5850–5859. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B. Interactions between vitamin C and nanocolloidal silver particles studied by cyclic voltammetry and UV−Vis spectrophotometry. Electroanalysis 2017, 29, 2498–2506. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Varut, R.-M.; Oprea, B.; Iordache, S. Interactions of some chemotherapeutic agents as epirubicin, gemcitabine and paclitaxel in multicomponent systems based on orange essential oil. Pharmaceuticals 2021, 14, 619. [Google Scholar] [CrossRef]
- Tutunaru, B.; Samide, A.; Spînu, C.-I.; Tigae, C.; Oprea, B. Induced effect of gold nanoparticles (AuNPs) and halide ions on pyridoxine molecule stability. Appl. Sci. 2021, 11, 9470. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Bratulescu, G.; Ionescu, C. Electrochemical synthesis and characterization of new electrodes based on poly-hematoxylin films. J. Appl. Polym. Sci. 2013, 130, 687–697. [Google Scholar] [CrossRef]
- Tutunaru, B.; Samide, A.; Iordache, S.; Tigae, C.; Simionescu, A.; Popescu, A. Ceftriaxone degradation in the presence of sodium halides investigated by electrochemical methods assisted by UV−Vis spectrophotometry. Appl. Sci. 2021, 11, 1376. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B.; Tigae, C.; Efrem, R.; Moanta, A.; Dragoi, M. Removal of Methylene Blue and Methyl Blue from wastewater by electrochemical degradation. Environ. Prot. Eng. 2014, 40, 93–104. [Google Scholar] [CrossRef]
- Tutunaru, B.; Tigae, C.; Spînu, C.; Prunaru, I. Spectrophotometry and electrochemistry of Brilliant Blue FCF in aqueous solution of NaX. Int. J. Electrochem. Sci. 2017, 12, 396–412. [Google Scholar] [CrossRef]
- Tutunaru, B.; Samide, A.; Moanta, A.; Ionescu, C.; Tigae, C. Electrochemical study of metribuzin pesticide degradation on bismuth electrode in aqueous solution. Int. J. Electrochem. Sci. 2015, 10, 223–234. [Google Scholar]
- Hubicka, U.; Padiasek, A.; Zuromska-Witek, B.; Szlósarczyk, M. Determination of vitamins K1, K2 MK-4, MK-7, MK-9 and D3 in pharmaceutical products and dietary supplements by TLC-densitometry. Processes 2020, 8, 870. [Google Scholar] [CrossRef]
- Hua, K.; Li, Y.; Ding, R.; Zhai, Y.; Chen, L.; Qian, W.; Yang, J. A simple, sensitive, and high-throughput LC-APCI-MS/MS method for simultaneous determination of vitamin K1, vitamin K1 2,3-epoxide in human plasma and its application to a clinical pharmacodynamics study of warfarin. J. Pharmaceut. Biomed. 2018, 159, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Balfourier, A.; Luciani, N.; Wang, G.; Gerald Lelon, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, D.; Florent Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103–113. [Google Scholar] [PubMed]


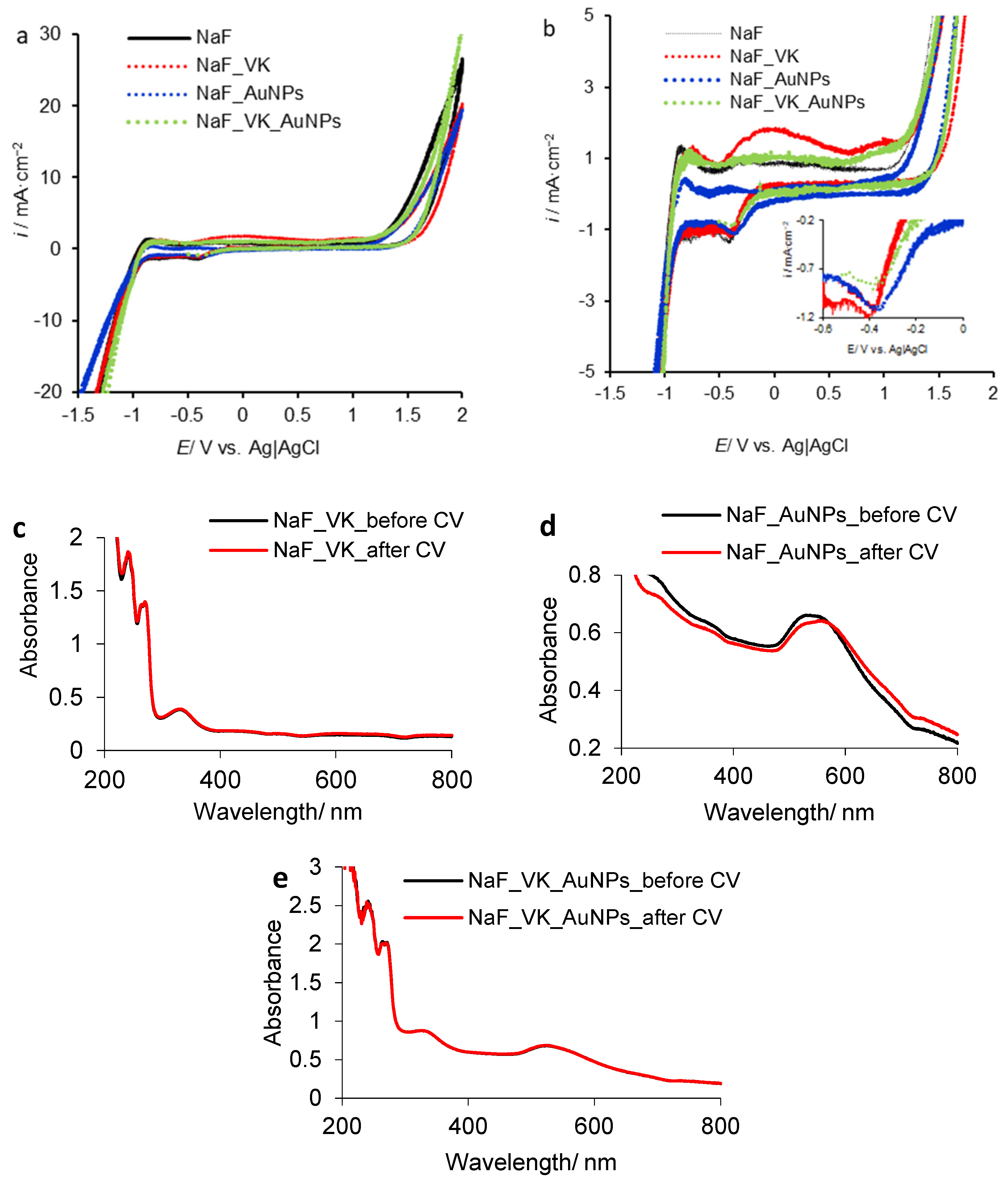
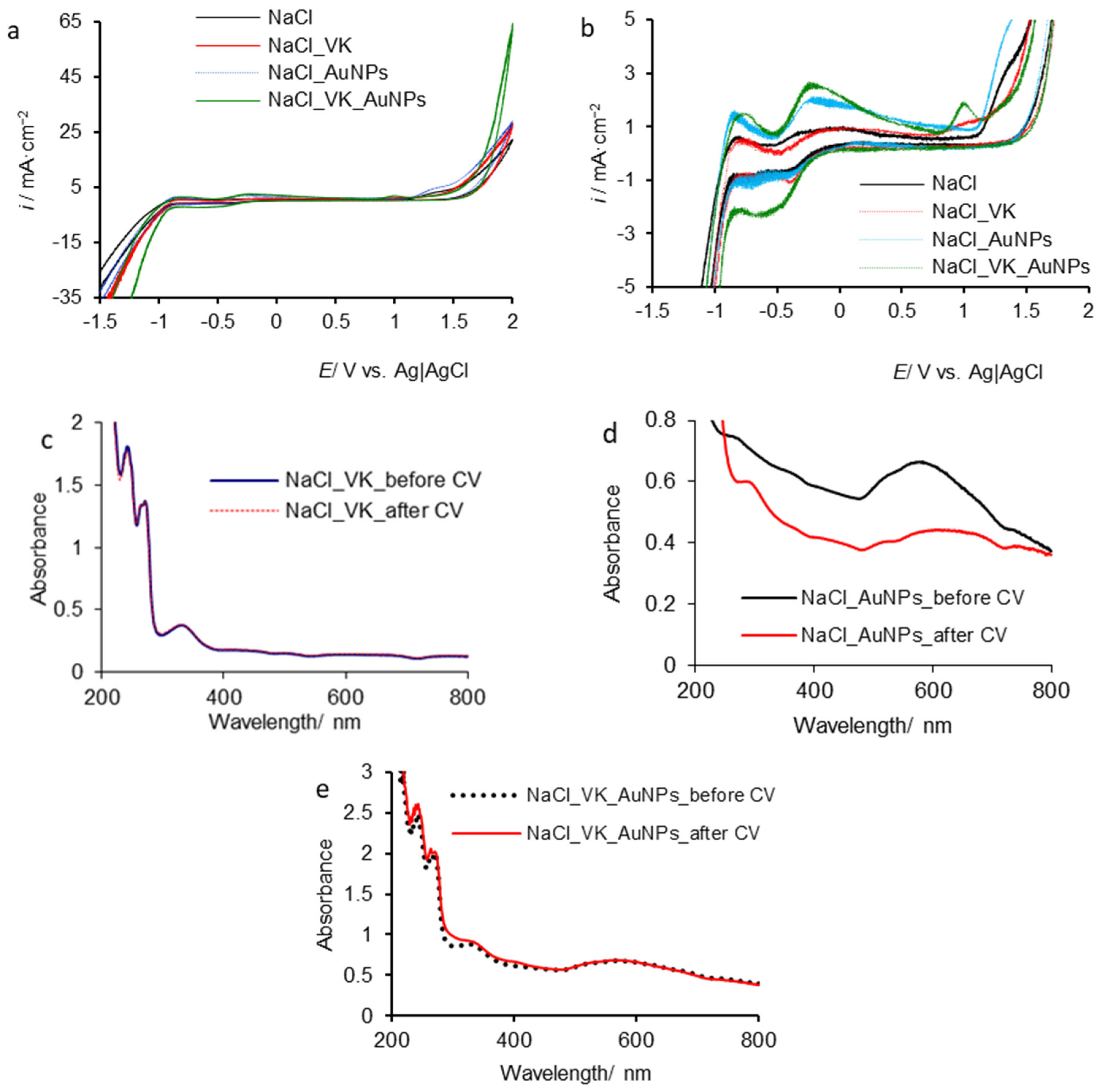

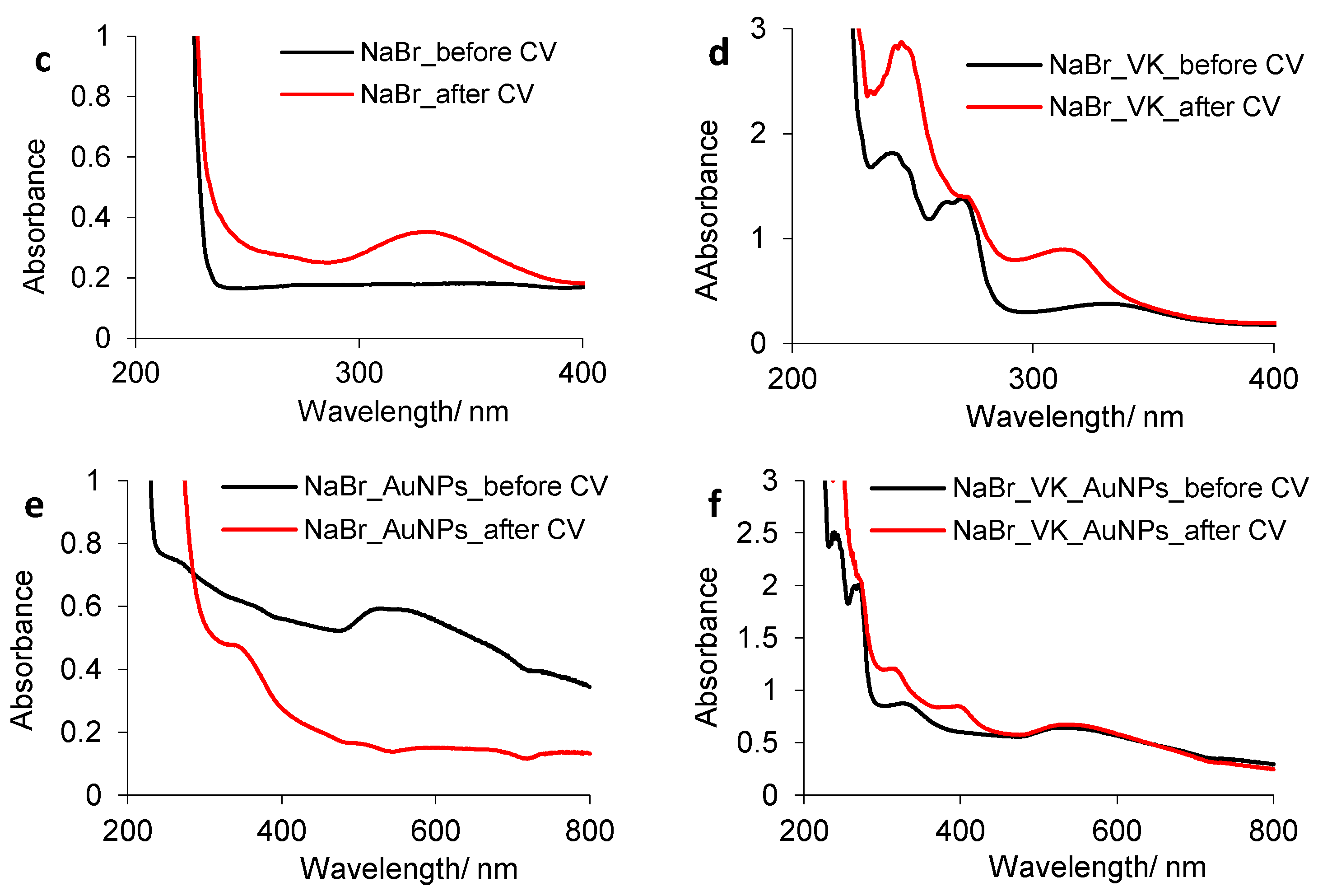


| Sample | ε (mV) | Time (s) | i × 103 (A cm−2) | q (C cm−2) | ||
|---|---|---|---|---|---|---|
| εa (mV) | εc (mV) | ia | ic | |||
| NaF | 2000 | 1000 | 10 | 26.9 | 0.375 | 0.266 |
| NaF–VK | 20.3 | 0.29 | 0.200 | |||
| NaF–AuNPs | 19.1 | −0.018 | 0.191 | |||
| NaF–VK–AuNPs | 30.1 | 0.17 | 0.299 | |||
| NaCl | 21.9 | 0.31 | 0.216 | |||
| NaCl–VK | 27.0 | 0.21 | 0.267 | |||
| NaCl–AuNPs | 29.1 | 0.18 | 0.289 | |||
| NaCl–VK–AuNPs | 63.4 | 0.15 | 0.632 | |||
| NaBr | 92.5 | 9.1 | 0.834 | |||
| NaBr–VK | 87.3 | 8.9 | 0.784 | |||
| NaBr–AuNPs | 90.8 | 7.1 | 0.830 | |||
| NaBr–VK–AuNPs | 59.6 | 7.1 | 0.498 | |||
| Compounds | Ions | ||||||
|---|---|---|---|---|---|---|---|
| F− | Cl− | Br− | I− | ||||
| Stability | Compatibility | Stability | Compatibility | Stability | Compatibility | Stability | |
| VK | good | - | relative | - | unstable | - | unstable from the first contact |
| AuNPS | good | - | low | - | unstable | - | unstable from the first contact |
| VK–AuNPS | good | good | for a short time | low (restrictive) | unstable | incompatible | unstable from the first contact/incompatible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samide, A.; Tutunaru, B.; Oprea, B. Processes and Interactions Impacting the Stability and Compatibility of Vitamin K and Gold Nanoparticles. Processes 2022, 10, 1805. https://doi.org/10.3390/pr10091805
Samide A, Tutunaru B, Oprea B. Processes and Interactions Impacting the Stability and Compatibility of Vitamin K and Gold Nanoparticles. Processes. 2022; 10(9):1805. https://doi.org/10.3390/pr10091805
Chicago/Turabian StyleSamide, Adriana, Bogdan Tutunaru, and Bogdan Oprea. 2022. "Processes and Interactions Impacting the Stability and Compatibility of Vitamin K and Gold Nanoparticles" Processes 10, no. 9: 1805. https://doi.org/10.3390/pr10091805
APA StyleSamide, A., Tutunaru, B., & Oprea, B. (2022). Processes and Interactions Impacting the Stability and Compatibility of Vitamin K and Gold Nanoparticles. Processes, 10(9), 1805. https://doi.org/10.3390/pr10091805






