The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review
Abstract
:1. Introduction
2. Classification of Water Pollutants
2.1. Organic Pollutants
2.2. Inorganic Pollutants:
2.3. Microbes/Pathogens
2.4. Suspended Solid and Sediments
3. Source of Pollution
3.1. Urban Pollution
3.2. Agricultural Pollution
3.3. Industrial Pollution
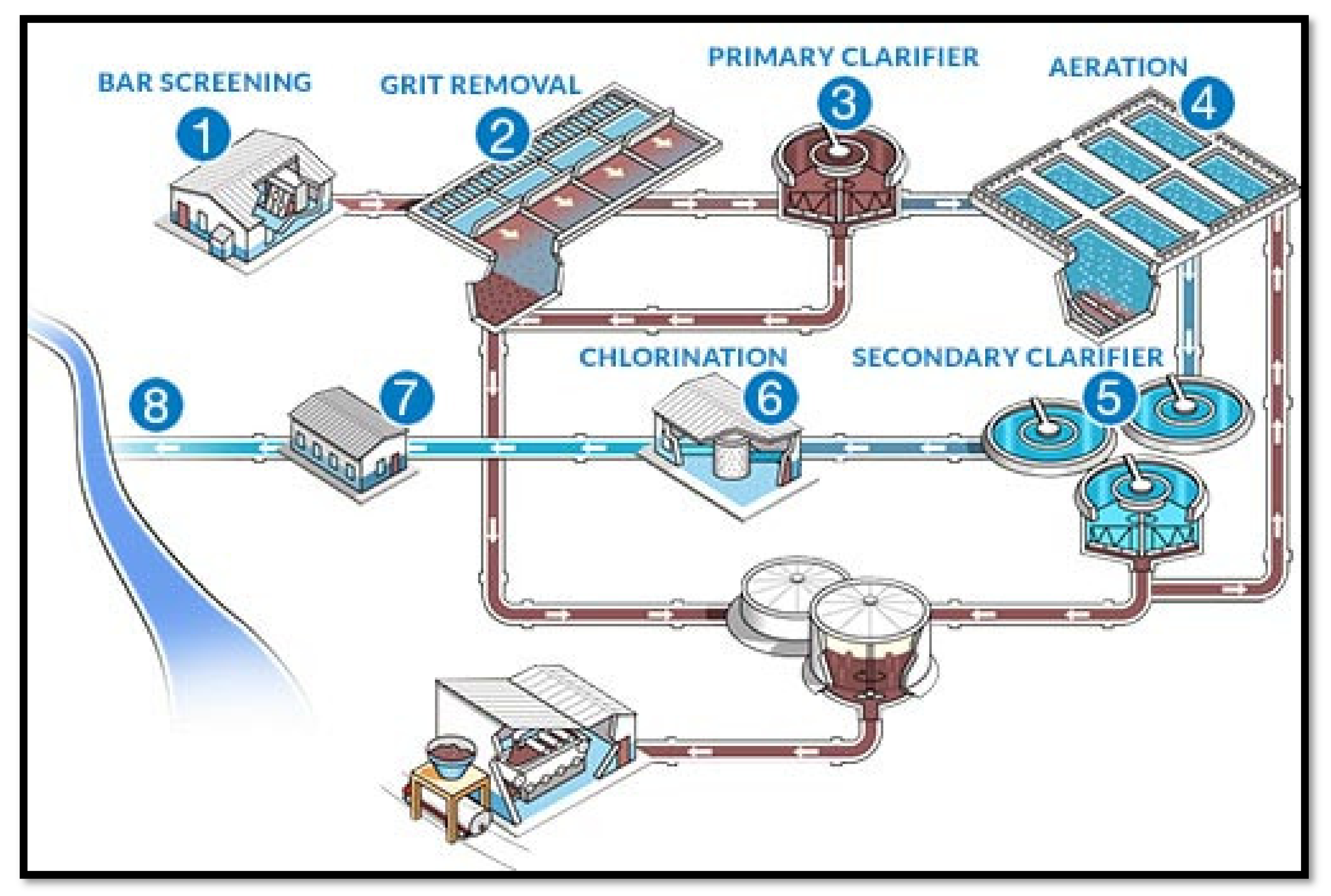
4. Wastewater Treatment Technology
4.1. Precipitation/Encapsulation
4.1.1. Primary Process
- Screening
- Centrifugal separation
- Coagulation & Flocculation
- Floatation
- Separation by gravity and sedimentation
4.1.2. Secondary Process
- Aerobic process
- Anaerobic process
- Encapsulation
4.1.3. Tertiary Process
- Oxidation/Advanced oxidation
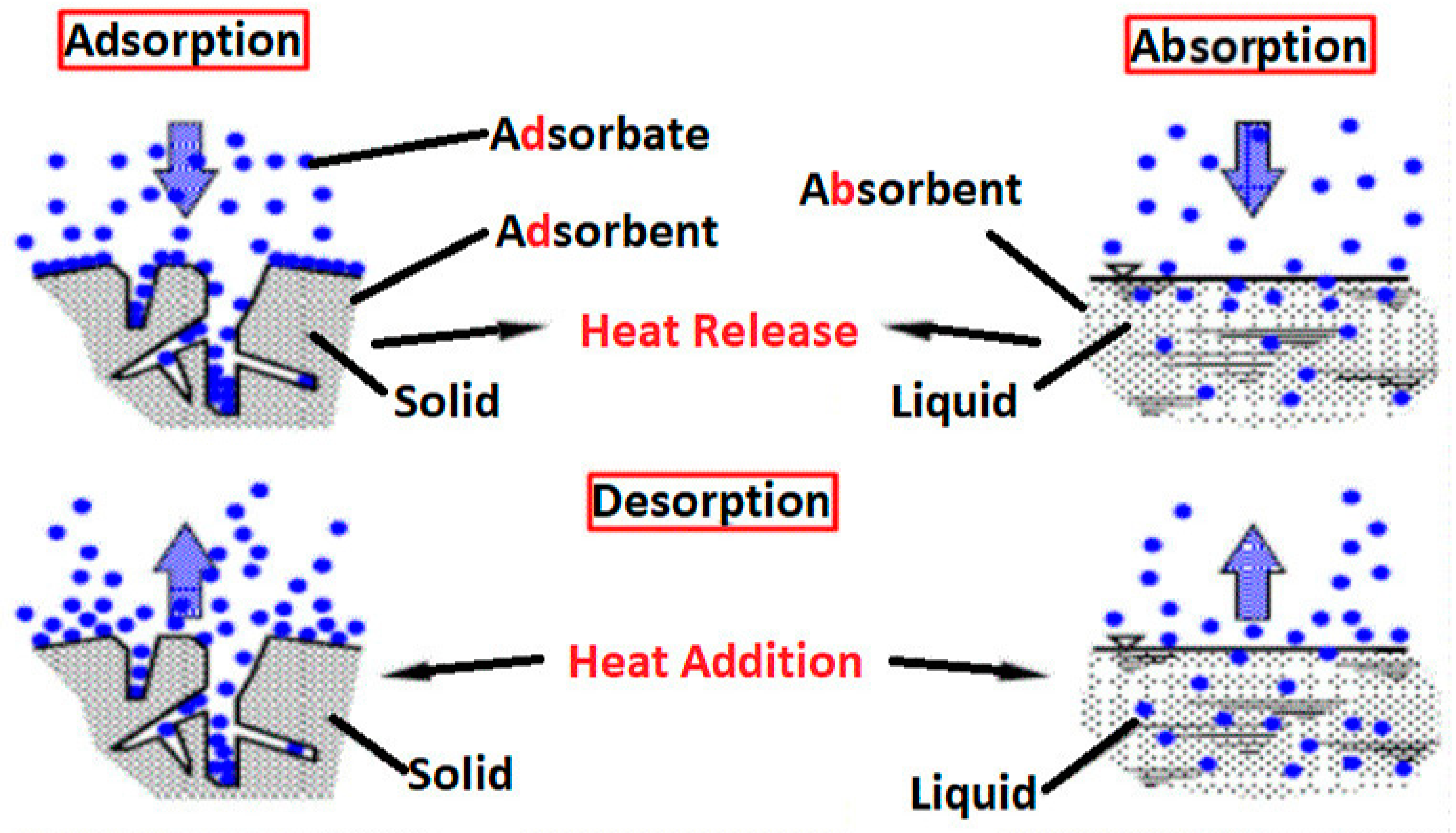
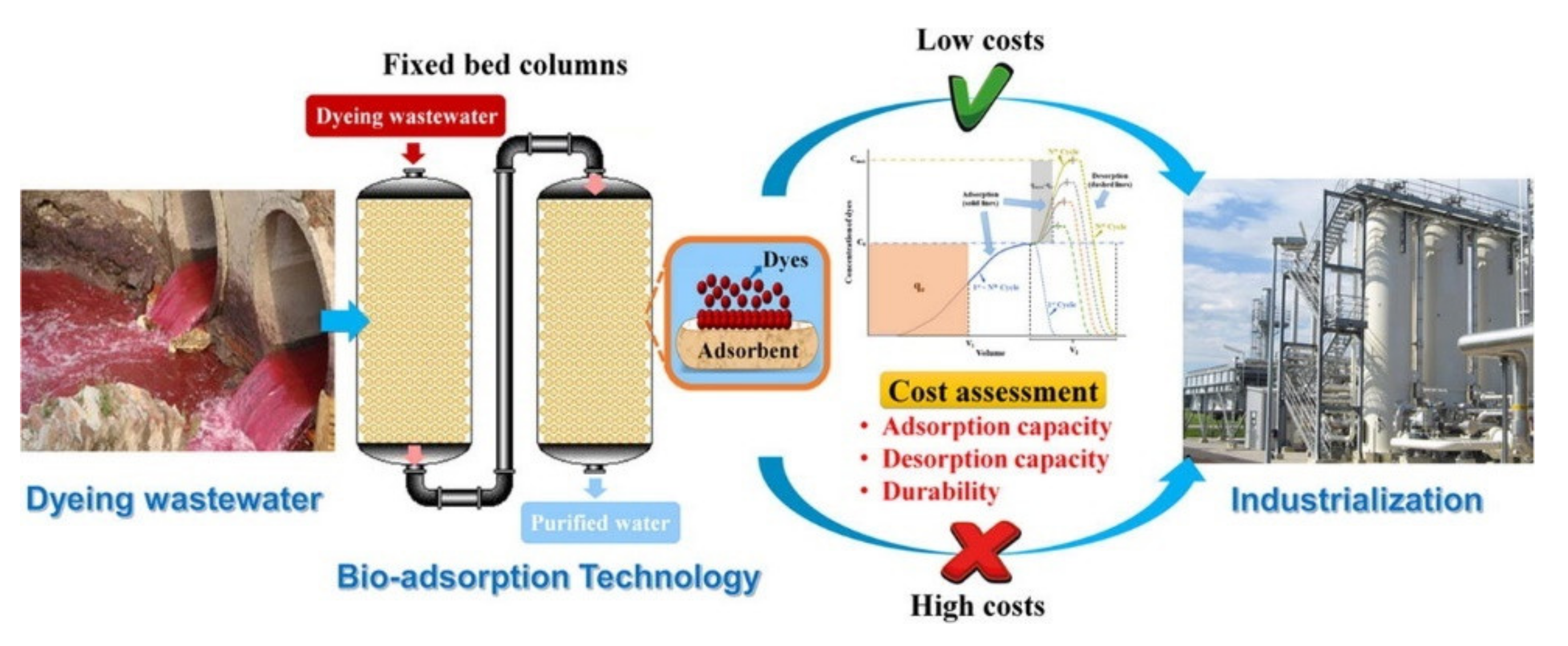
4.2. Adsorption
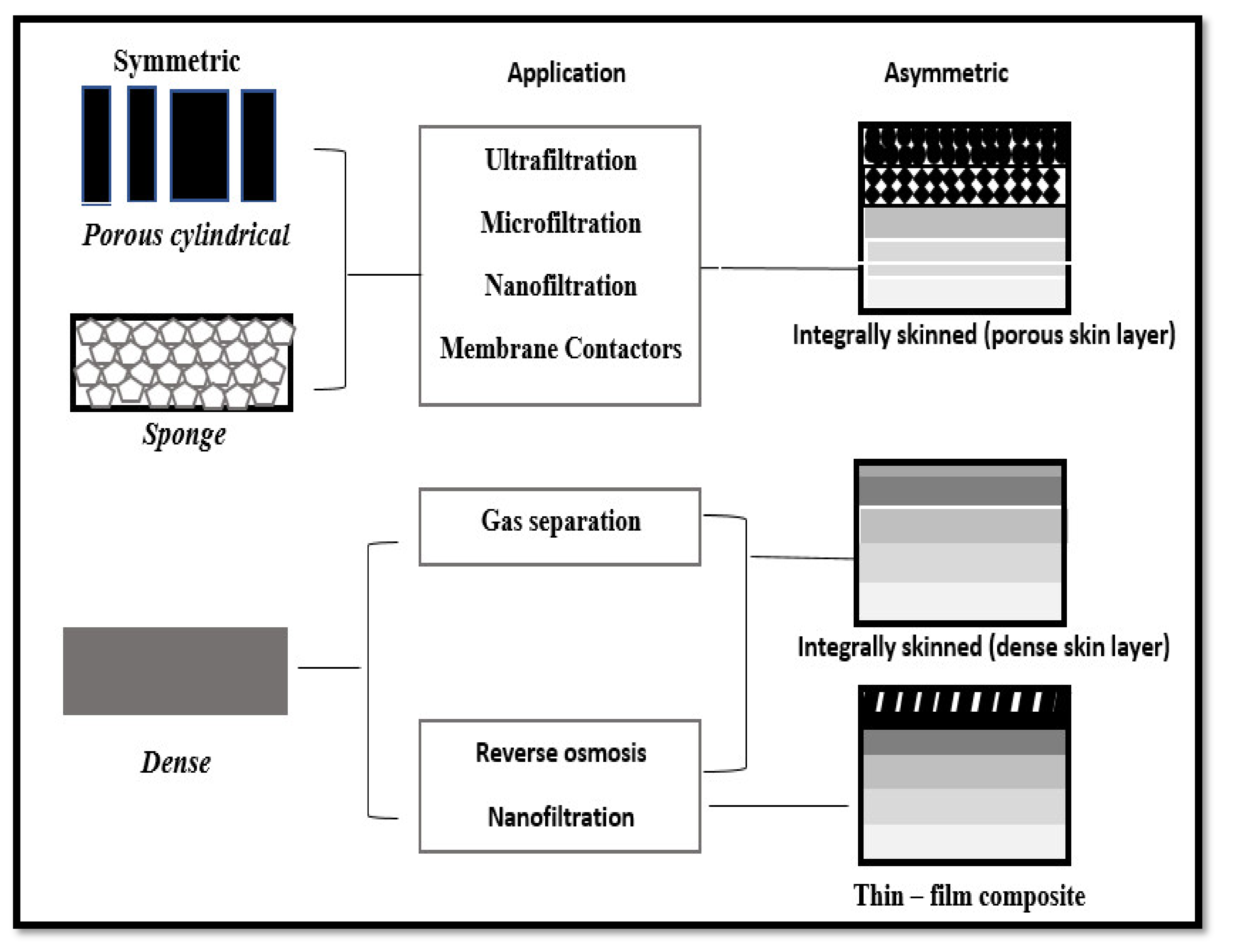
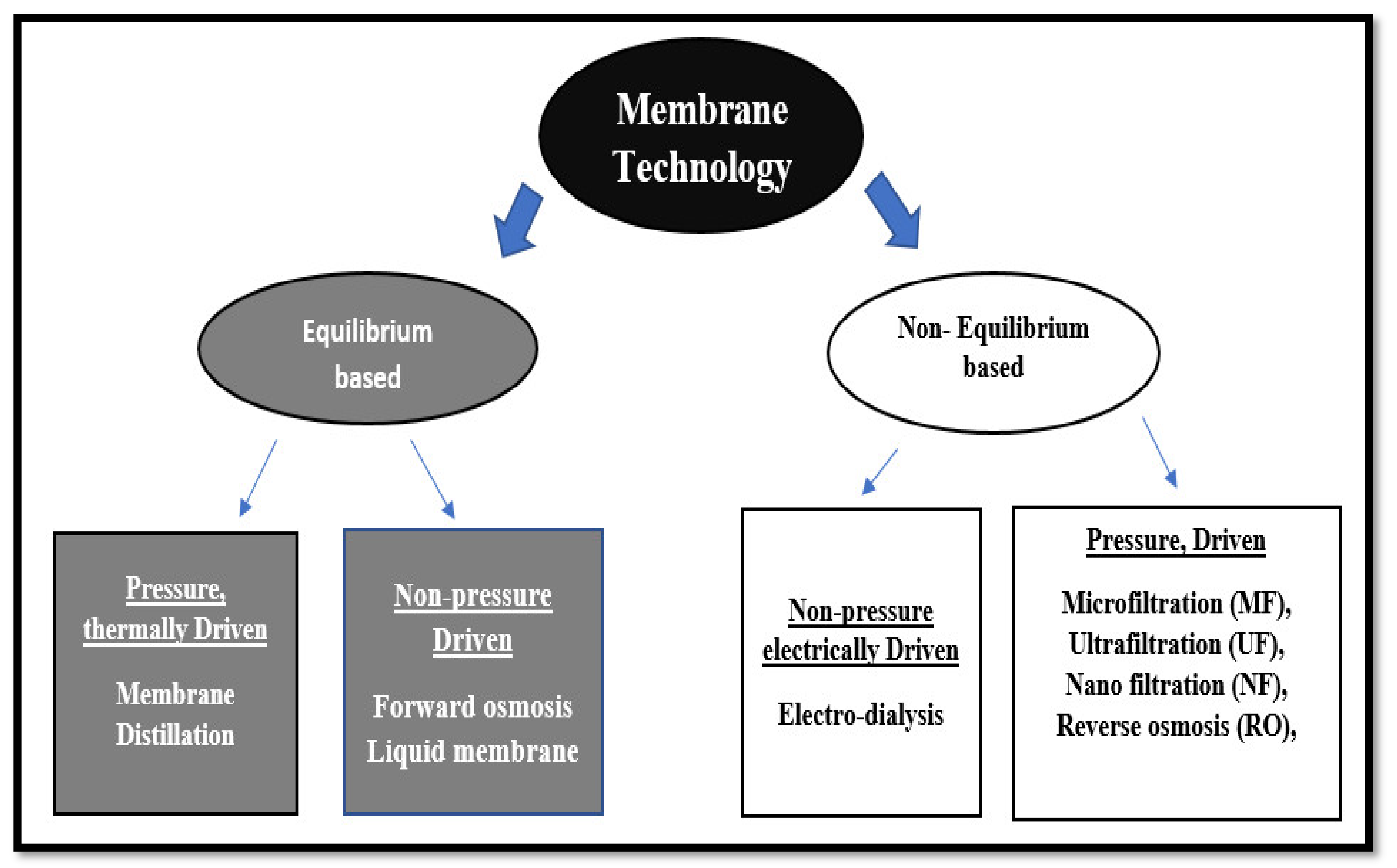
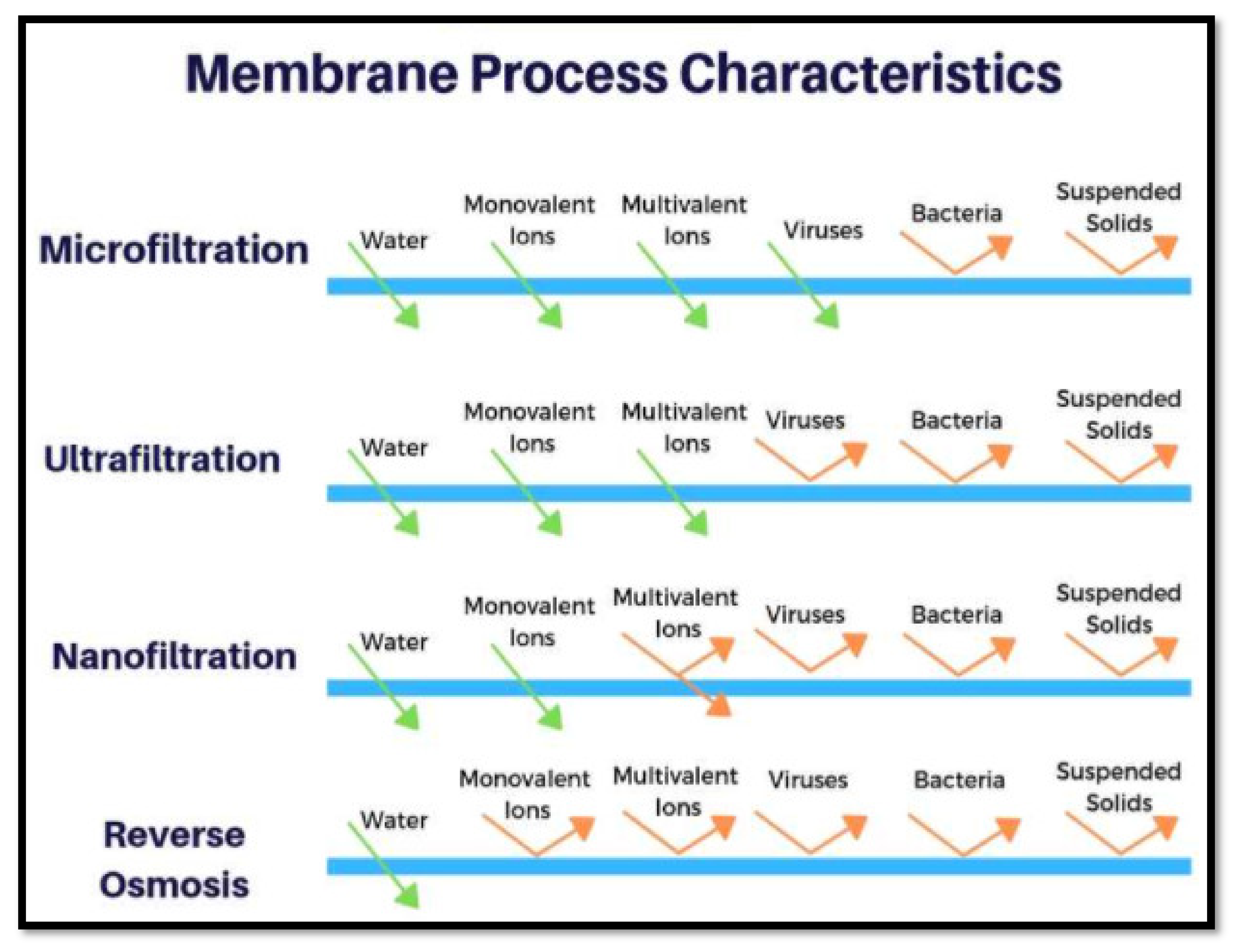
4.3. Membrane Technologies
- Micro and ultra-filtration.
- Membrane bioreactor (MBR).
- Reverse osmosis (RO).
- Electrolysis.
- Electrodialysis.
- Micro and ultra-filtration
- Membrane bioreactor (MBR)
- Reverse osmosis (RO)
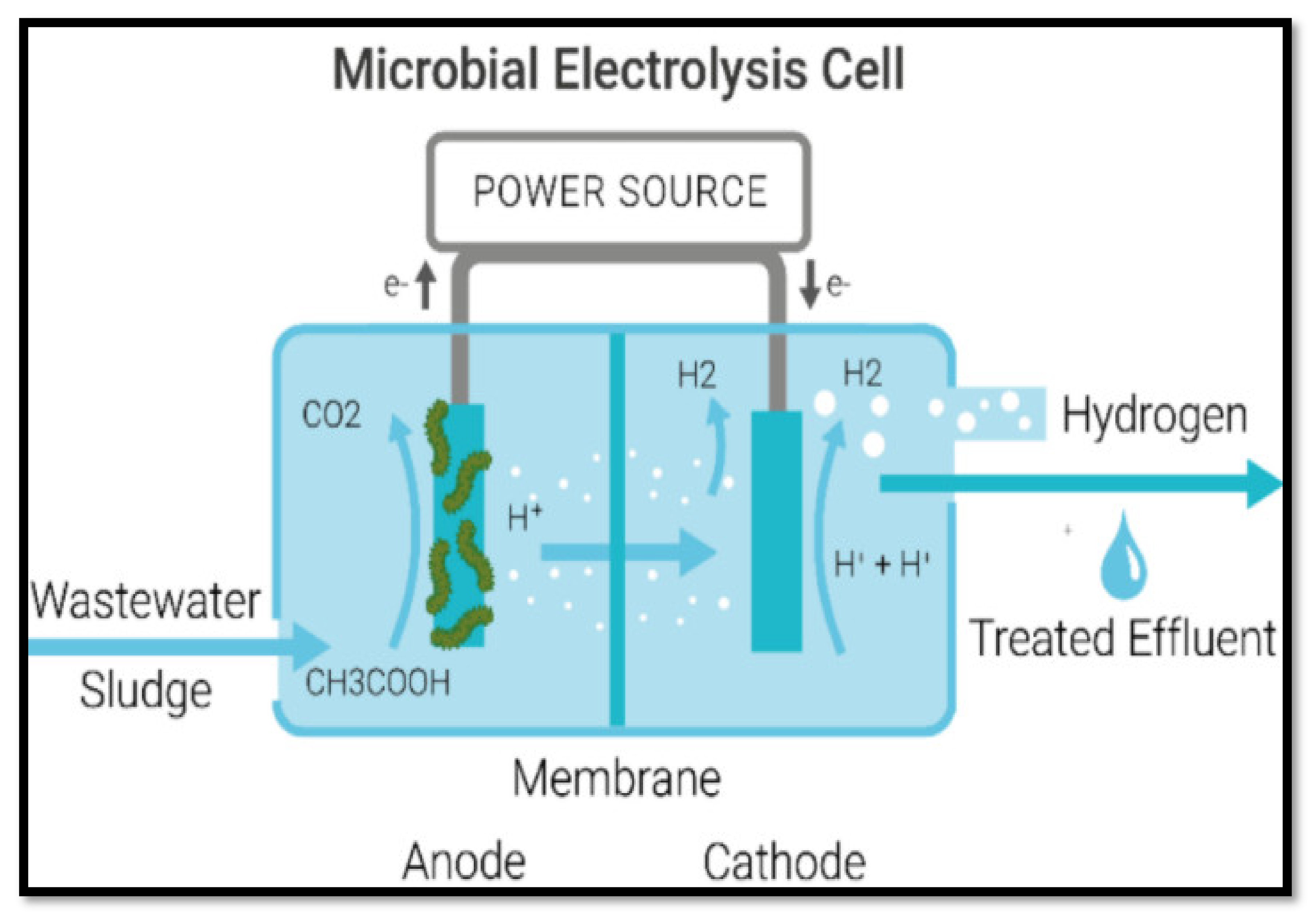
- Electrolysis
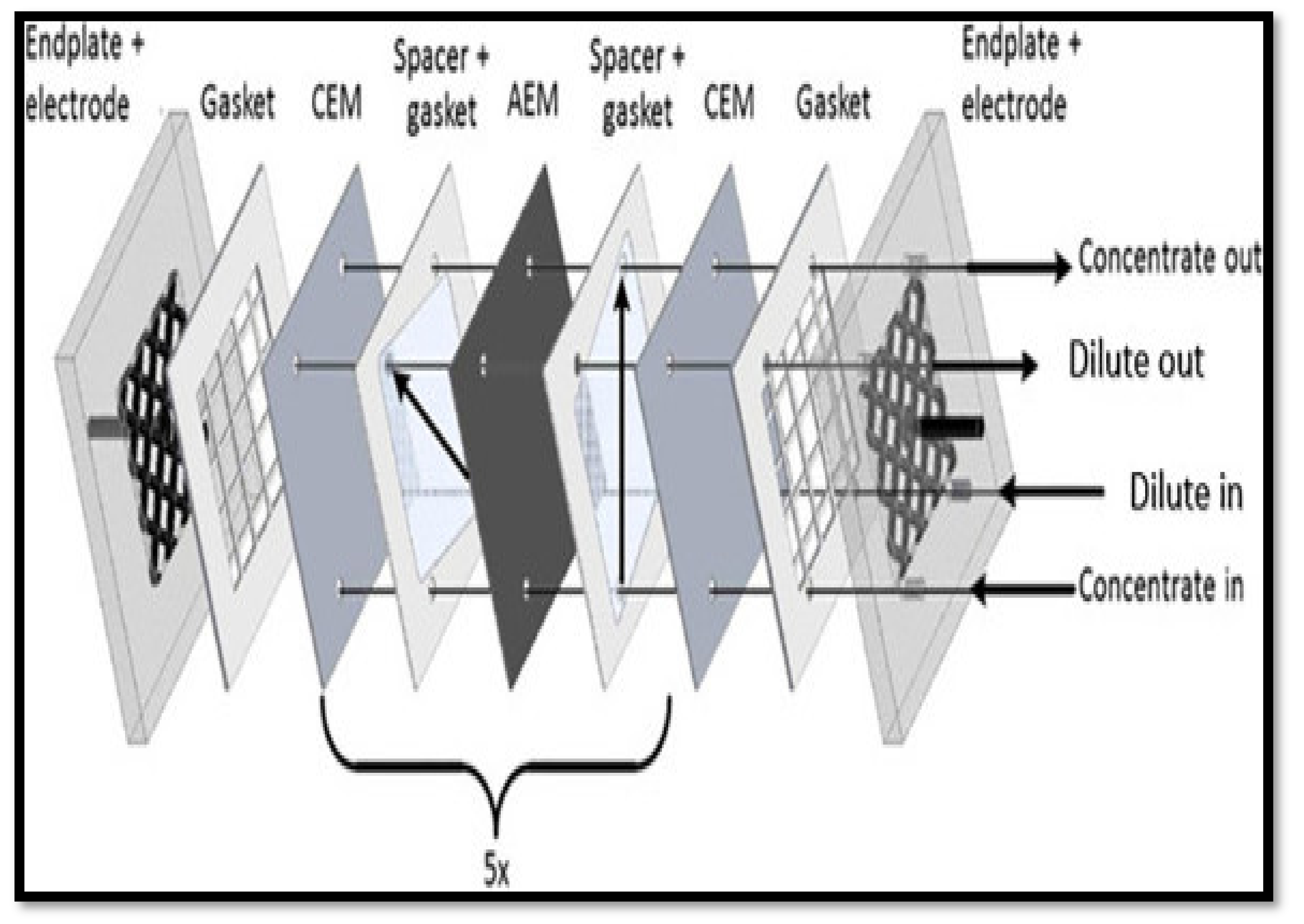
- Electrodialysis (ED)
4.4. Artificial Intelligence Models to Treat the Wastewater
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarfeen, N.; Nisa, K.U.; Hamid, B.; Bashir, Z.; Yatoo, A.M.; Dar, M.A.; Mohiddin, F.A.; Amin, Z.; Ahmad, R.a.A.; Sayyed, R. Microbial Remediation: A Promising Tool for Reclamation of Contaminated Sites with Special Emphasis on Heavy Metal and Pesticide Pollution: A Review. Processes 2022, 10, 1358. [Google Scholar] [CrossRef]
- Bani-Melhem, K.; Al-Shannag, M.; Alrousan, D.; Al-Kofahi, S.; Al-Qodah, Z.; Al-Kilani, M.R. Impact of soluble COD on grey water treatment by electrocoagulation technique. Desalination Water Treat. 2017, 89, 101–110. [Google Scholar] [CrossRef]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Munter, R. Industrial Wastewater Characteristics; The Baltic University Programme (BUP): Uppsala, Sweden, 2003; pp. 185–194. [Google Scholar]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Cheremisinoff, N.P. Handbook of Water and Wastewater Treatment Technologies; Butterworth-Heinemann: Oxford, UK, 2001. [Google Scholar]
- Bakar, N.A.; Othman, N.; Yunus, Z.M.; Altowayti, W.A.H.; Al-Gheethi, A.; Asharuddin, S.M.; Tahir, M.; Fitriani, N.; Mohd-Salleh, S.N.A. Nipah (Musa Acuminata Balbisiana) banana peel as a lignocellulosic precursor for activated carbon: Characterization study after carbonization process with phosphoric acid impregnated activated carbon. Biomass Convers. Biorefinery, 2021; in press. [Google Scholar] [CrossRef]
- Li, L.; Rong, S.; Wang, R.; Yu, S. Recent advances in artificial intelligence and machine learning for nonlinear relationship analysis and process control in drinking water treatment: A review. Chem. Eng. J. 2021, 405, 126673. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Shah, D.V. Role of Absorption and Adsorption in the Removal of Waste. In Emerging Trends in Environmental Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 33–47. [Google Scholar]
- Iqbal, J.; Howari, F.M.; Mohamed, A.-M.O.; Paleologos, E.K. Assessment of radiation pollution from nuclear power plants. In Pollution Assessment for Sustainable Practices in Applied Sciences and Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1027–1053. [Google Scholar]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Altowayti, W.; Othman, N.; Shahir, S.; Alshalif, A.; Al-Gheethi, A.; Al-Towayti, F.; Saleh, Z.; Haris, S. Removal of arsenic from wastewater by using different technologies and adsorbents: A review. Int. J. Environ. Sci. Technol. 2021, 9, 9243–9266. [Google Scholar] [CrossRef]
- Pitás, V.; Somogyi, V.; Kárpáti, Á.; Thury, P.; Fráter, T. Reduction of chemical oxygen demand in a conventional activated sludge system treating coke oven wastewater. J. Clean. Prod. 2020, 273, 122482. [Google Scholar] [CrossRef]
- Mahmood, T.; Momin, S.; Ali, R.; Naeem, A.; Khan, A. Technologies for removal of emerging contaminants from wastewater. In Wastewater Treatment; IntechOpen: London, UK, 2022. [Google Scholar]
- Zheng, C.; Zhao, L.; Zhou, X.; Fu, Z.; Li, A. Treatment technologies for organic wastewater. Water Treat. 2013, 11, 250–286. [Google Scholar]
- Zhang, H.; Zhang, H.; Zhao, L.; Zhou, B.; Li, P.; Liu, B.; Wang, Y.; Yang, C.; Huang, K.; Zhang, C. Ecosystem impact and dietary exposure of polychlorinated biphenyls (PCBs) and heavy metals in Chinese mitten crabs (Eriocheir sinensis) and their farming areas in Jiangsu, China. Ecotoxicol. Environ. Saf. 2021, 227, 112936. [Google Scholar] [CrossRef] [PubMed]
- Elijah, A.A. A Review of the Petroleum Hydrocarbons Contamination of Soil, Water and Air and the Available Remediation Techniques, Taking into Consideration the Sustainable Development Goals. Earthline J. Chem. Sci. 2022, 7, 97–113. [Google Scholar] [CrossRef]
- Pandit, D.N.; Kumari, R.; Shitanshu, S.K. A comparative assessment of the status of Surajkund and Rani Pond, Aurangabad, Bihar, India using overall Index of Pollution and Water Quality Index. Acta Ecol. Sin. 2022, 42, 149–155. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Allozy, H.G.A.; Shahir, S.; Goh, P.S.; Yunus, M.A.M. A novel nanocomposite of aminated silica nanotube (MWCNT/Si/NH 2) and its potential on adsorption of nitrite. Environ. Sci. Pollut. Res. 2019, 26, 28737–28748. [Google Scholar] [CrossRef]
- Wasewar, K.L. Process intensification in wastewater treatments: Basics of process intensification and inorganic pollutants. In Contamination of Water; Elsevier: Amsterdam, The Netherlands, 2021; pp. 313–337. [Google Scholar]
- Ankodia, V. Water Pollution. In Contemporary Global Issues and Challenges; Sunrise Publisher: Jaipur, India, 2021; 231p. [Google Scholar]
- Altowayti, W.A.H.; Othman, N.; Goh, P.S.; Alshalif, A.F.; Al-Gheethi, A.A.; Algaifi, H.A. Application of a novel nanocomposites carbon nanotubes functionalized with mesoporous silica-nitrenium ions (CNT-MS-N) in nitrate removal: Optimizations and nonlinear and linear regression analysis. Environ. Technol. Innov. 2021, 22, 101428. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Sulivan Jouanneau, A.A.; Durand, M.-J.; Thouand, G. Detection and Effects of Metal and Organometallic Compounds with Microbial Bioluminescence and Raman Spectroscopy. In Handbook of Cell Biosensors; Springer: Berlin/Heidelberg, Germany, 2022; p. 825. [Google Scholar]
- Haris, S.A.; Altowayti, W.A.H.; Ibrahim, Z.; Shahir, S. Arsenic biosorption using pretreated biomass of psychrotolerant Yersinia sp. strain SOM-12D3 isolated from Svalbard, Arctic. Environ. Sci. Pollut. Res. 2018, 25, 27959–27970. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Nachana’a Timothy, E.T.W. Environmental pollution by heavy metal: An overview. Chemistry 2019, 3, 72–82. [Google Scholar]
- Izah, S.C.; Ngun, C.T.; Richard, G. Microbial quality of groundwater in the Niger Delta region of Nigeria: Health implications and effective⇓ treatment technologies. In Current Directions in Water Scarcity Research; Elsevier: Amsterdam, The Netherlands, 2022; Volume 6, pp. 149–172. [Google Scholar]
- Sekyere, J.O.; Faife, S.L. Pathogens, Virulence and Resistance Genes Surveillance with Metagenomics Can Pre-Empt Dissemination and Escalation of Untreatable Infections: A Systematic Review and Meta-Analyses. bioRxiv 2021. [Google Scholar] [CrossRef]
- Devane, M.; Moriarty, E.; Weaver, L.; Cookson, A.; Gilpin, B. Fecal indicator bacteria from environmental sources; strategies for identification to improve water quality monitoring. Water Res. 2020, 185, 116204. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Zhong, C.; Martin, J.W.; Alessi, D.S.; Goss, G.G.; Ren, Y.; He, Y. Suspended solids-associated toxicity of hydraulic fracturing flowback and produced water on early life stages of zebrafish (Danio rerio). Environ. Pollut. 2021, 287, 117614. [Google Scholar] [CrossRef] [PubMed]
- Turjja, S.R. Controlling the Contamination: Preventing Environmental Impacts of Combined Sewage Overflows in NYC. Bachelor’s Thesis, Fordham University, New York, NY, USA, 2022. [Google Scholar]
- Hongyang, X.; Pedret, C.; Santin, I.; Vilanova, R. Decentralized model predictive control for N and P removal in wastewater treatment plants. In Proceedings of the 2018 22nd International Conference on System Theory, Control and Computing (ICSTCC), IEEE, Sinaia, Romania, 10–12 October 2018; pp. 224–230. [Google Scholar]
- Singh, N.; Poonia, T.; Siwal, S.S.; Srivastav, A.L.; Sharma, H.K.; Mittal, S.K. Challenges of water contamination in urban areas. In Current Directions in Water Scarcity Research; Elsevier: Amsterdam, The Netherlands, 2022; Volume 6, pp. 173–202. [Google Scholar]
- Arjen, Y.; Hoekstra, J.B.; van Ginkel, K.C.H. Urban water security: A review. Environ. Res. Lett. 2018, 13, 053002. [Google Scholar]
- Angelevska, B.; Atanasova, V.; Andreevski, I. Urban air quality guidance based on measures categorization in road transport. Civ. Eng. J. 2021, 7, 253–267. [Google Scholar] [CrossRef]
- Alexandra Muller, H.O.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Toatal Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Viktor, Z.; Anatolii, L.; Olha, Z.; Svetlana, M. Conceptual Principles of Reengineering of Agricultural Resources: Open Problems, Challenges and Future Trends. In The Digital Agricultural Revolution: Innovations and Challenges in Agriculture through Technology Disruptions; Wiley: Hoboken, NJ, USA, 2022; pp. 269–287. [Google Scholar]
- Evans, A.E.V.; Sagasta, J.M.-S.; Qadir, M.; Boelee, E.; Ippolito, A. Agriculture water pollution: Key knowledge gaps and research needs. Environ. Sustain. 2019, 36, 20–27. [Google Scholar]
- El-Sheekh, M.; Abdel-Daim, M.M.; Okba, M.; Gharib, S.; Soliman, A.; El-Kassas, H. Green technology for bioremediation of the eutrophication phenomenon in aquatic ecosystems: A review. Afr. J. Aquat. Sci. 2021, 46, 274–292. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Gojenko, B.; Yu, J.; Wei, L.; Lio, D.; Xiao, T. A review of water pollution arising from agriculture and mining activities in Central Asia: Facts, causes and effects. Environ. Pollut. 2021, 291, 118209. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Jiao, G.; Wang, J.; Li, J.; Li, M.; Jiang, H. Spatial Pattern of Technological Innovation in the Yangtze River Delta Region and Its Impact on Water Pollution. Int. J. Environ. Res. Public Health 2022, 19, 7437. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, J.; Zhou, N.; Zhang, T.; Zeng, H. Does the “10-Point Water Plan” reduce the intensity of industrial water pollution> Quasi-experimental evidence from China. J. Environ. Manag. 2021, 295, 113048. [Google Scholar] [CrossRef] [PubMed]
- Mroue, A.M.; Obkirchner, G.; Dargin, J.; Muell, J. Water-Energy Nexus: The Role of Hydraulic Fracturing. In Regulating Water Security in Unconventional Oil and Gas; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–38. [Google Scholar]
- Jafarinejad, S. Environmental impacts of the petroleum industry, protection options, and Regulations. Pet. Waste Treat. Pollut. Control. 2017, 85–116. [Google Scholar]
- Towne, W.W.; Tsivoglou, E.C. Sources and Control of Radioactive Water Pollutants. Sew. Ind. Wastes 1957, 29, 143–156. [Google Scholar]
- Bonavigo, L.; Zucchetti, M.; Mankolli, H. Water Radioactive Pollution and Related Environmental Aspects. J. Int. Environ. Appl. Sci. 2009, 4, 357–363. [Google Scholar]
- Reddy, A.S.; Nair, A.T. The fate of microplastics in wastewater treatment plants: An overview of source and remediation technologies. Environ. Technol. Innov. 2022, 28, 102815. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Zubrowska-Sudol, M.; Walczak, J.; Piechota, G. Disintegration of waste sludge as an element bio-circular economy in waste water treatment plant towards carbon recovery for biological nutrient removal. Bioresour. Technol. 2022, 360, 127622. [Google Scholar] [CrossRef]
- Cran, M.; Gray, S.; Schmidt, J.; Gao, L. Root cause analysis for membrane system validation failure at a full-scale recycled water treatment plant. Desalination 2022, 523, 115405. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Singh, S.; Kansal, S.K. Process intensification of treatment of inorganic water pollutants. In Inorganic Pollutants in Water; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–271. [Google Scholar]
- Asharuddin, S.M.; Othman, N.; Altowayti, W.A.H.; Bakar, N.A.; Hassan, A. Recent advancement in starch modification and its application as water treatment agent. Environ. Technol. Innov. 2021, 23, 101637. [Google Scholar] [CrossRef]
- Ayob, S.; Othman, N.; Altowayti, W.A.H.; Khalid, F.S.; Bakar, N.A.; Tahir, M.; Soedjono, E.S. A review on adsorption of heavy metals from wood-industrial wastewater by oil palm waste. J. Ecol. Eng. 2021, 22, 249–265. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; González-García, M.B.; Costa-García, A. Electrochemical determination of mercury: A review. Talanta 2013, 116, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Chaemiso, T.D.; Nefo, T. Removal methods of heavy metals from laboratory wastewater. J. Nat. Sci. Res. 2019, 9, 36–42. [Google Scholar]
- Birkett, J.W.; Lester, J.N. Endocrine Disrupters in Wastewater and Sludge Treatment Processes; IWA Publishing: London, UK, 2002. [Google Scholar]
- Altowayti, W.A.H.; Othman, N.; Tajarudin, H.A.; Al-Dhaqm, A.; Asharuddin, S.M.; Al-Gheethi, A.; Alshalif, A.F.; Salem, A.A.; Din, M.F.M.; Fitriani, N. Evaluating the pressure and loss behavior in water pipes using smart mathematical modelling. Water 2021, 13, 3500. [Google Scholar] [CrossRef]
- Lieser, K. Steps in precipitation reactions. Angew. Chem. Int. Ed. Engl. 1969, 8, 188–202. [Google Scholar] [CrossRef]
- Pankratz, T.M. Screening Equipment Handbook: For Industrial and Municipal Water and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lin, C.-C.; Wu, J.-M. A Novel Centrifugal Filtration Device. Separations 2022, 9, 129. [Google Scholar] [CrossRef]
- Amran, N.A.; Mustapha, S.N.A. Oil–Water Separation Techniques for Bilge Water Treatment. In Resources of Water; IntechOpen: London, UK, 2020. [Google Scholar]
- Zakaria, S.N.F.; Aziz, H.A.; Mohamad, M. Comparison Performance of Coagulation Flocculation Process and Combination with Ozonation Process of Stabilized Landfill Leachate Treatment. Water Environ. Res. 2022, 94, e10770. [Google Scholar] [CrossRef]
- Eng, L.Z.; Loo, K.P. Microwave-assisted extraction of banana peel bio-flocculant and its potential in wastewater treatment. Glob. J. Eng. Technol. Adv. 2019, 1, 001–009. [Google Scholar]
- Badawi, A.K.; Ismail, B.; Baaloudj, O.; Abdalla, K.Z. Advanced wastewater treatment process using algal photo-bioreactor associated with dissolved-air flotation system: A pilot-scale demonstration. J. Water Process Eng. 2022, 46, 102565. [Google Scholar] [CrossRef]
- Hamidi, S.; Banaee, M.; Pourkhabbaz, H.R.; Sureda, A.; Khodadoust, S.; Pourkhabbaz, A.R. Effect of petroleum wastewater treated with gravity separation and magnetite nanoparticles adsorption methods on the blood biochemical response of mrigal fish (Cirrhinus cirrhosus). Environ. Sci. Pollut. Res. 2022, 29, 3718–3732. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Nguyen, P.; Carvalho, G.; Reis, M.A.; Oehmen, A. A review of the biotransformations of priority pharmaceuticals in biological wastewater treatment processes. Water Res. 2021, 188, 116446. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- He, Z.-W.; Yang, W.-J.; Ren, Y.-X.; Jin, H.-Y.; Tang, C.-C.; Liu, W.-Z.; Yang, C.-X.; Zhou, A.-J.; Wang, A.-J. Occurrence, effect, and fate of residual microplastics in anaerobic digestion of waste activated sludge: A state-of-the-art review. Bioresour. Technol. 2021, 331, 125035. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Almeida-Naranjo, C.E.; Villamar Ayala, C.A. Improvement of nutrients removal from domestic wastewater by activated-sludge encapsulation with polyvinyl alcohol (PVA). J. Environ. Sci. Health Part A 2019, 54, 721–727. [Google Scholar] [CrossRef]
- Wu, C.P. Ammonia Wastewater Treatment by Immobilized Activated Sludge. Bachelor’s Thesis, Worcester Polytechnic Institute, Shanghai Jiao Tong University, Shanghai, China, 2010. [Google Scholar]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. Rsc Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Ahmed, S.; Dhingra, A.; Singh, C.P.; Khan, S.U.; Mohammadi, A.A.; Changani, F.; Yousefi, M.; Alam, S. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020, 269, 122411. [Google Scholar] [CrossRef]
- Guadarrama-Pérez, O.; Gutiérrez-Macías, T.; García-Sánchez, L.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: A review. Int. J. Energy Res. 2019, 43, 5106–5127. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Bakar, N.A.; Othman, N.; Yunus, Z.M.; Altowayti, W.A.H.; Tahir, M.; Fitriani, N.; Mohd-Salleh, S.N.A. An insight review of lignocellulosic materials as activated carbon precursor for textile wastewater treatment. Environ. Technol. Innov. 2021, 22, 101445. [Google Scholar] [CrossRef]
- Samer, M. Biological and chemical wastewater treatment processes. Wastewater Treat. Eng. 2015, 150, 212. [Google Scholar]
- Yang, S.; Guo, B.; Shao, Y.; Mohammed, A.; Vincent, S.; Ashbolt, N.J.; Liu, Y. The value of floc and biofilm bacteria for anammox stability when treating ammonia-rich digester sludge thickening lagoon supernatant. Chemosphere 2019, 233, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Altowayti, W.A.H.; Othman, N.; Al-Gheethi, A.; Dzahir, N.H.b.M.; Asharuddin, S.M.; Alshalif, A.F.; Nasser, I.M.; Tajarudin, H.A.; Al-Towayti, F.A.H. Adsorption of Zn2+ from Synthetic Wastewater Using Dried Watermelon Rind (D-WMR): An Overview of Nonlinear and Linear Regression and Error Analysis. Molecules 2021, 26, 6176. [Google Scholar] [PubMed]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut. Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Swanckaert, B.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. A review on ion-exchange nanofiber membranes: Properties, structure and application in electrochemical (waste) water treatment. Sep. Purif. Technol. 2022, 287, 120529. [Google Scholar] [CrossRef]
- Singh, R.; Hankins, N. Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cadee, K.; O’Leary, B.; Smith, P.; Slunjski, M.; Bourke, M. World’s first magnetic ion exchange (MIEX®) water treatment plant to be installed in Western Australia. In Proceedings of the American Water Works Association Conference, Denver, CO, USA, 11–15 June 2020; pp. 11–15. [Google Scholar]
- Sales, M.G.F.; Delerue-Matos, C.; Martins, I.; Serra, I.; Silva, M.; Morais, S. A waste management school approach towards sustainability. Resour. Conserv. Recycl. 2006, 48, 197–207. [Google Scholar] [CrossRef]
- Nqombolo, A.; Mpupa, A.; Moutloali, R.M.; Nomngongo, P.N. Wastewater treatment using membrane technology. In Wastewater Water Quality; InTechOpen: London, UK, 2018; p. 29. [Google Scholar]
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Aldana, J.C.; Acero, J.L.; Álvarez, P.M. Membrane filtration, activated sludge and solar photocatalytic technologies for the effective treatment of table olive processing wastewater. J. Environ. Chem. Eng. 2021, 9, 105743. [Google Scholar] [CrossRef]
- Kim, I.; Choi, D.-C.; Lee, J.; Chae, H.-R.; Jang, J.H.; Lee, C.-H.; Park, P.-K.; Won, Y.-J. Preparation and application of patterned hollow-fiber membranes to membrane bioreactor for wastewater treatment. J. Membr. Sci. 2015, 490, 190–196. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Li, X.; Hua, Z.; Wang, X.; Ren, Y. Self-generated electric field to suppress sludge production and fouling development in a membrane bioreactor for wastewater treatment. Chemosphere 2020, 261, 128046. [Google Scholar] [CrossRef] [PubMed]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: Membrane fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Anjoo Anna, S.J.; Sheela, A.M. Review of Modern Technologies in Biological Wastewater Treatment. Int. J. Sci. Res. 2018, 9, 1380–1393. [Google Scholar] [CrossRef]
- Fudge, T.; Bulmer, I.; Bowman, K.; Pathmakanthan, S.; Gambier, W.; Dehouche, Z.; Al-Salem, S.M.; Constantinou, A. Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps. Water 2021, 13, 445. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpää, M. A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, A. Simultaneous removal of nitrate and hardness ions from groundwater using electrodeionization. Sep. Purif. Technol. 2016, 164, 107–113. [Google Scholar] [CrossRef]
- Sosa-Fernandez, P.; Post, J.; Bruning, H.; Leermakers, F.; Rijnaarts, H. Electrodialysis-based desalination and reuse of sea and brackish polymer-flooding produced water. Desalination 2018, 447, 120–132. [Google Scholar] [CrossRef]
- Pan, Y.; Froese, F.; Liu, N.; Hu, Y.; Ye, M. The adoption of artificial intelligence in employee recruitment: The influence of contextual factors. Int. J. Hum. Resour. Manag. 2021, 33, 1125–1147. [Google Scholar] [CrossRef]
- Malviya, A.; Jaspal, D. Artificial intelligence as an upcoming technology in wastewater treatment: A comprehensive review. Environ. Technol. Rev. 2021, 10, 177–187. [Google Scholar] [CrossRef]
- Nourani, V.; Elkiran, G.; Abba, S. Wastewater treatment plant performance analysis using artificial intelligence—An ensemble approach. Water Sci. Technol. 2018, 78, 2064–2076. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, S. Machine-learning insights into nitrate-reducing communities in a full-scale municipal wastewater treatment plant. J. Environ. Manag. 2021, 300, 113795. [Google Scholar] [CrossRef] [PubMed]
- Hernández-del-Olmo, F.; Gaudioso, E.; Duro, N.; Dormido, R. Machine learning weather soft-sensor for advanced control of wastewater treatment plants. Sensors 2019, 19, 3139. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Nam, K.; Tariq, S.; Lim, J.Y.; Park, J.; Yoo, C. A hybrid machine learning–based multi-objective supervisory control strategy of a full-scale wastewater treatment for cost-effective and sustainable operation under varying influent conditions. J. Clean. Prod. 2021, 291, 125853. [Google Scholar] [CrossRef]
- Bernardelli, A.; Marsili-Libelli, S.; Manzini, A.; Stancari, S.; Tardini, G.; Montanari, D.; Anceschi, G.; Gelli, P.; Venier, S. Real-time model predictive control of a wastewater treatment plant based on machine learning. Water Sci. Technol. 2020, 81, 2391–2400. [Google Scholar] [CrossRef]
- Elkiran, G.; Nourani, V.; Abba, S. Multi-step ahead modelling of river water quality parameters using ensemble artificial intelligence-based approach. J. Hydrol. 2019, 577, 123962. [Google Scholar] [CrossRef]
- Yu, P.; Cao, J.; Jegatheesan, V.; Du, X. A real-time BOD estimation method in wastewater treatment process based on an optimized extreme learning machine. Appl. Sci. 2019, 9, 523. [Google Scholar] [CrossRef]
- Khademikia, S.; Haghizadeh, A.; Godini, H.; Shams, K.G. The performance evaluation of Khorramabad wastewater treatment plant by using artificial intelligence network. Yafte 2016, 18, 12–23. [Google Scholar]
- Zhu, J.-J.; Kang, L.; Anderson, P.R. Predicting influent biochemical oxygen demand: Balancing energy demand and risk management. Water Res. 2018, 128, 304–313. [Google Scholar] [CrossRef]
- Kang, H.; Yang, S.; Huang, J.; Oh, J. Time series prediction of wastewater flow rate by bidirectional LSTM deep learning. Int. J. Control Autom. Syst. 2020, 18, 3023–3030. [Google Scholar] [CrossRef]
- Mateo Pérez, V.; Mesa Fernández, J.M.; Villanueva Balsera, J.; Alonso Álvarez, C. A Random Forest Model for the Prediction of FOG Content in Inlet Wastewater from Urban WWTPs. Water 2021, 13, 1237. [Google Scholar] [CrossRef]
- Oliveira-Esquerre, K.P.; Seborg, D.E.; Mori, M.; Bruns, R.E. Application of steady-state and dynamic modeling for the prediction of the BOD of an aerated lagoon at a pulp and paper mill: Part II. Nonlinear approaches. Chem. Eng. J. 2004, 105, 61–69. [Google Scholar] [CrossRef]
- Akratos, C.S.; Papaspyros, J.N.; Tsihrintzis, V.A. An artificial neural network model and design equations for BOD and COD removal prediction in horizontal subsurface flow constructed wetlands. Chem. Eng. J. 2008, 143, 96–110. [Google Scholar] [CrossRef]
- Hamed, M.M.; Khalafallah, M.G.; Hassanien, E.A. Prediction of wastewater treatment plant performance using artificial neural networks. Environ. Model. Softw. 2004, 19, 919–928. [Google Scholar] [CrossRef]
- Talib, A.; Abu Hasan, Y.; Abdul Rahman, N. Predicting biochemical oxygen demand as indicator of river pollution using artificial neural networks. In Proceedings of the 18th World IMACS/MODSIM Congress, Citeseer, Cairns, Australia, 13–17 July 2009; pp. 13–17. [Google Scholar]
- Elmolla, E.S.; Chaudhuri, M.; Eltoukhy, M.M. The use of artificial neural network (ANN) for modeling of COD removal from antibiotic aqueous solution by the Fenton process. J. Hazard. Mater. 2010, 179, 127–134. [Google Scholar] [CrossRef]
- Nasr, M.; Ateia, M.; Hassan, K. Artificial intelligence for greywater treatment using electrocoagulation process. Sep. Sci. Technol. 2016, 51, 96–105. [Google Scholar] [CrossRef]
- Zhang, D.; Hølland, E.S.; Lindholm, G.; Ratnaweera, H. Hydraulic modeling and deep learning based flow forecasting for optimizing inter catchment wastewater transfer. J. Hydrol. 2018, 567, 792–802. [Google Scholar] [CrossRef]
- Mamandipoor, B.; Majd, M.; Sheikhalishahi, S.; Modena, C.; Osmani, V. Monitoring and detecting faults in wastewater treatment plants using deep learning. Environ. Monit. Assess. 2020, 192, 148. [Google Scholar] [CrossRef]
- Zhuang, Z.; Sun, Z.; Cheng, Y.; Yao, R.; Zhang, W. Modeling and optimization of paper-making wastewater treatment based on reinforcement learning. In Proceedings of the 2018 37th Chinese Control Conference (CCC), Wuhan, China, 25–27 July 2018; pp. 8342–8346. [Google Scholar]
- Granata, F.; Papirio, S.; Esposito, G.; Gargano, R.; De Marinis, G. Machine learning algorithms for the forecasting of wastewater quality indicators. Water 2017, 9, 105. [Google Scholar] [CrossRef]
- Barzegar, R.; Aalami, M.T.; Adamowski, J. Short-term water quality variable prediction using a hybrid CNN–LSTM deep learning model. Stoch. Environ. Res. Risk Assess. 2020, 34, 415–433. [Google Scholar] [CrossRef]
- Basu, A.; Ali, S.S.; Hossain, S.; Asif, M. A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes 2022, 10, 1154. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wu, H.; Pan, S.; Cheng, X.; Sun, Y.; Xu, Y. Effective and simultaneous removal of organic/inorganic arsenic using polymer-based hydrated iron oxide adsorbent: Capacity evaluation and mechanism. Sci. Total Environ. 2020, 742, 140508. [Google Scholar] [CrossRef]
- Das, T.K.; Bezbaruah, A.N. Comparative study of arsenic removal by iron-based nanomaterials: Potential candidates for field applications. Sci. Total Environ. 2021, 764, 142914. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Di Luca, C.; Mori, F.; Pintar, A.; Žerjav, G.; Valiente, M.; Palet, C. Synthesis and adsorption behavior of mesoporous alumina and Fe-doped alumina for the removal of dominant arsenic species in contaminated waters. J. Environ. Chem. Eng. 2019, 7, 102901. [Google Scholar] [CrossRef]
- Muedi, K.; Brink, H.; Masindi, V.; Maree, J. Effective removal of arsenate from wastewater using aluminium enriched ferric oxide-hydroxide recovered from authentic acid mine drainage. J. Hazard. Mater. 2021, 414, 125491. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Tran, H.N.; Vu, H.A.; Trinh, M.V.; Nguyen, T.V.; Loganathan, P.; Vigneswaran, S.; Nguyen, T.M.; Vu, D.L.; Nguyen, T.H.H. Laterite as a low-cost adsorbent in a sustainable decentralized filtration system to remove arsenic from groundwater in Vietnam. Sci. Total Environ. 2020, 699, 134267. [Google Scholar] [CrossRef]
- Murugesan, G.; Sathishkumar, M.; Swaminathan, K. Arsenic removal from groundwater by pretreated waste tea fungal biomass. Bioresour. Technol. 2006, 97, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Sinha, T.J.M.; Srivastava, S. Functionalized nanocrystalline cellulose: Smart biosorbent for decontamination of arsenic. Int. J. Miner. Processing 2015, 139, 51–63. [Google Scholar] [CrossRef]
- Bahari, Z.M.; Altowayti, W.A.H.; Ibrahim, Z.; Jaafar, J.; Shahir, S. Biosorption of As (III) by non-living biomass of an arsenic-hypertolerant Bacillus cereus strain SZ2 isolated from a gold mining environment: Equilibrium and kinetic study. Appl. Biochem. Biotechnol. 2013, 171, 2247–2261. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Khamesy, S.; Hamidian, A.; Atghia, O. Identification of the fungi absorbing heavy metals isolated from waste deposits of zinc factories. Mycol. Iran. 2016, 3, 65–73. [Google Scholar]
- Allozy, H.G.A.; Abd Karim, K.J. Removal of copper ions from aqueous solutions using poly (vinylbenzyl chloride). Malays. J. Anal. Sci. 2020, 24, 978–991. [Google Scholar]

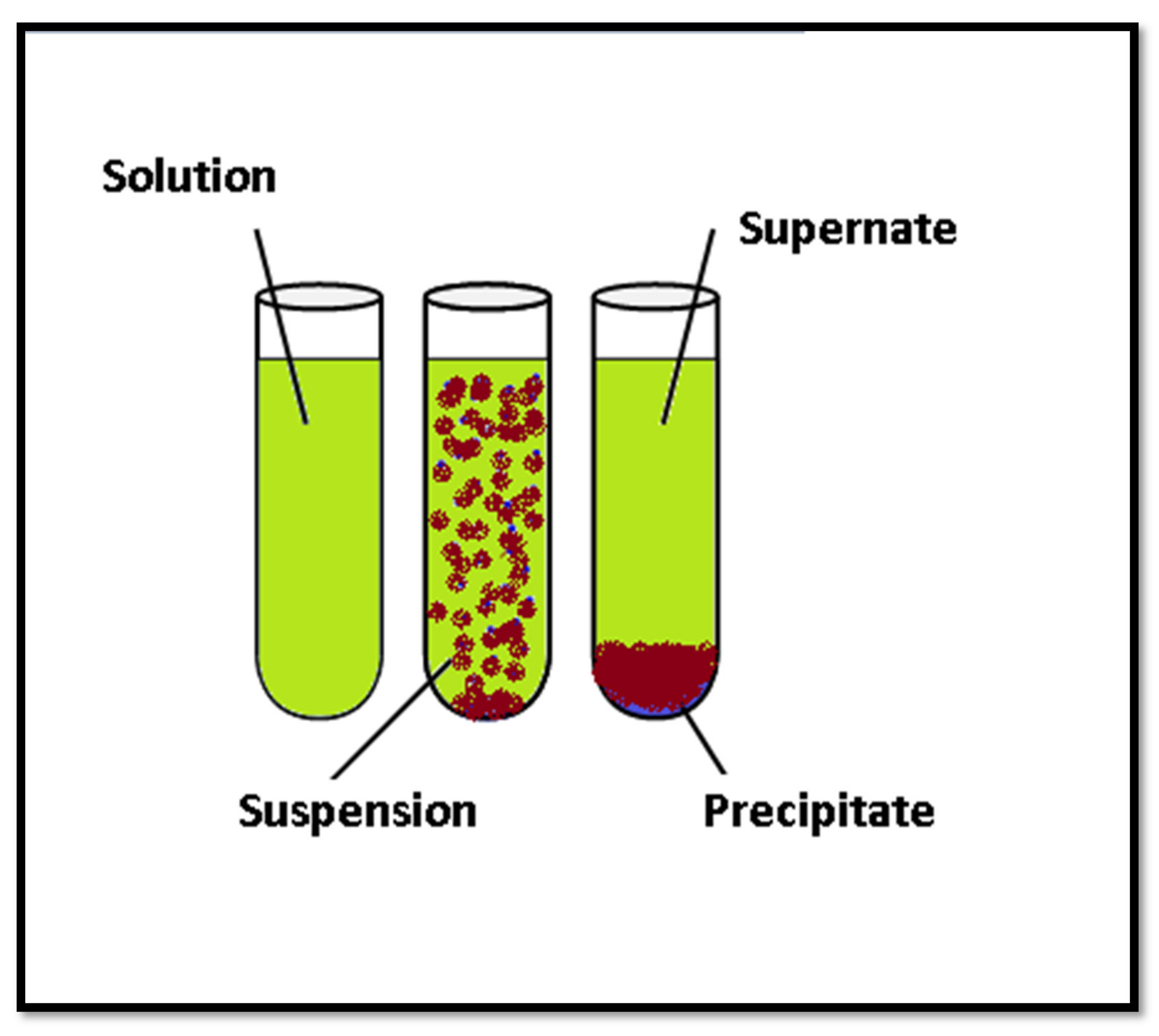


| Physical | Chemical | Biological |
|---|---|---|
| Solids Temperature Colour Odor | Organic substances include proteins, sugars, lipids, surfactants, phenols, and insecticides. Inorganics: heavy metals, hazardous substances, alkalinity, nitrogen, phosphorus, and pH. Gases: Oxygen, hydrogen sulfide, methane | Plants Animals Viruses |
| Heavy Metal | Main Sources | Main Organ and System Affected |
|---|---|---|
| Lead (Pb) | Lead-based batteries, leaded gasoline, alloys, cable sheathing pigments, rust inhibitors, ammunition, glazes, and plastic stabilizers. | Bones, liver, kidneys, brain, lungs, spleen. Immunological, hematological, cardiovascular, reproductive system. |
| Arsenic (As) | Electronics and glass production. | Skin, lungs, brain, kidneys. Metabolic, cardiovascular, immunological, and endocrine systems. |
| Copper (Cu) | Corroded plumbing systems, electronic and cables industry. | Liver, brain, kidneys, cornea. Gastrointestinal, lungs, immunological, hematological system. |
| Zinc (Zn) | Brass coating, rubber products, some cosmetics, and aerosol deodorants. | Stomach cramps, skin irritations, vomiting, nausea, anemia, and convulsions. |
| Chromium (Cr) | Steel and pulp mills and tanneries. | Skin, lungs, kidneys, liver, brain, pancreas. Tastes, gastrointestinal, reproductive system |
| Cadmium (Cd) | Batteries, paints, steel industry, plastic industries, metal refineries, and corroded galvanized pipes. | Bones, liver, kidneys, lungs, testes, brain. Immunological, cardiovascular system. |
| Mercury (Hg) | Electrolytic production of chlorine and caustic soda, runoff from landfills and agriculture, electrical appliances, Industrial and control instruments, laboratory apparatus, and refineries. | Brain, lungs, kidneys, liver. Immunological, cardiovascular, endocrine, and reproductive systems. |
| Nickel (Ni) | Manufacturing of nickel alloys and stainless steel. | Kidney, pulmonary fibrosis, gastrointestinal problems, lung, and skin. |
| Primary | Secondary | Tertiary | Sludge Treatment and Disposal |
|---|---|---|---|
| -Screening -Centrifugal separation -Coagulation & flocculation -Floatation -Sedimentation, gravity separation | -Aerobic -Anaerobic -Encapsulation | -Oxidation/Advanced oxidation -Adsorption -Micro and ultra-filtration -Membrane bioreactor (MBR) -Reverse osmosis -Electrolysis -Electrodialysis -Precipitation -Distillation -Ion exchange -Crystallization -Evaporation -Solvent extraction | -Thickening -Digestion -Dewatering -Disposal |
| Method | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Chemical precipitation | Simple/Inexpensive The majority of metals can be eliminated. | Significant sludge production led to disposal issues | [20] |
| Chemical coagulation | Dewatering and Sludge settling | High price, large chemical use | [55] |
| Ion—exchange | high rate of material regeneration a metal-specific | High price fewer metal ions are eliminated | [56] |
| Electrochemical Method | a metal-specific No chemical consumption Pure metals are attainable. | capital coat high high ongoing expenses the pH of the starting solution and current density | [57] |
| Adsorption Using activated carbon | Most metals are easily removed. high effectiveness (99%) | Activated carbon price lacking regeneration Performance is influenced by the adsorbent | [23] |
| Using natural zeolite | Metals may generally be removed. reasonably affordable materials | low effectiveness | [58] |
| Membrane process and ultrafiltration | created a less solid waste less use of chemicals high effectiveness (>95% for a single metal) | high start-up and operating costs minimal flow rates Removal (9%) falls off when additional metals are present. | [13] |
| Methods | Adsorption Conditions | Heavy Metal | Removal Capacity | Reference | |||
|---|---|---|---|---|---|---|---|
| Concentration | Temperature °C | pH | Dosage | ||||
| Iron oxide/nano-porous carbon magnetic composite | 5 mg/L | Room | 8 | 1.8 g/L | As (III) | 6.69 mg/g | [127] |
| Polymer-based hydrated iron oxide adsorbent | 50 mg/L | Room | 7 | 100 mg/L | As (V) | 71.5 mg/g | [128] |
| Graphene oxide supported nanoscale zero-valent iron | 5 mg/L | 22 ± 2 | 3–9 | 0.43 | As (III) | 36 mg/g | [129] |
| γ-Al2O3 | 100 | 25 | 4 | 0.5 | As (V) | 54 mg/g | [130] |
| Fe/AlO(OH) | 150 ppm | Room | 3 | 1 g | As (V) | 102 mg/g | [131] |
| Natural laterite from Thach That (NLTT) | 200 μg/L | 30 | 2–9 | 2.5 g | As (V) | 580 μg /g | [132] |
| Tea fungal biomass | 1.3–0.9 mg/L | 30 | 7.2 | 20 g/L | As (V) | 76% | [133] |
| Functionalized nanocrystalline cellulose | 50 mg/L | 7.5–2.5 | Room | 0.5 g/L | As (III) | 10.56 mg/g | [134] |
| B. cereus strain ZS2 | 80 μM | 30 | 7 | 0.5 g/L | As (III) | 153 mg/g | [135] |
| Yersinia sp. strain SOM-12D3) | 6.5 mg/L | 30 | 7 | 0.5 g/L | As (III) | 159 mg/g | [26] |
| Chemical precipitation (CaO) | 32 mg/L | 9–10 | Zn2+ | 99% | [136] | ||
| Membrane (RO) | 500 mg/L | Operation pressure 5 atm | Ni2+ | 99.5% | [137] | ||
| Aspergillus fumigatus (Dead) | 10 mg/L | 28 | 5 | 0.04 mg/L | Pb (II) | 102 mg/g | [138] |
| Aspergillus fumigatus (Dead) | 10 mg/L | 28 | 5 | 0.04 mg/L | Cd (II) | 120 mg/g | [138] |
| Poly(vinylbenzyl chloride) | 160 mg/L | room | 7 | 14 mg | Cu2+ | 263.15 mg/g | [139] |
| Dried Watermelon Rind | 400 mg/L | 30–40 | 8 | 1.5 mg/L | Zn2+ | 25 mg/g | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowayti, W.A.H.; Shahir, S.; Othman, N.; Eisa, T.A.E.; Yafooz, W.M.S.; Al-Dhaqm, A.; Soon, C.Y.; Yahya, I.B.; Che Rahim, N.A.N.b.; Abaker, M.; et al. The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review. Processes 2022, 10, 1832. https://doi.org/10.3390/pr10091832
Altowayti WAH, Shahir S, Othman N, Eisa TAE, Yafooz WMS, Al-Dhaqm A, Soon CY, Yahya IB, Che Rahim NANb, Abaker M, et al. The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review. Processes. 2022; 10(9):1832. https://doi.org/10.3390/pr10091832
Chicago/Turabian StyleAltowayti, Wahid Ali Hamood, Shafinaz Shahir, Norzila Othman, Taiseer Abdalla Elfadil Eisa, Wael M. S. Yafooz, Arafat Al-Dhaqm, Chan Yong Soon, Izzati Binti Yahya, Nur Anis Natasha binti Che Rahim, Mohammed Abaker, and et al. 2022. "The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review" Processes 10, no. 9: 1832. https://doi.org/10.3390/pr10091832
APA StyleAltowayti, W. A. H., Shahir, S., Othman, N., Eisa, T. A. E., Yafooz, W. M. S., Al-Dhaqm, A., Soon, C. Y., Yahya, I. B., Che Rahim, N. A. N. b., Abaker, M., & Ali, A. (2022). The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review. Processes, 10(9), 1832. https://doi.org/10.3390/pr10091832







