Characteristics of Bauxite Residue–Limestone Pellets as Feedstock for Fe and Al2O3 Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
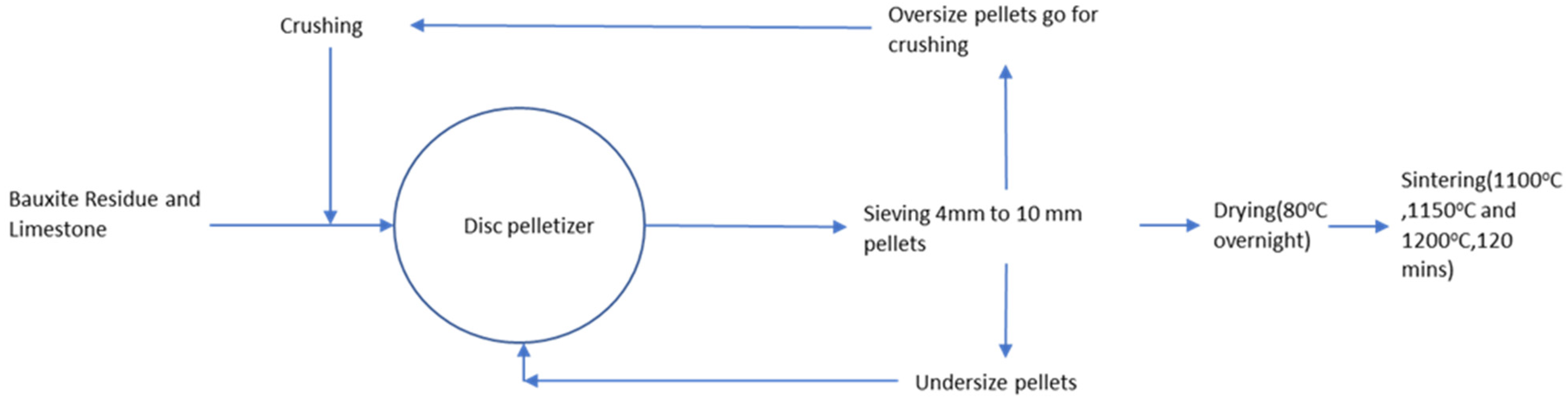
2.2. Pelletizing and Sintering
2.3. Characterization
3. Results
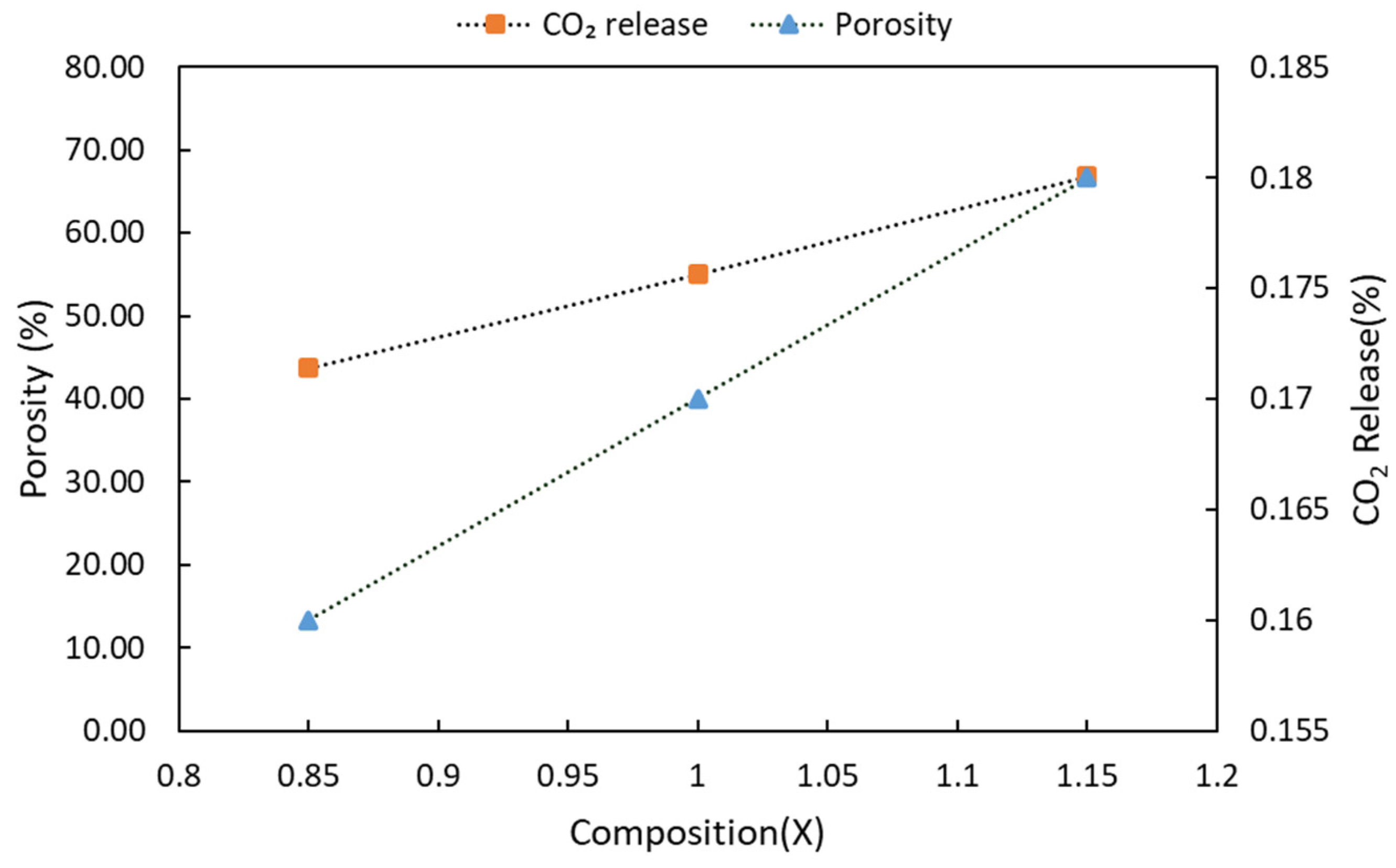
3.1. Physical Properties
3.2. Chemical Analysis
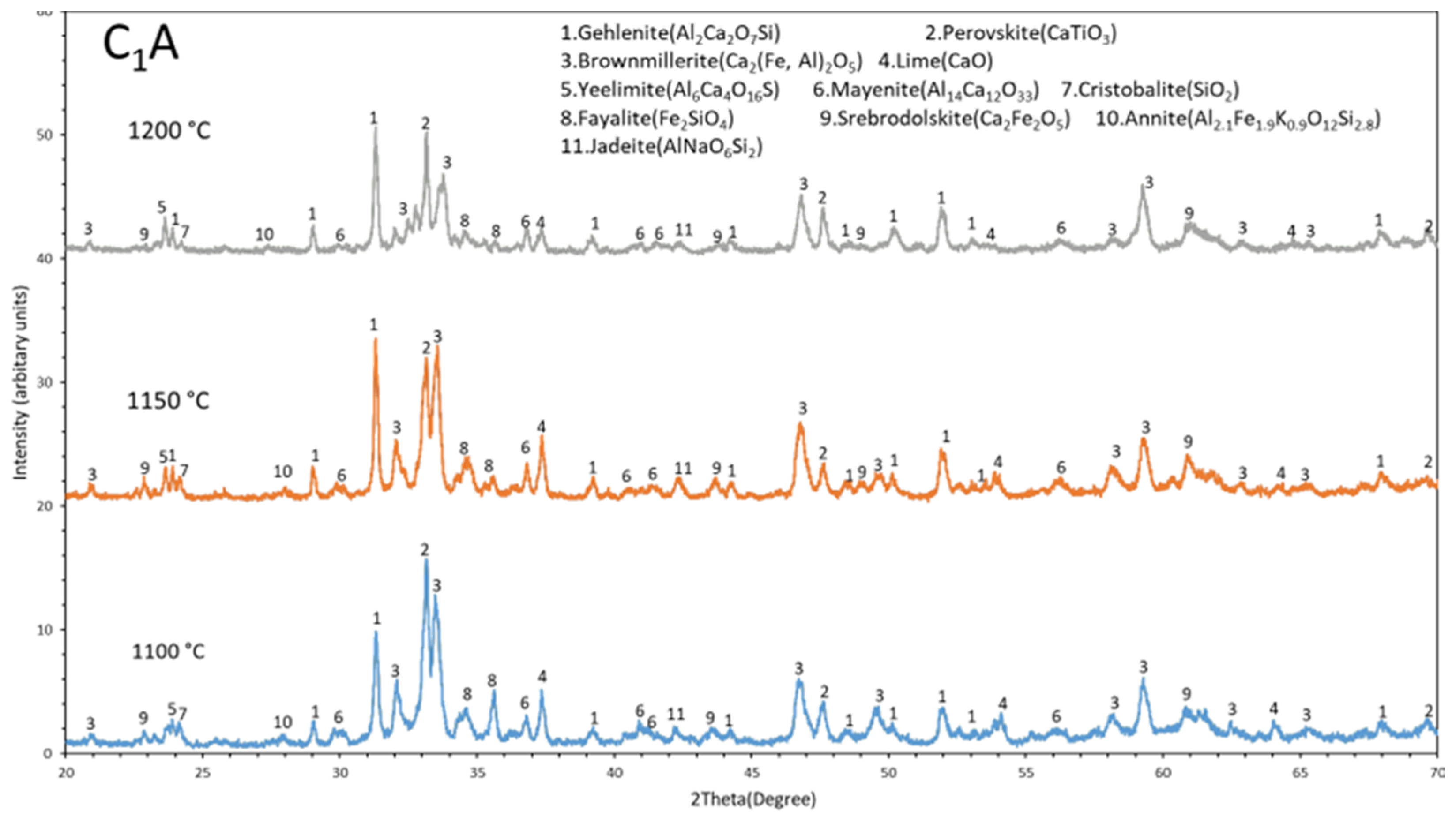
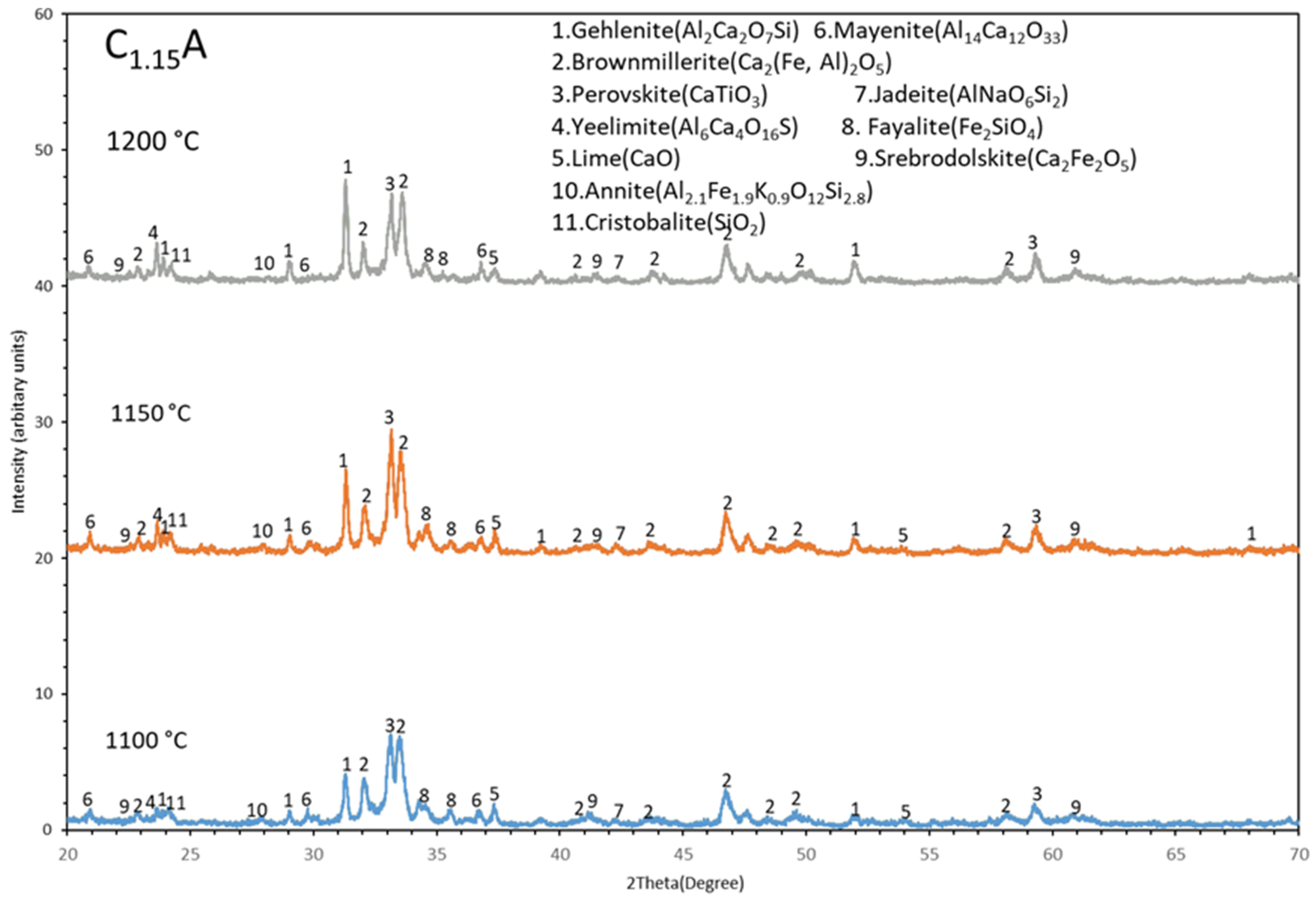
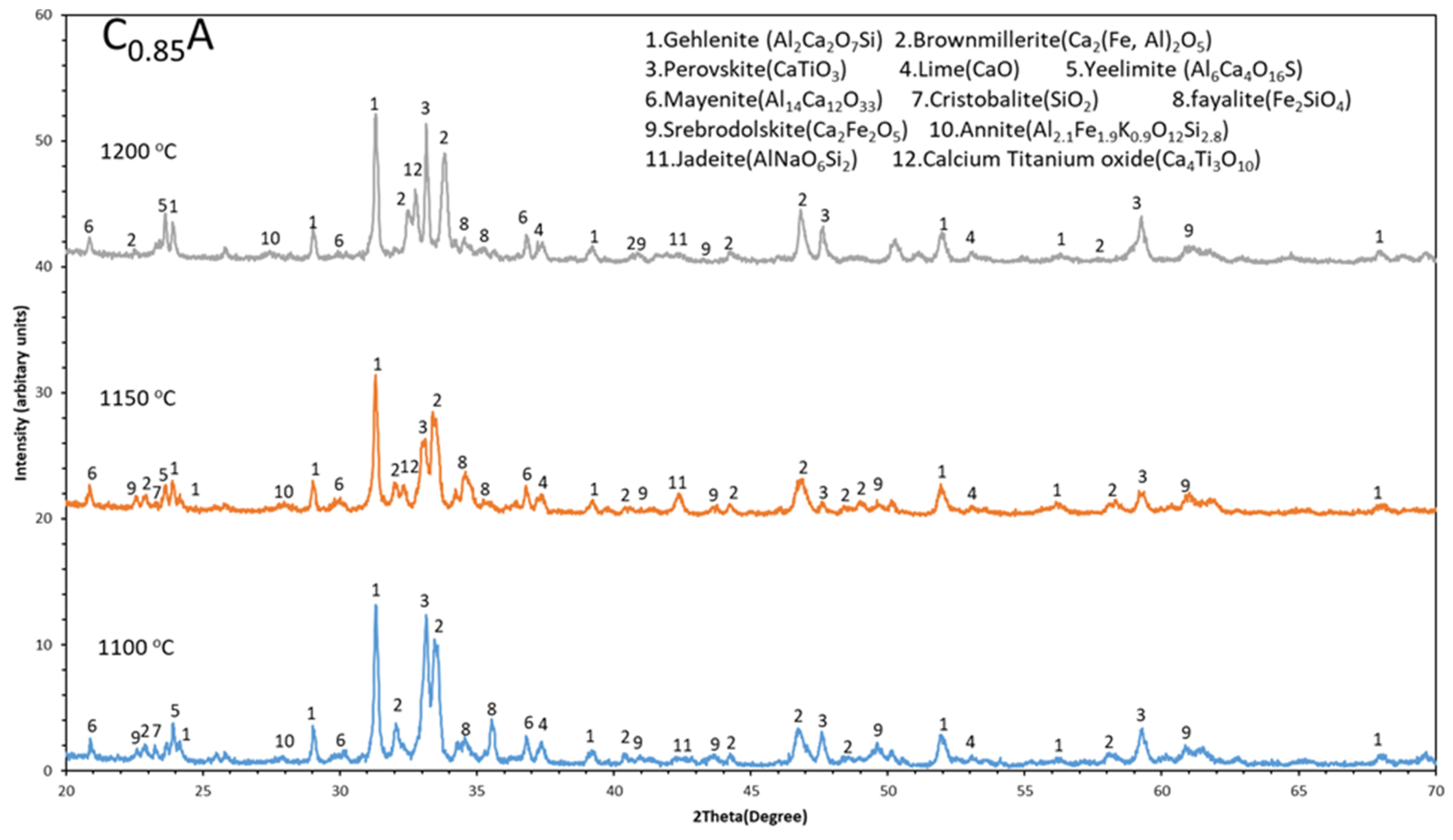
3.3. Phase Analysis (XRD)
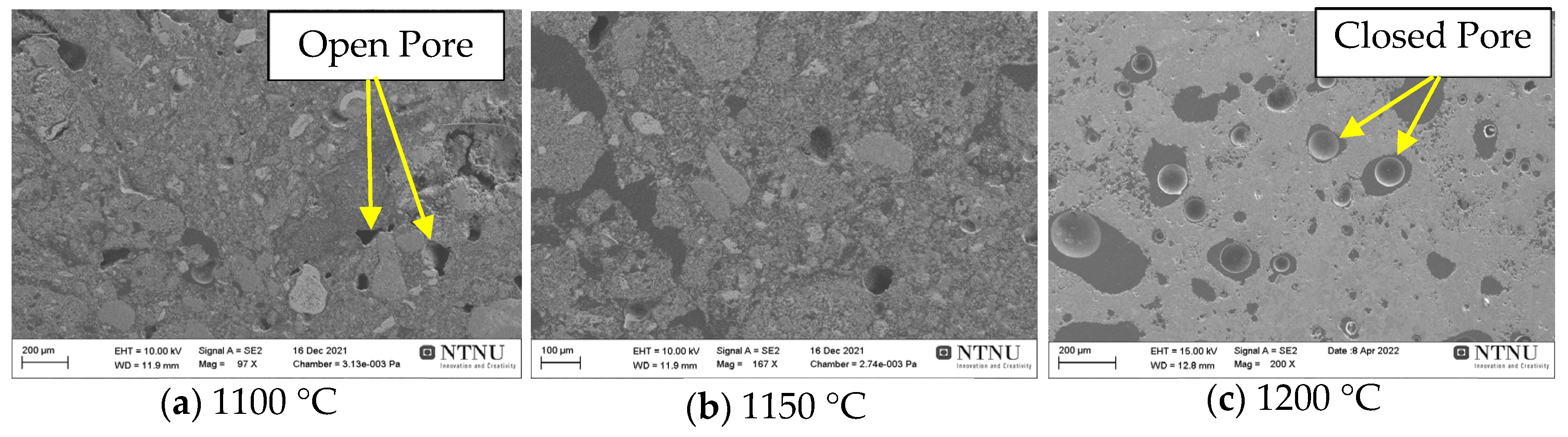
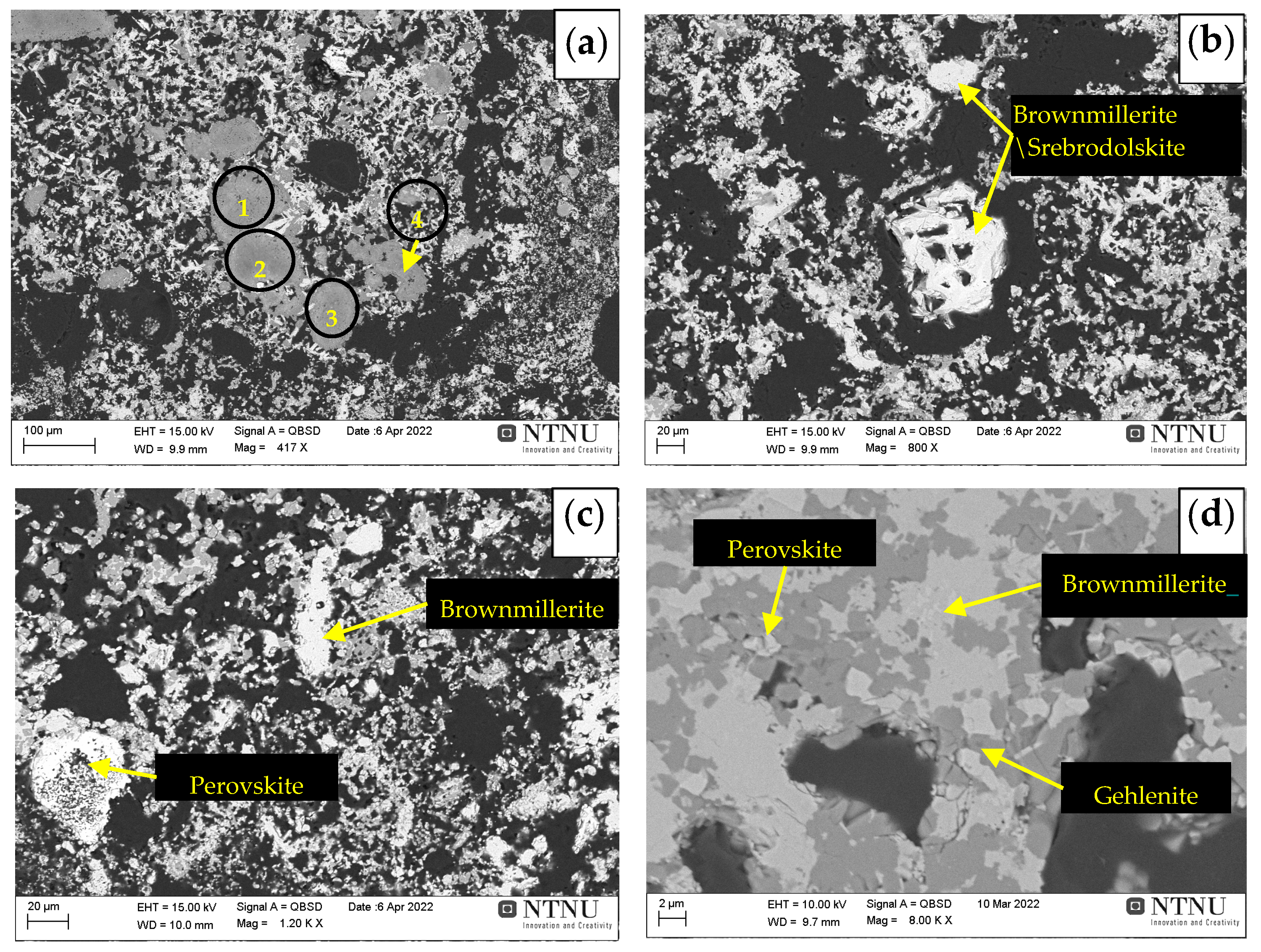
3.4. Microstructural Analysis
4. Discussion
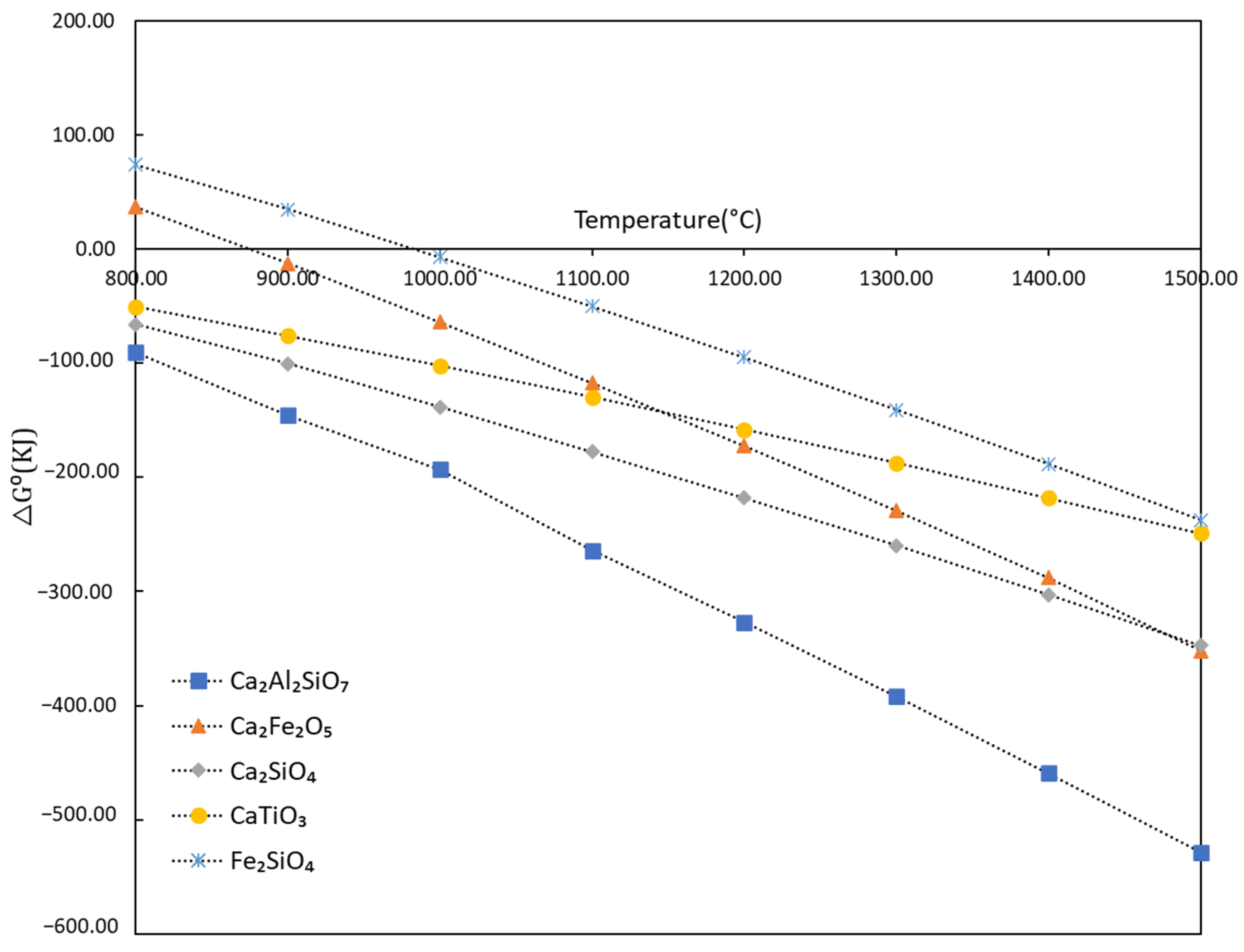
4.1. Evolution of Phases
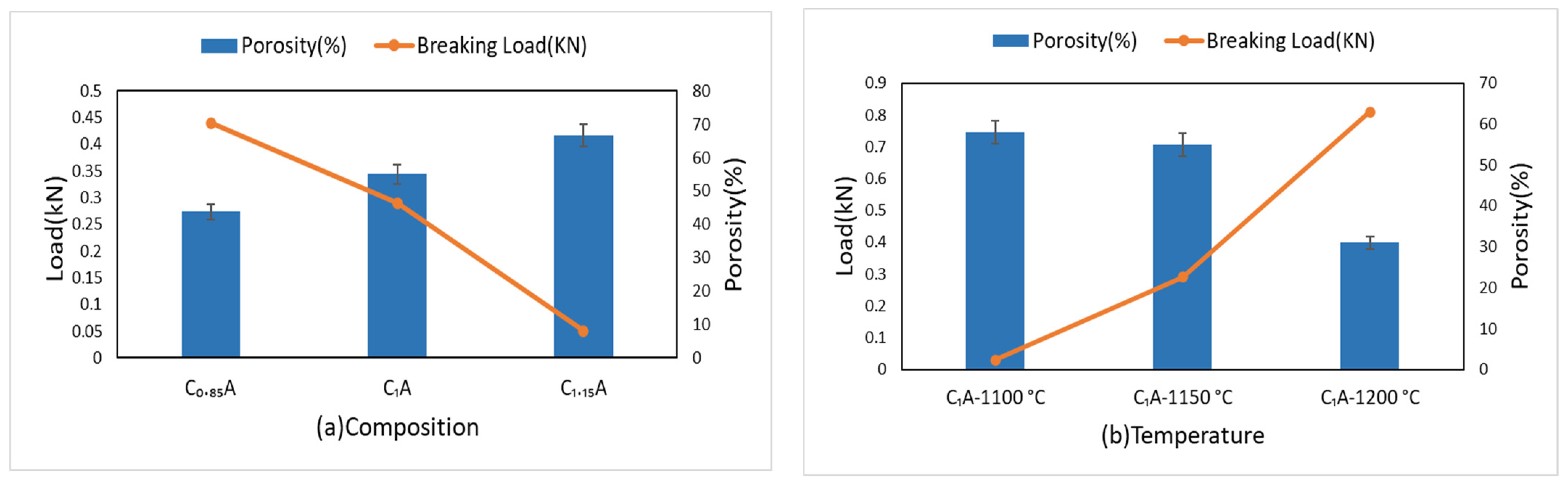
4.2. Physical Properties of Pellets
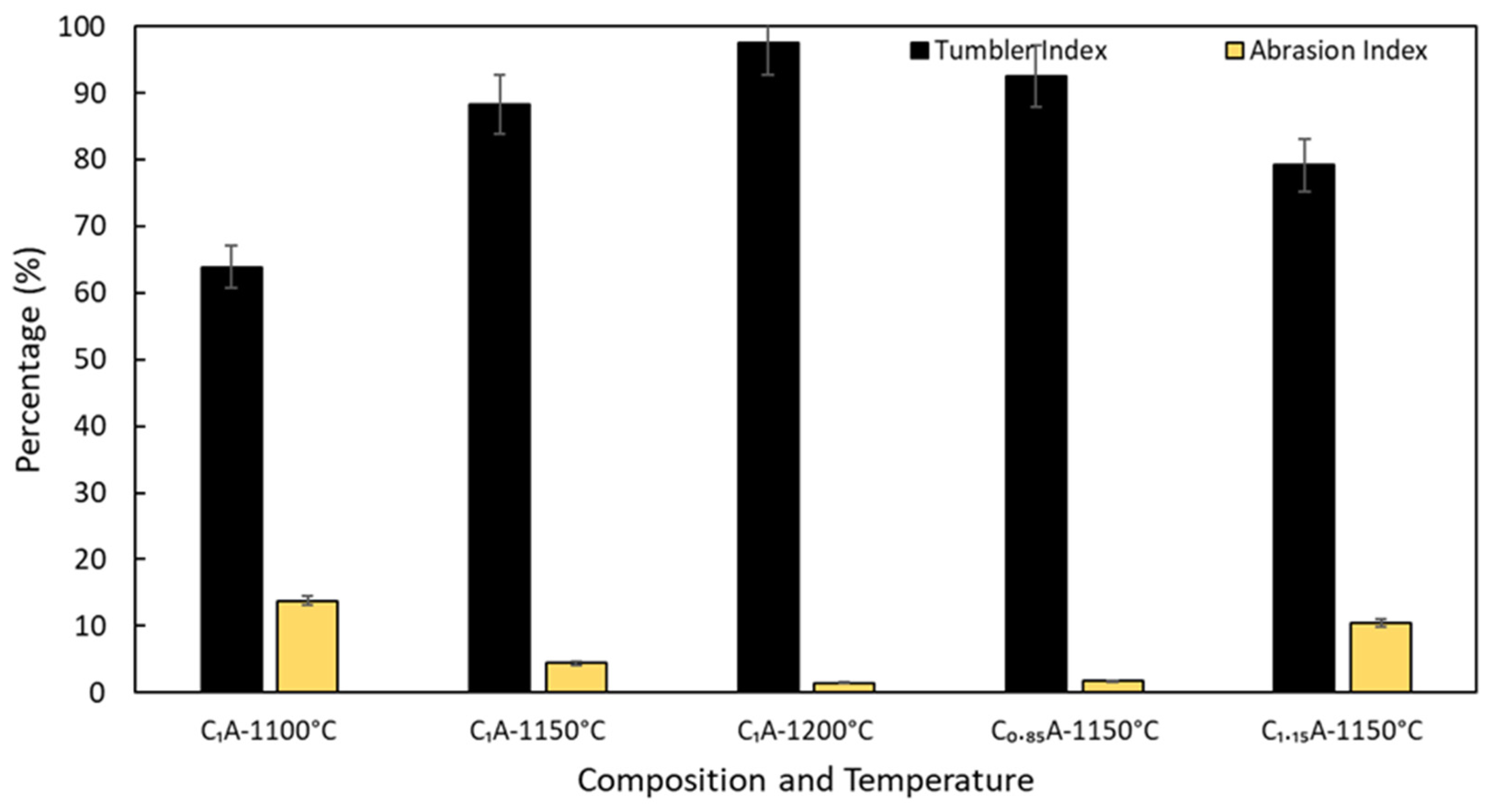
4.3. Mechanical Properties of Pellets
5. Conclusions
- Pellet density and weight loss are increased with higher sintering temperature application, which is partly explained by the decrease in BET surface area and porosity; on the other hand, BET surface area and porosity are increased with more CaCO3 addition.
- It was found that in view of porosity and pellet strength, sintering temperature of 1150 °C yields acceptable pellets with about 90% tumbler index and 55% porosity.
- Tumble and abrasion indexes are better for the pellets sintered at higher temperatures in range of 1100 °C to 1200 °C and the less CaCO3 added pellets.
- There is inverse correlation between the strength of the pellets and the porosity, with porosity decreases the strength of pellets is increased, for both varying sintering temperature in range of 1100 °C to 1200 °C and composition of pellets.
- Iron present in the sintered pellets mainly in three different phases such as brownmillerite (Ca2(Fe,Al)2O5), srebrodolskite (Ca2Fe2O5), and fayalite (Fe2SiO4). Among these brownmillerite (Ca2(Fe,Al)2O5) is the major iron containing phase.
- The Al fraction in the Ca2(Fe,Al)2O5 increases with increases in the sintering temperature in range of 1100 °C to 1200 °C, which may be due to more mass transport in sintering via high temperature diffusion.
- Gehlenite, brownmillerite, and perovskite are the major spectrums in the sintered pellets and gehlenite spectrum becomes more intense with increases in sintering temperature (1100 °C to 1200 °C).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balomenos, E.; Panias, D.; Paspaliaris, I. Energy and Exergy Analysis of the Primary Aluminum Production Processes: A Review on Current and Future Sustainability. Miner. Process. Extr. Metall. Rev. 2011, 32, 69–89. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Other Valuable Metals from Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef] [Green Version]
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Safarian, J.; Kolbeinsen, L. Sustainability in Alumina Production from Bauxite. Sustain. Ind. Process. Summit 2016, 5, 75–82. [Google Scholar]
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative Methodologies for the Utilisation of Wastes from Metallurgical and Allied Industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X. Applications of Red Mud as an Environmental Remediation Material: A Review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization Processes of Bauxite Residue: A Comprehensive Review. J. Hazard. Mater. 2021, 403, 123671. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite Residue Issues: I. Current Management, Disposal and Storage Practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Cardenia, C.; Xakalashe, B.; Balomenos, E.; Panias, D.; Friedrich, B. Reductive roasting process for the recovery of iron oxides from bauxite residue through rotary kiln furnace and magnetic separation. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; pp. 2–5. [Google Scholar]
- Yin, P.T.; Buhle Xakalashe, B.F.; Panias, D.; Vassiliadou, V. Carbothermic reduction of bauxite residue for iron recovery and subsequent aluminium recovery from slag leaching. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; pp. 603–613. [Google Scholar]
- Archambo, M.S.; Kawatra, S.K. Utilization of Bauxite Residue: Recovering Iron Values Using the Iron Nugget Process. Miner. Process. Extr. Metall. Rev. 2021, 42, 222–230. [Google Scholar] [CrossRef]
- Lazou, A.; Van Der Eijk, C.; Tang, K.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. The Utilization of Bauxite Residue with a Calcite-Rich Bauxite Ore in the Pedersen Process for Iron and Alumina Extraction. Metall. Mater. Trans. B 2021, 52, 1255–1266. [Google Scholar] [CrossRef]
- Lazou, A.; Van der Eijk, C.; Balomenos, E.; Safarian, J. Smelting Reduction of Bauxite Residue and Beneficiation By-Product in View of a Leachable Slag and Pig Iron Production. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 23–26 June 2019; pp. 17–34. [Google Scholar]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite Residue Issues: III. Alkalinity and Associated Chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Wagner, G.H. Making Concrete Articles and the Like; 1932. Available online: https://patents.google.com/patent/US1865732A/en (accessed on 22 November 2022).
- Jaquays, C. Building and Other Materials Containing Treated Bauxite Tailings and Process for Making Same. U.S. Patent 20050087107, 28 April 2005. [Google Scholar]
- Azof, F.I.; Yang, Y.; Panias, D.; Kolbeinsen, L.; Safarian, J. Leaching Characteristics and Mechanism of the Synthetic Calcium-Aluminate Slags for Alumina Recovery. Hydrometallurgy 2019, 185, 273–290. [Google Scholar] [CrossRef]
- Blake, H.E. Adaptation of the Pedersen Process to the Ferruginous Bauxites of the Pacific Northwest; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1967; p. 6939. [Google Scholar]
- Azof, F.I.; Safarian, J.; Kolbeinsen, L. The leachability of calcium aluminate phases in slags for the extraction of alumina. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017. [Google Scholar]
- ISO 3271; Iron Ores for Blast Furnace and Direct Reduction Feedstocks–Determination of the Tumble and Abrasion Indices. ISO: Geneva, Switzerland, 1995.
- Monsen, B.E.; Thomassen, E.S.; Bragstad, I.; Ringdalen, E.; Hoegaas, P.H. Characterization of DR pellets for DRI applications. In Proceedings of the Association for Iron and Steel Technology Conference Proceedings, Cleveland, OH, USA, 4–7 May 2015. [Google Scholar]
- Qu, Y.; Yang, Y.; Zou, Z.; Zeilstra, C.; Meijer, K.; Boom, R. Melting and Reduction Behaviour of Individual Fine Hematite Ore Particles. ISIJ Int. 2015, 55, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ishigaki, T. Thermodynamic Analysis of Nucleation of Anatase and Rutile from TiO2 Melt. J. Cryst. Growth 2002, 242, 511–516. [Google Scholar] [CrossRef]
- Poirier, J. 1P Lindemann Law and the Melting Temperature of Perovskites. Phys. Earth Planet. Inter. 1989, 54, 364–369. [Google Scholar] [CrossRef]
- Charlu, T.V.; Newton, R.C.; Kleppa, O.J. Thermochemistry of Synthetic Ca2Al2SiO7 (Gehlenite)-Ca2MgSi2O7 (Åkermanite) Melilites. Geochim. Cosmochim. Acta 1981, 45, 1609–1617. [Google Scholar] [CrossRef]
- Jackson, W.E.; De Leon, J.M.; Brown, G.E., Jr.; Waychunas, G.A.; Conradson, S.D.; Combes, J.-M. High-Temperature XAS Study of Fe2SiO4 Liquid: Reduced Coordination of Ferrous Iron. Science 1993, 262, 229–233. [Google Scholar] [CrossRef]
- Luna Vera, F.; Guancha Chalapud, M.; Castillo Viveros, I.; Vásquez Medina, E.A. From Eggshells to Quicklime: Using Carbonate Cycle as an Integrating Concept to Introduce Students to Materials Analysis by TGA and FTIR. J. Chem. Educ. 2018, 95, 625–630. [Google Scholar] [CrossRef]
- Tolkacheva, A.S.; Shkerin, S.N.; Korzun, I.V.; Plaksin, S.V.; Khrustov, V.R.; Ordinartsev, D.P. Phase Transition in Mayenite Ca12Al14O33. Russ. J. Inorg. Chem. 2012, 57, 1014–1018. [Google Scholar] [CrossRef]
- Ceretti, M.; Corallini, S.; Paulus, W. Influence of Phase Transformations on Crystal Growth of Stoichiometric Brownmillerite Oxides: Sr2ScGaO5 and Ca2Fe2O5. Crystals 2016, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Kar, M.K.; Van Der Eijk, C.; Safarian, J. Hydrogen reduction of high temperature sintered and self-hardened pellets of bauxite residue produced via the addition of limestone and quicklime. In Proceedings of the 40th International ICSOBA Conference, Athens, Greece, 10–14 October 2022; p. 11. [Google Scholar]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.; Rajapakse, R.M.G. Thermal Decomposition of Calcium Carbonate (Calcite Polymorph) as Examined by in-Situ High-Temperature X-Ray Powder Diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ruan, H.D.; Frost, R.L. Thermal Decomposition of Bauxite Minerals: Infrared Emission Spectroscopy of Gibbsite, Boehmite and Diaspore. J. Mater. Sci. 2002, 37, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Jamil, N.H.; Abdullah, M.M.A.B.; Ibrahim, W.M.A.W.; Rahim, R.; Sandu, A.V.; Vizureanu, P.; Castro-Gomes, J.; Gómez-Soberón, J.M. Effect of Sintering Parameters on Microstructural Evolution of Low Sintered Geopolymer Based on Kaolin and Ground-Granulated Blast-Furnace Slag. Crystals 2022, 12, 1553. [Google Scholar] [CrossRef]




| Composition/Temperature (°C) | 1100 °C | 1150 °C | 1200 °C |
|---|---|---|---|
| C0.85A | 21.4 | 21.6 | 21.8 |
| C1A | 21.5 | 21.8 | 21.9 |
| C1.15A | 23.1 | 23.7 | 23.8 |
| Temperature (°C) | BET Surface Area (m2/g) | Porosity (vol%) |
|---|---|---|
| 1100 | 1.06 | 58.85 |
| 1150 | 0.29 | 54.99 |
| 1200 | 0.25 | 31.45 |
| Composition (CxA) | BET Surface Area (m2/g) | Porosity (vol%) |
|---|---|---|
| C0.85A | 0.25 | 43.35 |
| C1A | 0.29 | 54.99 |
| C1.15A | 0.39 | 66.78 |
| Ratio/Temperature | 1100 °C | 1150°C | 1200°C |
|---|---|---|---|
| C1.15A | 0.0133 | 0.05 | 0.5 |
| SD | 0.01 | 0.01 | 0.1 |
| C1A | 0.0366 | 0.2933 | 0.8166 |
| SD | 0.01 | 0.08 | 0.8 |
| C0.85A | 0.0733 | 0.4466 | 1.266 |
| SD | 0.02 | 0.04 | 0.08 |
| Samples/Oxides | Al2O3 | CaO | Fe2O3 | K2O | MnO | MgO | Na2O | P2O5 | SO3 | SiO2 | TiO2 | Cr2O3 | V2O5 | NiO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BR | 22.00 | 8.80 | 41.71 | 0.09 | 0.08 | 0.23 | 3.10 | 0.11 | 0.95 | 6.10 | 5.00 | … | … | … | 11.83 |
| Limestone | 0.90 | 52.70 | 0.15 | 0.12 | … | 0.95 | … | 0.01 | 0.06 | 2.07 | 0.03 | … | … | … | 42.60 |
| BR + CaCO3 (Dry pellets) | 13.20 | 27.50 | 23.20 | 0.15 | 0.04 | 0.59 | 2.55 | 0.07 | 0.58 | 6.05 | 3.25 | 0.14 | 0.11 | 0.07 | 22.40 |
| Sintered (C1A-1150 °C) | 19.80 | 32.80 | 29.60 | 0.19 | 0.05 | 0.51 | 2.31 | 0.12 | 0.96 | 8.30 | 4.07 | 0.20 | 0.15 | 0.10 | 0.72 |
| Elements | Area 1 | Area 2 | Area 3 | Point 4 |
|---|---|---|---|---|
| Al | 35.0 | 41.1 | 35.5 | 31.7 |
| O | 42.1 | 43.8 | 43.1 | 37.9 |
| Fe | 5.3 | 1.4 | 3.2 | 4.5 |
| Ca | 7.1 | 5.6 | 7.9 | 7.7 |
| Na | 9.3 | 6.7 | 9.0 | 14.2 |
| Compounds | BR | C0.85A-1150 °C | C1A-1150 °C | C1.15A-1150 °C | C1A-1100 °C | C1A-1200 °C | Melting Points |
|---|---|---|---|---|---|---|---|
| Fe2O3 (hematite) | Major XRD | … | … | … | … | … | 1565 °C [22] |
| AlOOH (diaspore) | Major XRD | … | … | … | … | … | … |
| TiO2 (anatase) | Major XRD | … | … | … | … | … | 1784 °C [23] |
| CaTiO3 (perovskite) | Minor XRD | Major XRD, SEM | Major XRD, SEM | Major XRD, SEM | Major XRD, SEM | Major XRD, SEM | 1975 °C [24] |
| Ca2Al2SiO7 (gehlenite) | … | Major XRD and SEM | Major XRD and SEM | Major XRD and SEM | Major XRD and SEM | Major XRD and SEM | 1593 °C [25] |
| Ca2(Al,Fe)2O5 (brownmillerite) | … | Major XRD and SEM | Major XRD and SEM | Major XRD and SEM | Major XRD and SEM | Major XRD and SEM | … |
| Fe2SiO4 (fayelite) | … | Moderate XRD | Moderate XRD | Minor XRD | Moderate XRD | Minor XRD | 1204 °C [26] |
| CaO (calcium oxide) | … | Moderate XRD and SEM | Moderate XRD and SEM | Moderate XRD and SEM | Moderate XRD and SEM | Minor in XRD and SEM | 2572 °C [27] |
| Al6Ca4O16S (yellimite) | … | Moderate XRD | Moderate XRD | Moderate XRD | Moderate XRD | Moderate XRD | … |
| 12CaO.7Al2O3 (mayenite) | Minor XRD | Minor XRD | Minor XRD | Minor XRD | Minor XRD | 1373 °C [28] | |
| Ca2Fe2O5 (srebrodolskite) | … | Minor XRD and SEM | Minor XRD and SEM | Minor in XRD and SEM | Minor in XRD and SEM | Minor in XRD and SEM | 1350 °C [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, M.K.; Safarian, J. Characteristics of Bauxite Residue–Limestone Pellets as Feedstock for Fe and Al2O3 Recovery. Processes 2023, 11, 137. https://doi.org/10.3390/pr11010137
Kar MK, Safarian J. Characteristics of Bauxite Residue–Limestone Pellets as Feedstock for Fe and Al2O3 Recovery. Processes. 2023; 11(1):137. https://doi.org/10.3390/pr11010137
Chicago/Turabian StyleKar, Manish Kumar, and Jafar Safarian. 2023. "Characteristics of Bauxite Residue–Limestone Pellets as Feedstock for Fe and Al2O3 Recovery" Processes 11, no. 1: 137. https://doi.org/10.3390/pr11010137






