Seasonal Hypoxia Enhances Benthic Nitrogen Fixation and Shapes Specific Diazotrophic Community in the Eutrophic Marine Ranch
Abstract
1. Introduction
2. Materials and Methods
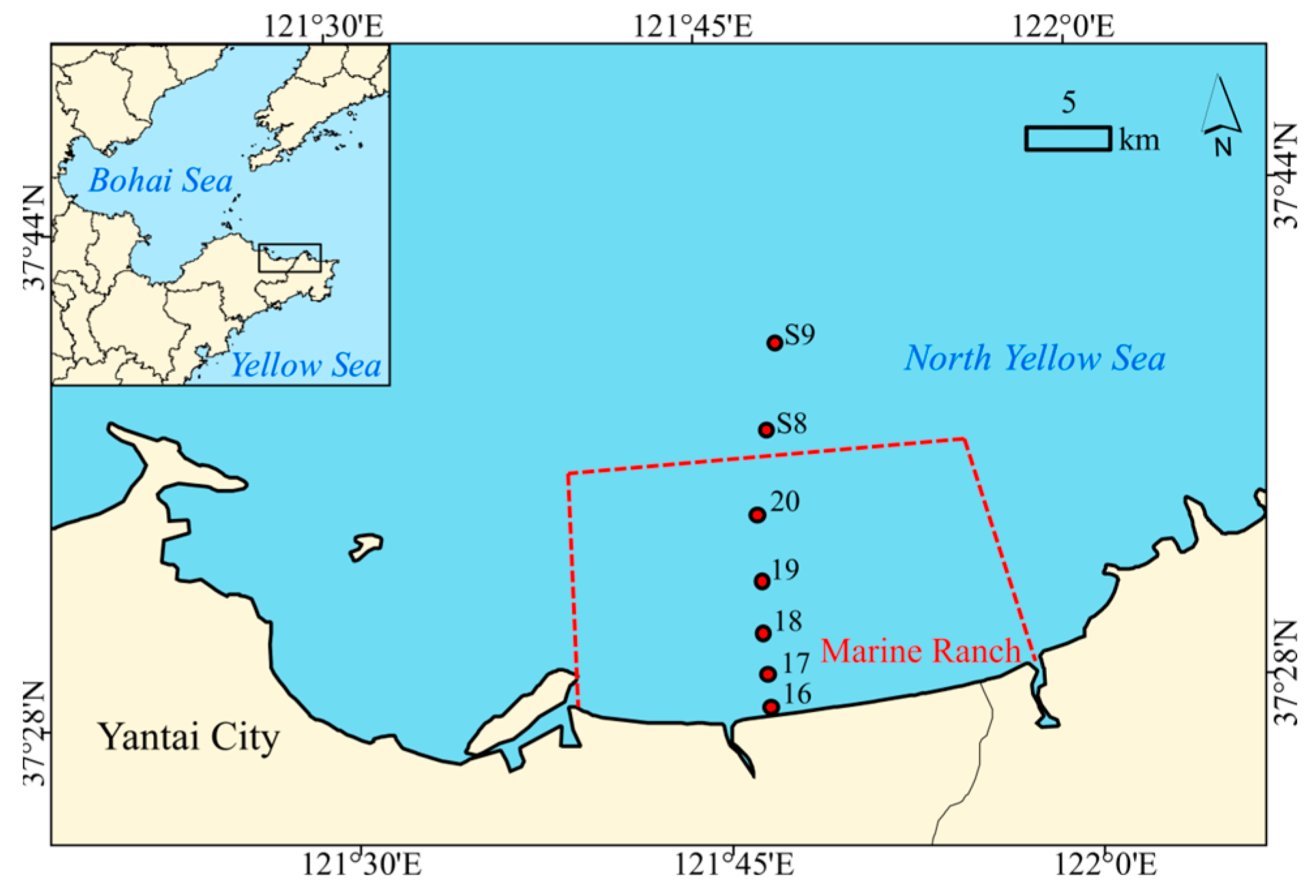
2.1. Sample Collection
2.2. Environmental Parameters Analysis
2.3. Nitrogen Fixation Rates Determination
2.4. DNA Extraction and nifH Gene Quantification
2.5. High Throughput Sequencing and Data Processing
2.6. Statistical Analysis
3. Results
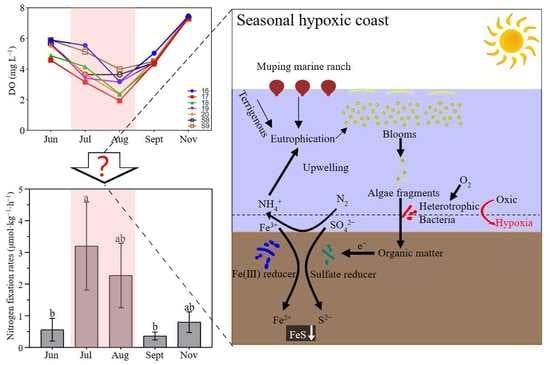
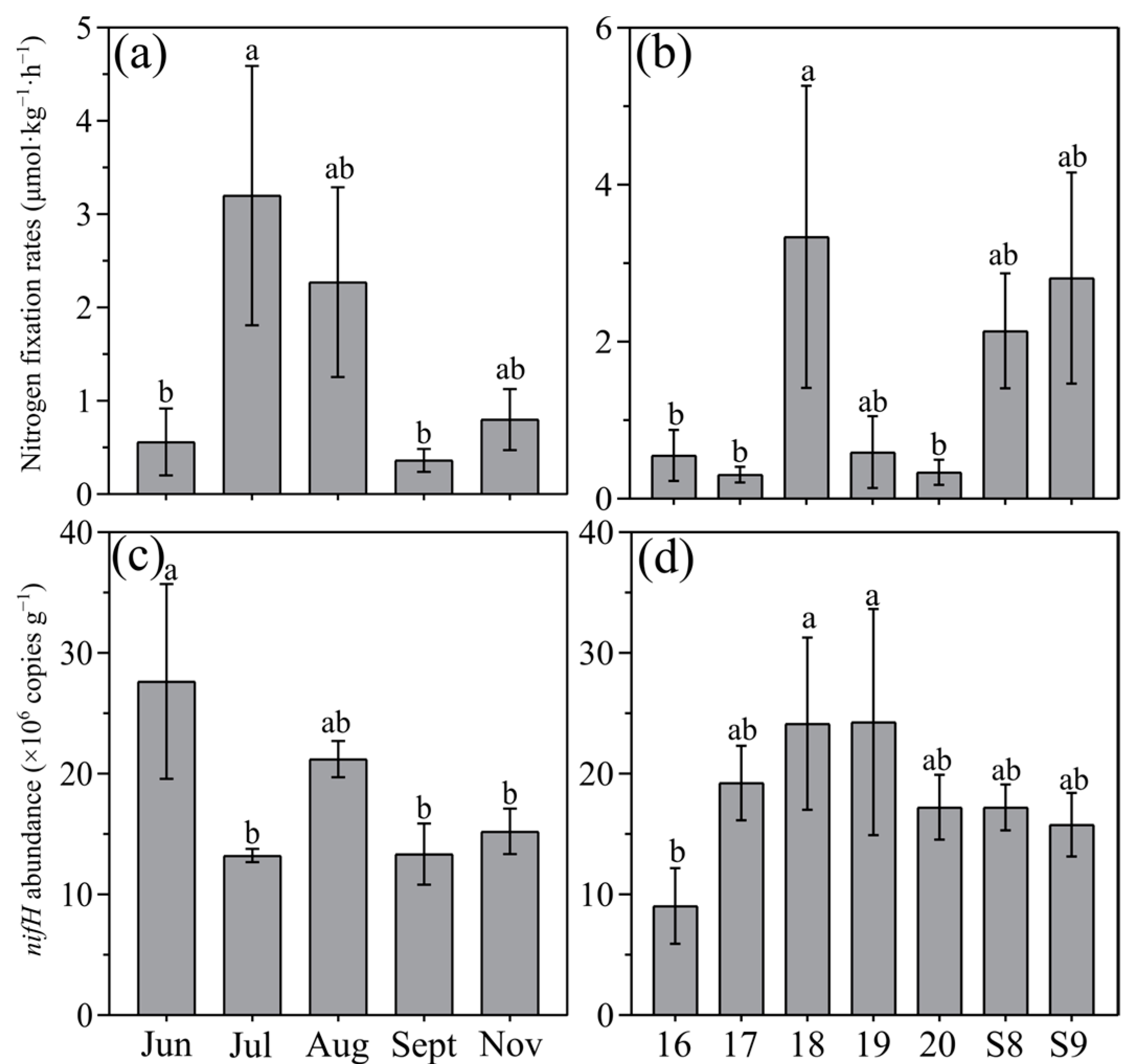
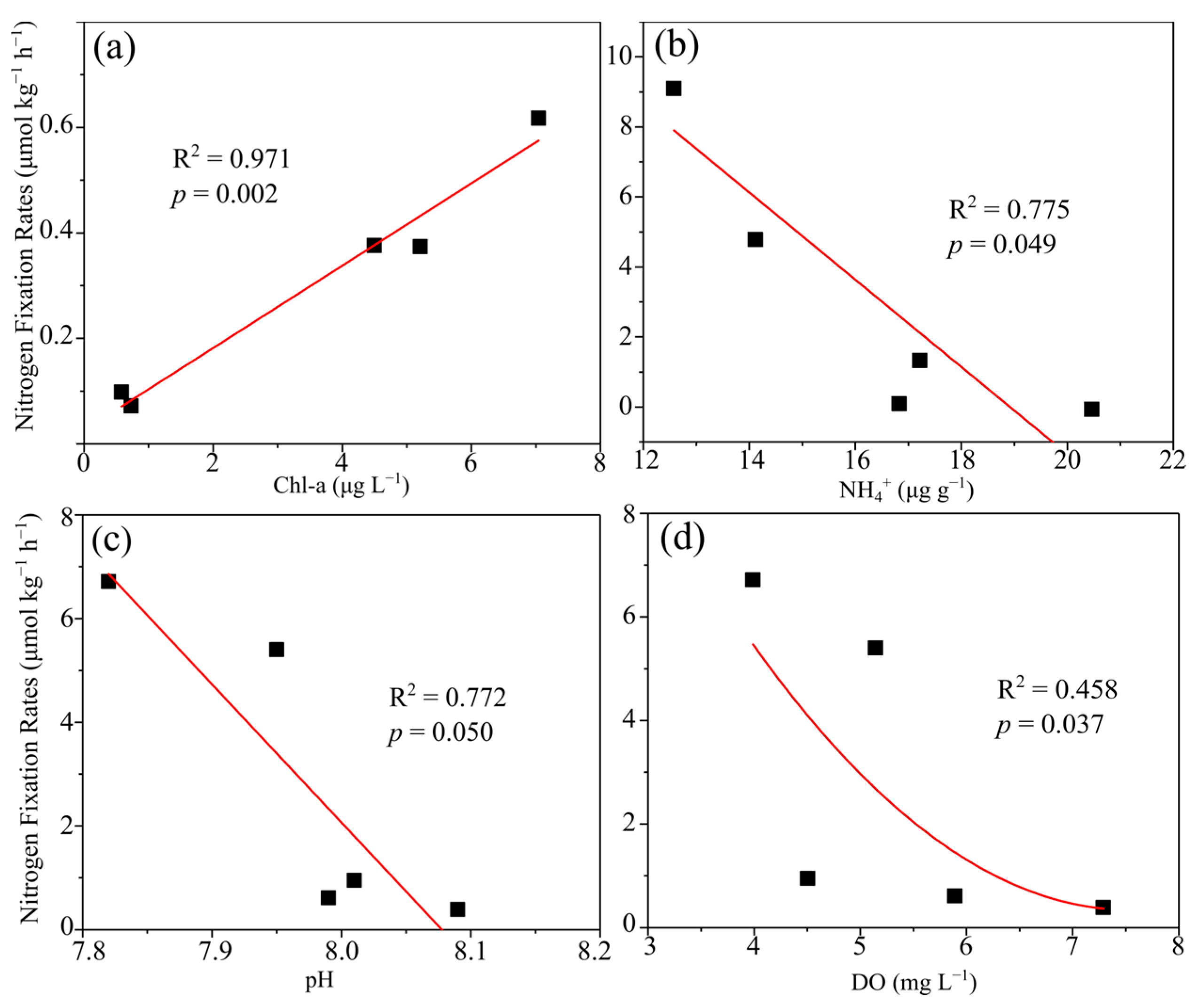
3.1. Potential Nitrogen Fixation Rates
3.2. Abundance of nifH Gene
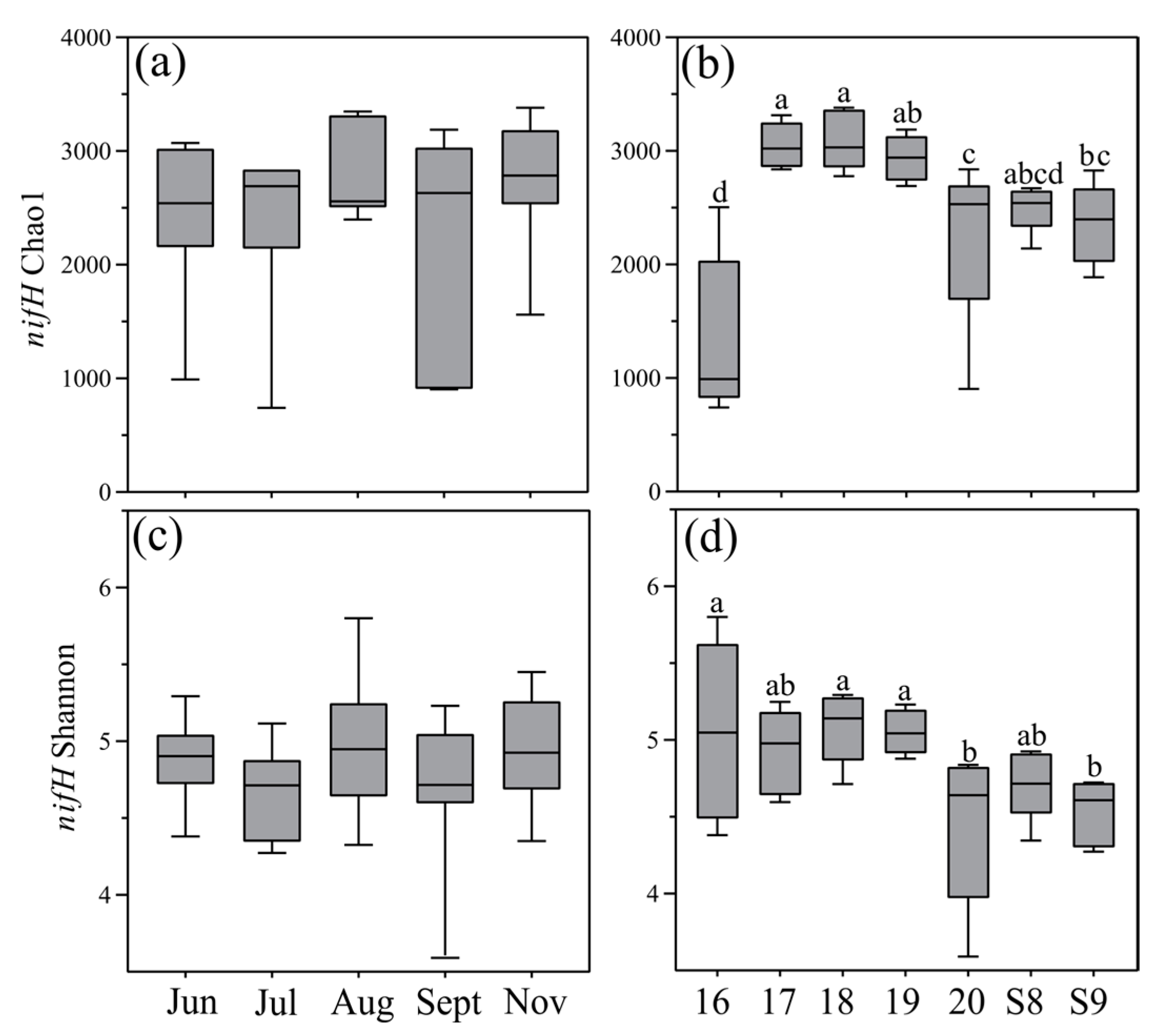
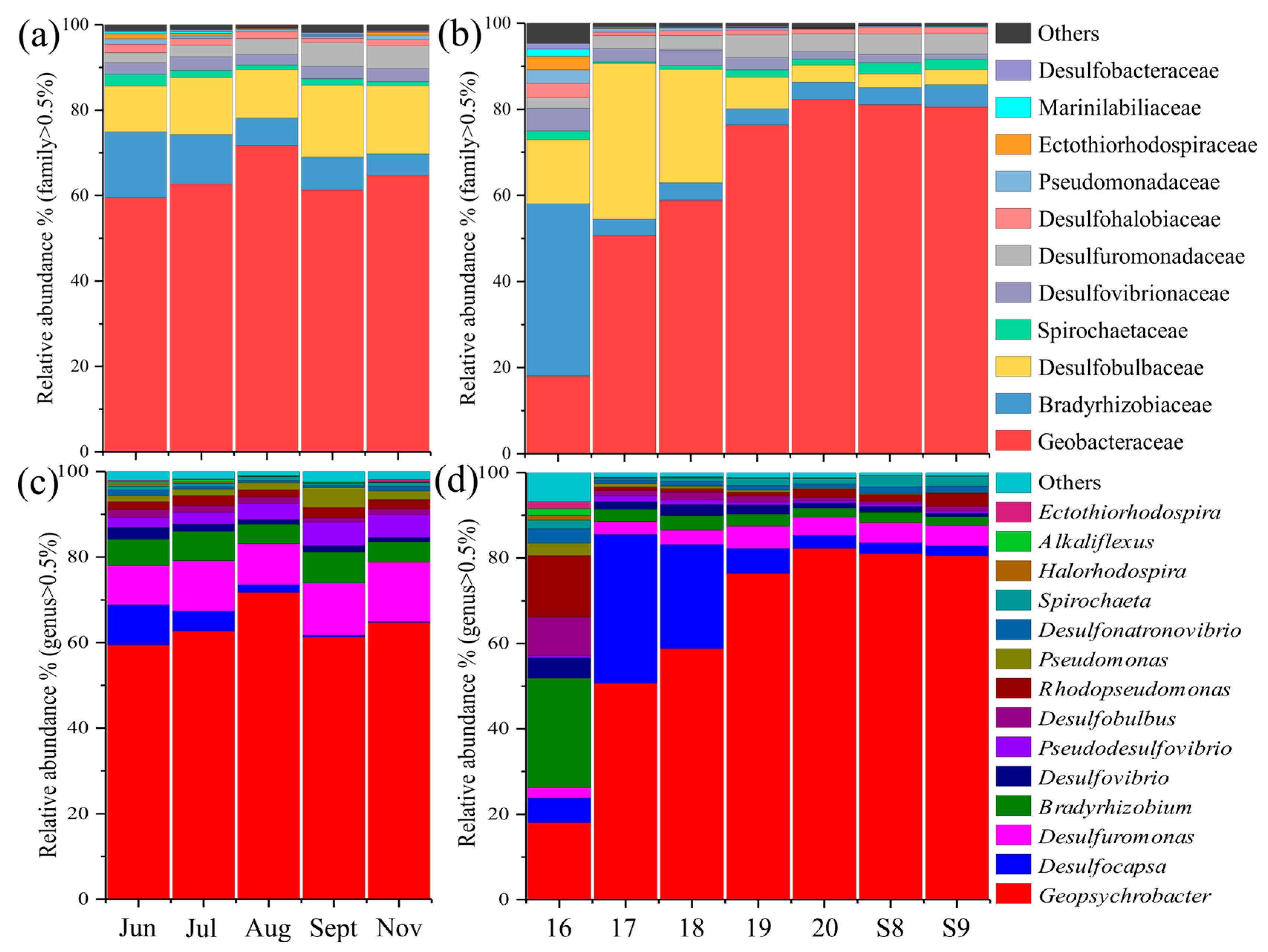
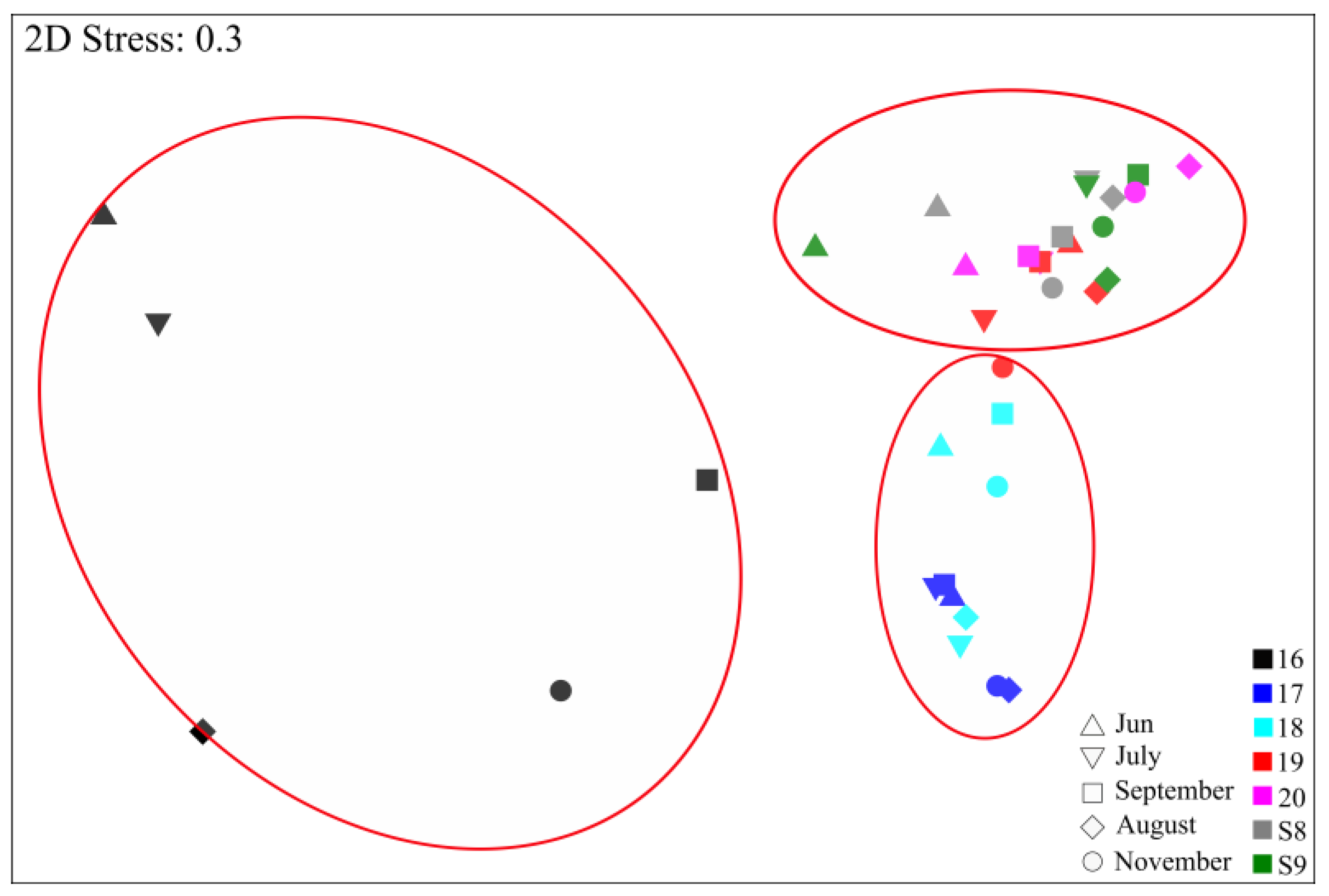
3.3. Diversity, Composition, and Distribution of nifH Gene Community
3.4. Abundance of nifH Gene
4. Discussion
4.1. Benthic Nitrogen Fixation Was Stimulated by Seasonal Hypoxia in Coastal Sediments
4.2. Iron-Reducing Bacteria Contributed Primarily to Benthic Nitrogen Fixation in the Coastal System
4.3. Ecological Implication
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gruber, N.; Galloway, J.N. An earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Moore, J.K.; Martiny, A.C.; Primeau, F.W. Convergent estimates of marine nitrogen fixation. Nature 2019, 566, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.A.; Dutkiewicz, S.; Moore, C.M.; Follows, M.J. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol. Oceanogr. 2013, 58, 2059–2075. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, H.; Li, D.; Luo, Y.; Mo, J. Substrate stoichiometry determines nitrogen fixation throughout succession in southern Chinese forests. Ecol. Lett. 2020, 23, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Bentzon-Tilia, M.; Traving, S.J.; Mantikci, M.; Knudsen-Leerbeck, H.; Hansen, J.L.S.; Markager, S.; Riemann, L. Significant N2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J. 2015, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Geisler, E.; Bogler, A.; Bar-Zeev, E.; Rahav, E. Heterotrophic nitrogen fixation at the hyper-eutrophic qishon river and estuary system. Front. Microbiol. 2020, 11, 1370. [Google Scholar] [CrossRef]
- Hou, L.; Wang, R.; Yin, G.; Liu, M.; Zheng, Y. Nitrogen fixation in the intertidal sediments of the Yangtze estuary: Occurrence and environmental implications. J. Geophys. Res. Biogeosci. 2018, 123, 936–944. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Díaz, R.J.; Levin, L.A.; Turner, R.E.; Gilbert, D.; Zhang, J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 2010, 7, 585–619. [Google Scholar] [CrossRef]
- Diaz Robert, J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Newell, S.E.; Carini, S.A.; Gardner, W.S. Denitrification dominates sediment nitrogen removal and is enhanced by bottom-water hypoxia in the northern gulf of Mexico. Estuaries Coast 2015, 38, 2279–2294. [Google Scholar] [CrossRef]
- Ward, B.B.; Devol, A.H.; Rich, J.J.; Chang, B.X.; Bulow, S.E.; Naik, H.; Pratihary, A.; Jayakumar, A. Denitrification as the dominant nitrogen loss process in the Arabian Sea. Nature 2009, 461, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Lavik, G.; Woebken, D.; Schmid, M.; Fuchs, B.M.; Amann, R.; Jørgensen, B.B.; Jetten, M.S.M. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc. Natl. Acad. Sci. USA 2005, 102, 6478–6483. [Google Scholar] [CrossRef] [PubMed]
- Thamdrup, B.; Dalsgaard, T.; Jensen, M.M.; Ulloa, O.; Farías, L.; Escribano, R. Anaerobic ammonium oxidation in the oxygen-deficient waters off northern Chile. Limnol. Oceanogr. 2006, 51, 2145–2156. [Google Scholar] [CrossRef]
- Fernandez, C.; Farias, L.; Ulloa, O. Nitrogen fixation in denitrified marine waters. PLoS ONE 2011, 6, e20539. [Google Scholar] [CrossRef] [PubMed]
- Gier, J.; Sommer, S.; Löscher, C.R.; Dale, A.W.; Schmitz, R.A.; Treude, T. Nitrogen fixation in sediments along a depth transect through the Peruvian oxygen minimum zone. Biogeosciences 2016, 13, 4065–4080. [Google Scholar] [CrossRef]
- Bertics, V.J.; Löscher, C.R.; Salonen, I.; Dale, A.W.; Gier, J.; Schmitz, R.A.; Treude, T. Occurrence of benthic microbial nitrogen fixation coupled to sulfate reduction in the seasonally hypoxic Eckernförde Bay, Baltic Sea. Biogeosciences 2013, 10, 1243–1258. [Google Scholar] [CrossRef]
- Messer, L.F.; Brown, M.V.; Van Ruth, P.D.; Doubell, M.; Seymour, J.R. Temperate southern Australian coastal waters are characterised by surprisingly high rates of nitrogen fixation and diversity of diazotrophs. PeerJ 2021, 9, e10809. [Google Scholar] [CrossRef]
- Emil, V.; Daniel, J.C.; Bo, G.G.; Harri, K.; Heikki, P.; Oleg, P.S.; Timo, T.; Markku, V.; Maren, V.; Norbert, W.; et al. Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the baltic sea. AMBIO 2007, 36, 186–194. [Google Scholar]
- Hamersley, M.R.; Turk, K.A.; Leinweber, A.; Gruber, N.; Zehr, J.P.; Gunderson, T.; Capone, D.G. Nitrogen fixation within the water column associated with two hypoxic basins in the Southern California Bight. Aquat. Microb. Ecol. 2011, 63, 193–205. [Google Scholar] [CrossRef]
- Yang, B.; Gao, X.; Zhao, J.; Lu, Y.; Gao, T. Biogeochemistry of dissolved inorganic nutrients in an oligotrophic coastal mariculture region of the northern Shandong Peninsula, north Yellow Sea. Mar. Pollut. Bull. 2020, 150, 110693. [Google Scholar] [CrossRef]
- Sun, X.; Sun, X.; Zhu, L.; Li, X.; Sun, S. Seasonal and spatial variation in abundance of the copepod Calanus sinicus: Effects of decreasing dissolved oxygen and small jellyfish bloom in northern Yellow Sea, China, nearshore waters. Mar. Pollut. Bull. 2020, 161, 111653. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Z.; Guo, Z.; Cong, W.; Song, M.; Wang, Y.; Jiang, Z. Response mechanism of microbial community to seasonal hypoxia in marine ranching. Sci. Total Environ. 2022, 811, 152387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Lin, X.; Cheng, L.; Li, Y.; Hu, X. Evidence of nitrogen loss from anaerobic ammonium oxidation coupled with ferric iron reduction in an intertidal wetland. Environ. Sci. Technol. 2015, 49, 11560–11568. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.W.A.; Lam, P.; Narvenkar, G.; Sarkar, A.; Naik, H.; Pratihary, A.; Shenoy, D.M.; Gauns, M.; Kurian, S.; Damare, S.; et al. Methane stimulates massive nitrogen loss from freshwater reservoirs in India. Nat. Commun. 2018, 9, 1265. [Google Scholar] [CrossRef] [PubMed]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.; Costello, E.; Fierer, N.; Pena, A.; Goodrich, J.; Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Fish, J.A.; Chai, B.; Wang, Q.; Sun, Y.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The functional gene pipeline and repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, R.; Gladkikh, V. Assessment of density variations of marine sediments with ocean and sediment depths. Sci. World J. 2014, 2014, 823296. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, X.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Yang, Y. Nitrogen fixation in surface sediments of the East China Sea: Occurrence and environmental implications. Mar. Pollut. Bull. 2018, 137, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.M.; Charette, M.A. Benthic nitrogen fixation in an eutrophic estuary affected by groundwater discharge. J. Coast Res. 2012, 28, 477–485. [Google Scholar] [CrossRef]
- Dekas, A.E.; Fike, D.A.; Chadwick, G.L.; Green-Saxena, A.; Fortney, J.; Connon, S.A.; Dawson, K.S.; Orphan, V.J. Widespread nitrogen fixation in sediments from diverse deep-sea sites of elevated carbon loading. Environ. Microbiol. 2018, 20, 4281–4296. [Google Scholar] [CrossRef]
- Whaley-Martin, K.J.; Mailloux, B.J.; van Geen, A.; Bostick, B.C.; Silvern, R.F.; Kim, C.; Ahmed, K.M.; Choudhury, I.; Slater, G.F. Stimulation of microbially mediated arsenic release in bangladesh aquifers by young carbon indicated by radiocarbon analysis of sedimentary bacterial lipids. Environ. Sci. Technol. 2016, 50, 7353–7363. [Google Scholar] [CrossRef]
- Spinette, R.F.; Brown, S.M.; Ehrlich, A.L.; Puggioni, G.; Deacutis, C.; Jenkins, B.D. Diazotroph activity in surface Narragansett Bay sediments in summer is stimulated by hypoxia and organic matter delivery. Mar. Ecol. Prog. Ser. 2019, 614, 35–50. [Google Scholar] [CrossRef]
- Selden, C.R.; Mulholland, M.R.; Widner, B.; Bernhardt, P.; Jayakumar, A. Toward resolving disparate accounts of the extent and magnitude of nitrogen fixation in the Eastern Tropical South Pacific oxygen deficient zone. Limnol. Oceanogr. 2021, 66, 1950–1960. [Google Scholar] [CrossRef]
- Luo, Y.W.; Lima, I.D.; Karl, D.M.; Deutsch, C.A.; Doney, S.C. Data-based assessment of environmental controls on global marine nitrogen fixation. Biogeosciences 2014, 11, 691–708. [Google Scholar] [CrossRef]
- Muyzer, G.; Stams, A.J.M. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef]
- Urdaci, M.C.; Stal, L.J.; Marchand, M. Occurrence of nitrogen fixation among Vibrio spp. Arch. Microbiol. 1988, 150, 224–229. [Google Scholar] [CrossRef]
- Lam, P.; Kuypers, M.M.M. Microbial nitrogen cycling processes in oxygen minimum zones. Annu. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Sulu-Gambari, F.; Hagens, M.; Behrends, T.; Seitaj, D.; Meysman, F.J.R.; Middelburg, J.; Slomp, C.P. Phosphorus cycling and burial in sediments of a seasonally hypoxic marine basin. Estuaries Coast 2018, 41, 921–939. [Google Scholar] [CrossRef]
- Černá, B.; Rejmánková, E.; Snyder, J.M.; Šantrůčková, H. Heterotrophic nitrogen fixation in oligotrophic tropical marshes: Changes after phosphorus addition. Hydrobiologia 2009, 627, 55–65. [Google Scholar] [CrossRef][Green Version]
- Fulweiler, R.W.; Brown, S.M.; Nixon, S.W.; Jenkins, B.D. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Mar. Ecol. Prog. Ser. 2013, 482, 57–68. [Google Scholar] [CrossRef]
- Newell, S.E.; Pritchard, K.R.; Foster, S.Q.; Fulweiler, R.W. Molecular evidence for sediment nitrogen fixation in a temperate New England estuary. PeerJ 2016, 4, e1615. [Google Scholar] [CrossRef]
- Jabir, T.; Vipindas, P.V.; Jesmi, Y.; Divya, P.S.; Adarsh, B.M.; Nafeesathul Miziriya, H.S.; Mohamed Hatha, A.A. Influence of environmental factors on benthic nitrogen fixation and role of sulfur reducing diazotrophs in a eutrophic tropical estuary. Mar. Pollut. Bull. 2021, 165, 112126. [Google Scholar] [CrossRef]
- Andersson, B.; Sundbäck, K.; Hellman, M.; Hallin, S.; Alsterberg, C. Nitrogen fixation in shallow-water sediments: Spatial distribution and controlling factors. Limnol. Oceanogr. 2014, 59, 1932–1944. [Google Scholar] [CrossRef]
- Knapp, A.N.; Casciotti, K.L.; Berelson, W.M.; Prokopenko, M.G.; Capone, D.G. Low rates of nitrogen fixation in eastern tropical South Pacific surface waters. Proc. Natl. Acad. Sci. USA 2016, 113, 4398–4403. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Wang, M.; Nie, M.; Qiu, S.; Wang, Q.; Quan, Z.; Xiao, M.; Li, B. Responses of soil nitrogen fixation to Spartina alterniflora invasion and nitrogen addition in a Chinese salt marsh. Sci. Rep. 2016, 6, 20384. [Google Scholar] [CrossRef]
- Tian, L.; Yan, Z.; Wang, C.; Xu, S.; Jiang, H. Habitat heterogeneity induces regional differences in sediment nitrogen fixation in eutrophic freshwater lake. Sci. Total Environ. 2021, 772, 145594. [Google Scholar] [CrossRef] [PubMed]
- Caton, I.R.; Caton, T.M.; Schneegurt, M.A. Nitrogen-fixation activity and the abundance and taxonomy of nifH genes in agricultural, pristine, and urban prairie stream sediments chronically exposed to different levels of nitrogen loading. Arch. Microbiol. 2018, 200, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Arashida, H.; Kugenuma, T.; Watanabe, M.; Maeda, I. Nitrogen fixation in Rhodopseudomonas palustris co-cultured with Bacillus subtilis in the presence of air. J. Biosci. Bioeng. 2019, 127, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, J.-L.; Luo, X.-Q.; Ahmad, M.; Duan, L.; Yin, L.-Z.; Fang, B.-Z.; Li, S.-H.; Yang, Y.; Jiang, L.; et al. Biogeographical distributions of nitrogen-cycling functional genes in a subtropical estuary. Funct. Ecol. 2022, 36, 187–201. [Google Scholar] [CrossRef]
- Holmes, D.E.; Nevin, K.P.; Lovley, D.R. Comparison of 16S rRNA, nifD, recA, gyrB, rpoB and fusA genes within the family Geobacteraceae fam. Nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1591–1599. [Google Scholar] [CrossRef]
- Li, L.; Jia, R.; Qu, Z.; Li, T.; Shen, W.; Qu, D. Coupling between nitrogen-fixing and iron (III)-reducing bacteria as revealed by the metabolically active bacterial community in flooded paddy soils amended with glucose. Sci. Total Environ. 2020, 716, 137056. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, H.; Shiratori, Y.; Isobe, K.; Otsuka, S.; Senoo, K. Predominant but previously-overlooked prokaryotic drivers of reductive nitrogen transformation in paddy soils, revealed by metatranscriptomics. Microbes Environ. 2017, 32, 180–183. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Q.; Feng, J.; Yang, Z.; Yu, C.; Zhang, J.; Ling, J.; Dong, J. Introduction of exotic species Sonneratia apetala alters diazotrophic community and stimulates nitrogen fixation in mangrove sediments. Ecol. Indic. 2022, 142, 109179. [Google Scholar] [CrossRef]
- Lin, B.; Braster, M.; Röling, W.F.M.; van Breukelen, B.M. Iron-reducing microorganisms in a landfill leachate-polluted aquifer: Complementing culture-independent information with enrichments and isolations. Geomicrobiol. J. 2007, 24, 283–294. [Google Scholar] [CrossRef]
- Jabir, T.; Vipindas, P.V.; Krishnan, K.P.; Mohamed Hatha, A.A. Abundance and diversity of diazotrophs in the surface sediments of Kongsfjorden, an Arctic fjord. World J. Microb. Biotechnol. 2021, 37, 41. [Google Scholar] [CrossRef]
- Luo, Z.; Zhong, Q.; Han, X.; Hu, R.; Liu, X.; Xu, W.; Wu, Y.; Huang, W.; Zhou, Z.; Zhuang, W.; et al. Depth-dependent variability of biological nitrogen fixation and diazotrophic communities in mangrove sediments. Microbiome 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.M.; Jenkins, B.D. Profiling gene expression to distinguish the likely active diazotrophs from a sea of genetic potential in marine sediments. Environ. Microbiol. 2014, 16, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Westerhoff, H.V.; Roling, W.F. How Geobacteraceae may dominate subsurface biodegradation: Physiology of Geobacter metallireducens in slow-growth habitat-simulating retentostats. Environ. Microbiol. 2009, 11, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Jilbert, T.; Conley, D.J.; Slomp, C.P. Hypoxia-driven variations in iron and manganese shuttling in the Baltic Sea over the past 8 kyr. Geochem. Geophys. Geosystems 2015, 16, 3754–3766. [Google Scholar] [CrossRef]
- Jessen, G.L.; Lichtschlag, A.; Ramette, A.; Pantoja, S.; Rossel, P.E.; Schubert, C.J.; Struck, U.; Boetius, A. Hypoxia causes preservation of labile organic matter and changes seafloor microbial community composition (Black Sea). Sci. Adv. 2017, 3, e1601897. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Holguin, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- Noisangiam, R.; Nuntagij, A.; Pongsilp, N.; Boonkerd, N.; Denduangboripant, J.; Ronson, C.; Teaumroong, N. Heavy metal tolerant Metalliresistens boonkerdii gen. nov., sp. nov., a new genus in the family Bradyrhizobiaceae isolated from soil in Thailand. Syst. Appl. Microbiol. 2010, 33, 374–382. [Google Scholar] [CrossRef]
- Jin, C.-Z.; Wu, X.-W.; Zhuo, Y.; Yang, Y.; Li, T.; Jin, F.-J.; Lee, H.-G.; Jin, L. Genomic insights into a free-living, nitrogen-fixing but non nodulating novel species of Bradyrhizobium sediminis from freshwater sediment: Three isolates with the smallest genome within the genus Bradyrhizobium. Syst. Appl. Microbiol. 2022, 45, 126353. [Google Scholar] [CrossRef]
- Thajudeen, J.; Yousuf, J.; Veetil, V.P.; Varghese, S.; Singh, A.; Abdulla, M.H. Nitrogen fixing bacterial diversity in a tropical estuarine sediments. World J. Microbiol. 2017, 33, 41. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, K.-M.; Yin, C.-R.; Lee, K.-Y.; Im, W.-T.; Lim, J.H.; Lee, S.-T. Odorous swine wastewater treatment by purple non-sulfur bacteria, Rhodopseudomonas palustris, isolated from eutrophicated ponds. Biotechnol. Lett. 2004, 26, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Satsuma, K.; Masuda, M.; Sato, K. A role of bradyrhizobium elkanii and closely related strains in the degradation of methoxychlor in soil and surface water environments. Biosci. Biotechnol. Biochem. 2013, 77, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, C.; Zhang, Y.; Zhao, H.; Wang, J.; Liu, H.; Yin, K. Low dissolved oxygen in the Pearl River estuary in summer: Long-term spatio-temporal patterns, trends, and regulating factors. Mar. Pollut. Bull. 2020, 151, 110814. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Q.; Chen, J.; Xie, L. Annual cycle of hypoxia off the Changjiang (Yangtze River) estuary. Mar. Environ. Res. 2012, 77, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.R.; Kemp, W.M.; Ball, W.P. Long-term trends in chesapeake bay seasonal hypoxia, stratification, and nutrient loading. Estuaries Coast 2011, 34, 1293–1309. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Liu, M.; Gong, J.; Zhang, X.; Yin, G.; You, L. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. J. Geophys. Res. Biogeosci. 2013, 118, 1237–1246. [Google Scholar] [CrossRef]
- Carpenter, J.H. The Chesapeake Bay Institute Technique for The Winkler Dissolved Oxygen Method. Limnol. Oceanogr. 1965, 10, 141–143. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, L.; Yang, G.; Li, T.; Yang, R.; Yin, G.; You, L. Reactive iron and its buffering capacity towards dissolved sulfide in sediments of Jiaozhou Bay, China. Mar. Environ. Res. 2012, 80, 46–55. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Zhang, Q.; Lin, X.; Zhao, J.; Zhang, X. Seasonal Hypoxia Enhances Benthic Nitrogen Fixation and Shapes Specific Diazotrophic Community in the Eutrophic Marine Ranch. Processes 2023, 11, 138. https://doi.org/10.3390/pr11010138
Yao C, Zhang Q, Lin X, Zhao J, Zhang X. Seasonal Hypoxia Enhances Benthic Nitrogen Fixation and Shapes Specific Diazotrophic Community in the Eutrophic Marine Ranch. Processes. 2023; 11(1):138. https://doi.org/10.3390/pr11010138
Chicago/Turabian StyleYao, Cheng, Qianqian Zhang, Xianbiao Lin, Jianmin Zhao, and Xiaoli Zhang. 2023. "Seasonal Hypoxia Enhances Benthic Nitrogen Fixation and Shapes Specific Diazotrophic Community in the Eutrophic Marine Ranch" Processes 11, no. 1: 138. https://doi.org/10.3390/pr11010138
APA StyleYao, C., Zhang, Q., Lin, X., Zhao, J., & Zhang, X. (2023). Seasonal Hypoxia Enhances Benthic Nitrogen Fixation and Shapes Specific Diazotrophic Community in the Eutrophic Marine Ranch. Processes, 11(1), 138. https://doi.org/10.3390/pr11010138