Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons: Sources, Quantification, Incidence, Toxicity, and Fate in Soil—A Review Study
Abstract
:1. Introduction
2. Sources
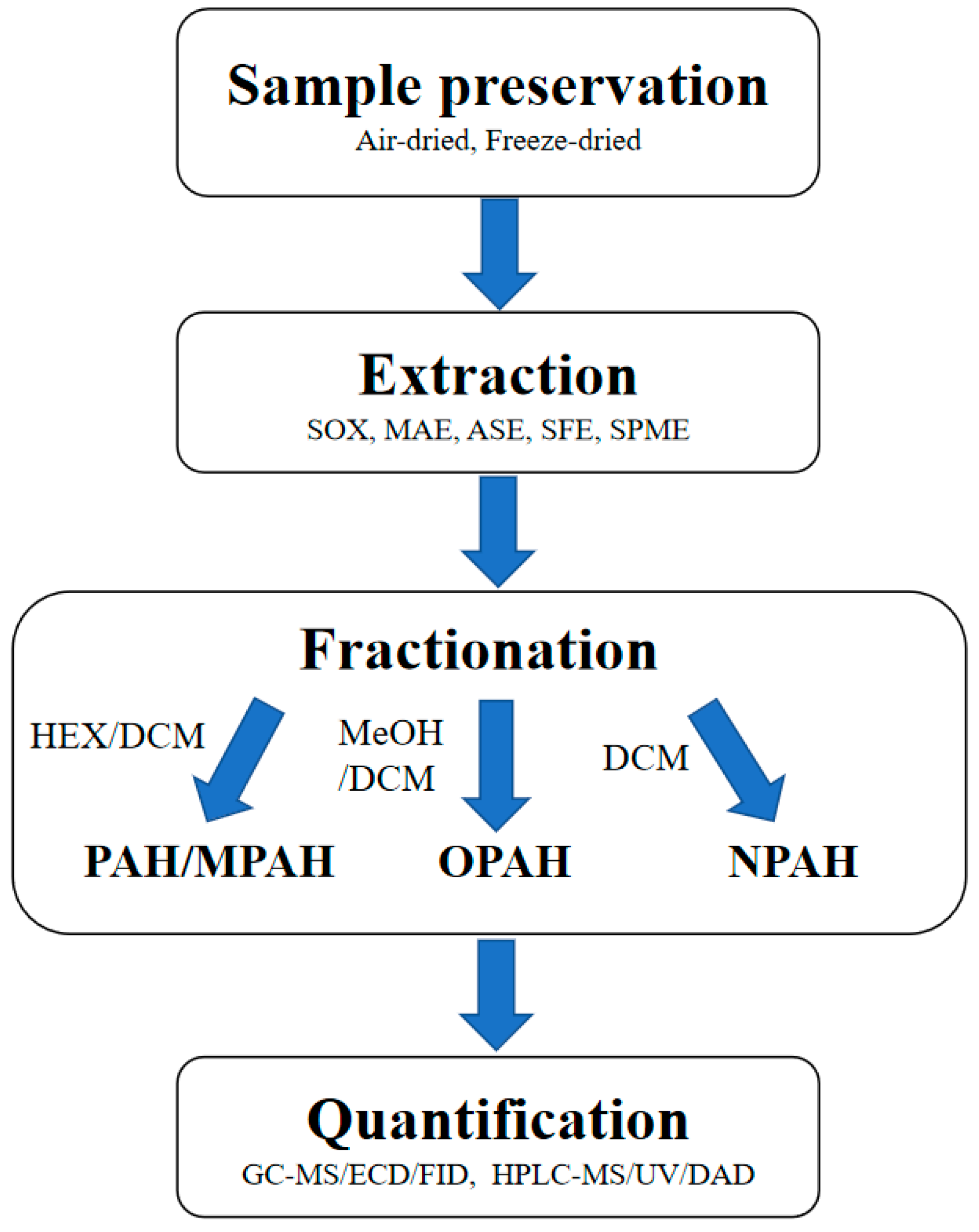
3. Quantification
4. Incidence
4.1. OPAHs
4.2. NPAHs
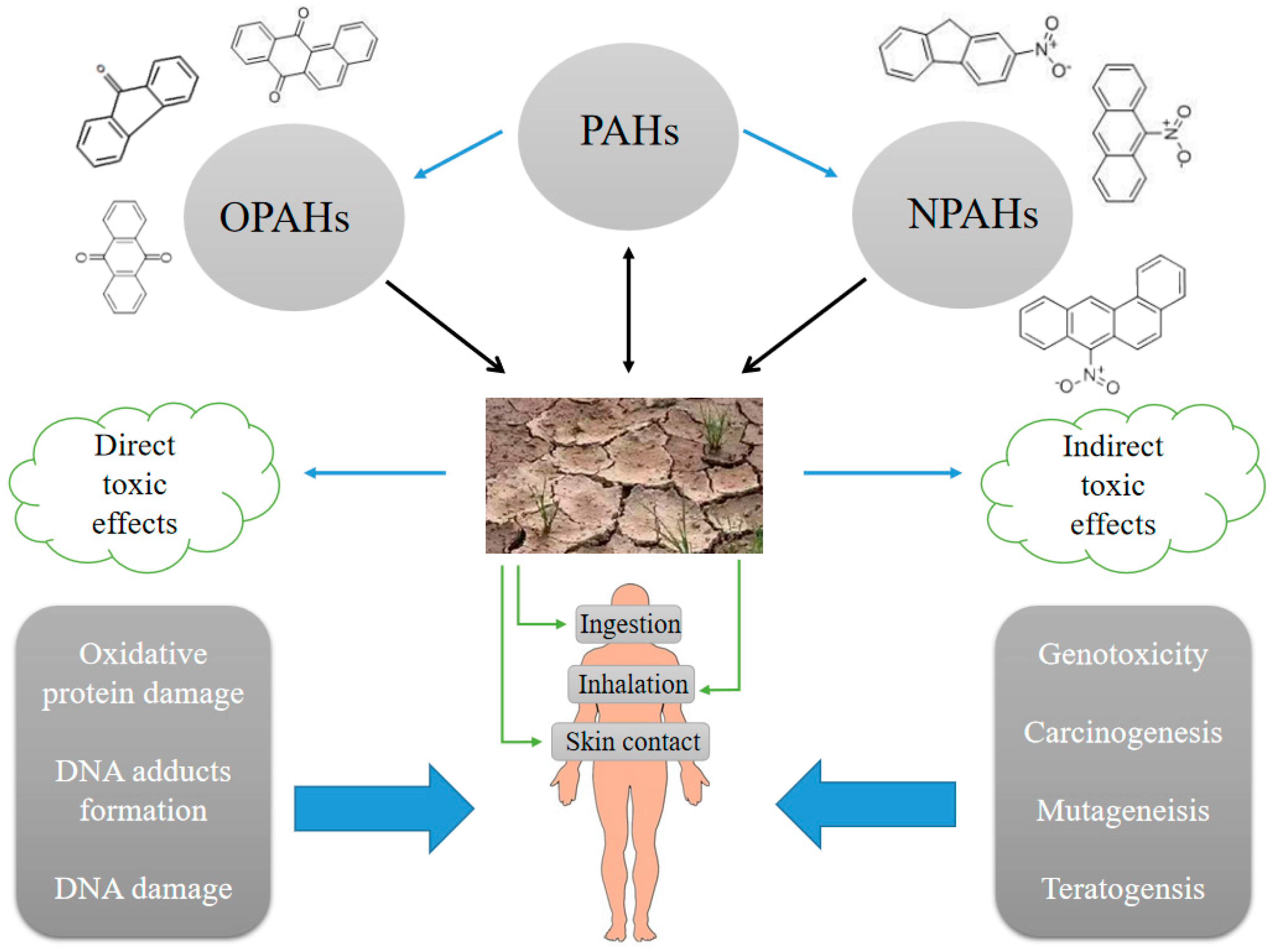
5. Toxicity and Human Health Risk
5.1. Exposure
5.2. Mutagenicity, Carcinogenicity, and Teratogenicity
5.3. Stress and Genotoxicity
6. Transport and Transformation
7. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Blumer, M. Benzpyrenes in soil. Science 1961, 134, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, K.; Sokhi, R.; Van Grieken, R. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.F.; Tao, S.; Wei, S.Y.; Chen, Y.C.; Zhang, Y.Y.; Shen, H.Z.; Huang, Y.; Zhu, D.; Yuan, C.Y.; Wang, H.C.; et al. Field measurement of emission factors of PM, EC, OC, parent; nitro-; and oxy-polycyclic aromatic hydrocarbons for residential briquette, coal cake; and wood in Rural Shanxi; China. Environ. Sci. Technol. 2013, 47, 2998–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafamme, R.E.; Hites, R.A. Global distribution of polycyclic aromatic hydrocarbons in recent sediments. Geochim. Cosmochim. Acta 1978, 42, 289–303. [Google Scholar] [CrossRef]
- Masclet, P.; Hoyau, V.; Jaffrezo, J.L.; Cachier, H. Polycyclic aromatic hydrocarbon deposition on the ice sheet of Greenland. Part I: Superficial snow. Atmos. Environ. 2000, 34, 3195–3207. [Google Scholar] [CrossRef]
- Cai, C.; Li, J.; Wu, D.; Wang, X.; Tsang, D.C.W.; Li, X.; Sun, J.; Zhu, L.; Shen, H.; Tao, S.; et al. Spatial distribution; emission source and health risk of parent PAHs and derivatives in surface soils from the Yangtze River Delta, eastern China. Chemosphere 2017, 178, 301–308. [Google Scholar] [CrossRef]
- McCarrick, S.; Cunha, V.; Zapletal, O.; Vondráček, J.; Dreij, K. In vitro and in vivo genotoxicity of oxygenated polycyclic aromatic hydrocarbons. Environ. Pollut. 2019, 246, 678–687. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: Formation of atmospheric mutagens. Environ. Health Perspect. 1994, 102, 117–126. [Google Scholar]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, L.B.; Oberg, L.; Haglund, P.; Tysklind, M. Sources; fate; and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. Ambio 2007, 36, 475–485. [Google Scholar] [CrossRef]
- Jung, D.J.K.; Klaus, T.; Fent, K. Cytochrome P450 induction by nitrated polycyclic aromatic hydrocarbons; azaarenes; and binary mixtures in fish hepatoma cell line PLHC-1. Environ. Toxicol. Chem. 2001, 20, 149–159. [Google Scholar] [CrossRef]
- De Guidi, G.; Falciglia, P.P.; Catalfo, A.; De Guidi, G.; Fagone, S.; Vagliasindi, F.G.A. Soil contaminated with PAHs and nitro-PAHs: Contamination levels in an urban area of Catania (Sicily, southern Italy) and experimental results from simulated decontamination treatment. Clean Technol. Environ. Policy 2017, 19, 1121–1132. [Google Scholar] [CrossRef]
- Musa Bandowe, B.A.; Wei, C.; Han, Y.; Cao, J.; Zhan, C.; Wilcke, W. Polycyclic aromatic compounds (PAHs, oxygenated PAHs, nitrated PAHs and azaarenes) in soils from China and their relationship with geographic location, land use and soil carbon fractions. Sci. Total Environ. 2019, 690, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Du, W.; Shen, G.; Li, B.; Liu, J.; Cheng, H.; Xing, B.; Tao, S. Estimating relative contributions of primary and secondary sources of ambient nitrated and oxygenated polycyclic aromatic hydrocarbons. Atmos. Environ. 2017, 159, 126–134. [Google Scholar] [CrossRef]
- Librando, V.; Fazzino, S. Quantification of polycyclic aromatic hydrocarbons and their nitro derivatives in atmospheric particulate matter of Augusta City. Chemosphere 1993, 27, 1649–1656. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Akutsu, K.; Murahashi, T.; Kakimoto, H.; Kizu, R. Comparison of polycyclic aromatic hydrocarbons and nitro polycyclic aromatic hydrocarbons in airborne particulates collected in downtown and suburban Kanazawa, Japan. Atmos. Environ. 2002, 36, 5535–5541. [Google Scholar] [CrossRef]
- Albinet, A.; Leoz-Garziandia, E.; Budzinski, H.; ViIlenave, E. Polycyclic aromatic hydrocarbons (PAHs); nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (South of France): Concentrations and sources. Sci. Total Environ. 2007, 384, 280–292. [Google Scholar] [CrossRef] [Green Version]
- De Castro Vasconcellos, P.; Sanchez-Ccoyllo, O.; Balducci, C.; Mabilia, R.; Cecinato, A. Occurrence and concentration levels of nitro-PAH in the air of three Brazilian cities experiencing different emission impacts. Water Air Soil Pollut. 2008, 190, 87–94. [Google Scholar] [CrossRef]
- Wild, S.R.; Jones, K.C. Polynuclear aromatic-hydrocarbons in the United-Kingdom environment—A preliminary source inventory and budget. Environ. Pollut. 1995, 88, 91–108. [Google Scholar] [CrossRef]
- Bandowe, B.A.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef]
- Hoffmann, T.; Oelmann, Y.; Wilcke, W. Plant diversity enhances the natural attenuation of polycyclic aromatic compounds (PAHs and oxygenated PAHs) in grassland soils. Soil Biol. Biochem. 2019, 129, 60–70. [Google Scholar]
- Burgos, W.D.; Pisutpaisal, N.; Mazzarese, M.C.; Chorover, J. Adsorption of quinolone to kaolinite and montmorillonite. Environ. Eng. Sci. 2002, 19, 59–64. [Google Scholar] [CrossRef]
- Weigand, H.; Totsche, K.U.; Kögel-Knabner, I.; Annweiler, E.; Richnow, H.H.; Michaelis, W. Fate of anthracene in contaminated soil: Transport and biochemical transformation under unsaturated flow conditions. Eur. J. Soil Sci. 2002, 53, 71–81. [Google Scholar] [CrossRef]
- Wilcke, W.; Kiesewetter, M.; Bandowe, B.A.M. Microbial formation and degradation of oxygen-containing polycyclic aromatic hydrocarbons (OPAHs) in soil during short-term incubation. Environ. Pollut. 2014, 184, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Schlanges, I.; Meyer, D.; Palm, W.U.; Ruck, W. Identification; quantification and distribution of PAC-metabolites, heterocyclic PAC and substituted PAC in groundwater samples of tar-contaminated sites from Germany. Polycycl. Arom. Compd. 2008, 28, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Bandowe, B.A.M.; Sobocka, J.; Wilcke, W. Oxygen-containing polycyclic aromatic hydrocarbons (OPAHs) in urban soils of Bratislava; Slovakia: Patterns; relation to PAHs and vertical distribution. Environ. Pollut. 2011, 159, 539–549. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J.; Zielinska, B.; Aschmann, S.M. Kinetics and nitro-products of the gas-phase OH and NO3 radical-initiated reactions of naphthalene-d8; fluoranthene-d10, and pyrene. Int. J. Chem. Kinet. 1990, 22, 999–1014. [Google Scholar] [CrossRef]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons—A review. Chem. Soc. Rev. 2013, 42, 9333–9391. [Google Scholar] [CrossRef] [Green Version]
- Wilcke, W.; Amelung, W.; Martius, C.; Garcia, M.V.B.; Zech, W. Biological sources of polycyclic aromatic hydrocarbons (PAHs) in the Amazonian rain forest. J. Plant Nutr. Soil Sci. 2000, 163, 27–30. [Google Scholar] [CrossRef]
- Moeckel, C.; Nizzetto, L.; Di Guardo, A.; Steinnes, E.; Freppaz, M.; Filippa, G.; Camporini, P.; Benner, J.; Jones, K.C. Persistent organic pollutants in boreal and montane soil profiles: Distribution; evidence of processes and implications for global cycling. Environ. Sci. Technol. 2008, 42, 8374–8380. [Google Scholar] [CrossRef]
- Okere, U.V.; Semple, K.T. Biodegradation of PAHs in ‘Pristine’ Soils from different climatic regions. J. Bioremed. Biodegrad. 2012, S1, 6. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Weber, W.J. Interactions of soil-derived dissolved organic matter with phenol in peroxidase-catalyzed oxidative coupling reactions. Environ. Sci. Technol. 2004, 38, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.J.; Thomas, G.O.; Jaward, F.M.; Steinnes, E.; Gustafsson, O.; Jones, K.C. PAHs in background soils from western Europe: Influence of atmospheric deposition and soil organic matter. Chemosphere 2008, 70, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Morriss, A.W.J.; Paton, G.I. Bioavailability of hydrophobic organic contaminants in soils, fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Wei, C.; Han, Y.; Bandowe, B.A.M.; Cao, J.; Huang, R.-J.; Ni, H.; Tian, J.; Wilcke, W. Occurrence, gas/particle partitioning and carcinogenic risk of polycyclic aromatic hydrocarbons and their oxygen- and nitrogen- containing derivatives in Xi’an, central China. Sci. Total Environ. 2015, 505, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Titaley, I.A.; Chlebowski, A.; Truong, L.; Tanguay, R.L.; Massey Simonich, S.L. Identification and toxicological evaluation of unsubstituted PAHs and novel PAH derivatives in pavement sealcoat products. Environ. Sci. Technol. Lett. 2016, 3, 234–242. [Google Scholar] [CrossRef]
- Krauss, M.; Wilcke, W.; Zech, W. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in forest soils, depth distribution as indicator of different fate. Environ. Pollut. 2000, 110, 79–88. [Google Scholar] [CrossRef]
- Arroyo, L.J.; Li, H.; Teppen, B.J.; Johnston, C.T.; Boyd, S.A. Oxidation of 1- naphthol coupled to reduction of structural Fe3+ in smectite. Clays Clay Miner. 2005, 53, 587–596. [Google Scholar] [CrossRef]
- Bamforth, S.M.; Singleton, I. Bioremediation of polycyclic aromatic hydrocarbons, current knowledge and future directions. J. Chem. Technol. Biotechnol. 2005, 80, 723–736. [Google Scholar] [CrossRef]
- Lemieux, C.L.; Lambert, I.B.; Lundstedt, S.; Tysklind, M.; White, P.A. Mutagenic hazards of complex polycyclic aromatic hydrocarbon mixtures in contaminated soil. Environ. Toxicol. Chem. 2008, 27, 978–990. [Google Scholar] [CrossRef]
- Murakami, M.; Yamada, J.; Kumata, H.; Takada, H. Sorptive behavior of nitro-PAHs in street runoff and their potential as indicators of diesel vehicle exhaust particles. Environ. Sci. Technol. 2008, 42, 1144–1150. [Google Scholar] [CrossRef]
- Qiao, M.; Bai, Y.H.; Cao, W.; Huo, Y.; Zhao, X.; Liu, D.; Li, Z. Impact of secondary effluent from wastewater treatment plants on urban rivers, polycyclic aromatic hydrocarbons and derivatives. Chemosphere 2018, 211, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, W.; Bandowe, B.A.M.; Lueso, M.G.; Ruppenthal, M.; del Valle, H.; Oelmann, Y. Polycyclic aromatic hydrocarbons (PAHs) and their polar derivatives (oxygenated PAHs; azaarenes) in soils along a climosequence in Argentina. Sci. Total Environ. 2014, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Bandowe, B.A.M.; Han, Y.; Cao, J.; Zhan, C.; Wilcke, W. Polycyclic aromatic hydrocarbons (PAHs) and their derivatives (alkyl-PAHs; oxygenated-PAHs; nitrated-PAHs and azaarenes) in urban road dusts from Xi’an; Central China. Chemosphere 2015, 134, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Lueso, M.G.; Wilcke, W. Oxygenated polycyclic aromatic hydro- carbons and azaarenes in urban soils, a comparison of a tropical city (Bangkok) with two temperate cities (Bratislava and Gothenburg). Chemosphere 2014, 107, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Bandowe, B.A.M.; Nkansah, M.A. Occurrence; distribution and health risk from polycyclic aromatic compounds (PAHs; oxygenated-PAHs and azaarenes) in street dust from a major West African Metropolis. Sci. Total Environ. 2016, 553, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, M.; Maletz, S.; Krauss, M.; Bluhm, K.; Schiwy, S.; Kuckelkorn, J.; Tiehm, A.; Brack, W.; Hollert, H. Heterocyclic aromatic hydrocarbons show estrogenic activity upon metabolization in a recombinant transactivation assay. Environ. Sci. Technol. 2014, 48, 5892–5901. [Google Scholar] [CrossRef]
- Chibwe, L.; Geier, M.C.; Nakamura, J.; Tanguay, R.L.; Aitken, M.D.; Simonich, S.L. Aerobic bioremediation of PAH contaminated soil results in increased genotoxicity and developmental toxicity. Environ. Sci. Technol. 2015, 49, 13889–13898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.-T.; Liu, H.; Liu, Y.; Teng, Y. Application Trends of Nanofibers in Analytical Chemistry. TrAC Trends Anal. Chem. (Regul. Ed.) 2020, 131, 115992. [Google Scholar] [CrossRef]
- Qiao, M.; Fu, L.J.; Cao, W.; Bai, Y.H.; Huang, Q.X.; Zhao, X. Occurrence and removal of polycyclic aromatic hydrocarbons and their derivatives in an ecological wastewater treatment plant in South China and effluent impact to the receiving river. Environ. Sci. Pollut. Res. 2019, 26, 5638–5644. [Google Scholar] [CrossRef]
- Han, Y.M.; Bandowe, B.A.M.; Wei, C.; Cao, J.J.; Wilcke, W.; Wang, G.H.; Ni, H.Y.; Jin, Z.D.; An, Z.S.; Yan, B.Z. Stronger association of polycyclic aromatic hydrocarbons with soot than with char in soils and sediments. Chemosphere 2015, 119, 1335–1345. [Google Scholar] [CrossRef] [Green Version]
- Brorström-Lundén, E.; Remberger, M.; Kaj, L.; Hansson, K.; Palm-Cousins, A.; Andersson, H. Results from the Swedish National Screening Programme 2008, Screening of Unintentionally Produced Organic Contaminants; Report B1944; Swedish Environmental Research Institute (IVL): Göteborg, Sweden, 2010. [Google Scholar]
- Sun, Z.; Zhu, Y.; Zhuo, S.; Liu, W.; Zeng, E.Y.; Wang, X.; Tao, S. Occurrence of nitro- and oxy-PAHs in agricultural soils in eastern China and excess lifetime cancer risks from human exposure through soil ingestion. Environ. Int. 2017, 108, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Singh, V.K.; Li, J.; Zhang, G. Concentrations, sources and health risk of nitrated- and oxygenated-polycyclic aromatic hydrocarbon in urban indoor air and dust from four cities of Nepal. Sci. Total Environ. 2018, 643, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavaman, P. Analysis of polycyclic aromatic hydrocarbons (PAHs) and their polar derivatives in soils of an industrial heritage city of Australia. Sci. Total Environ. 2020, 699, 134303. [Google Scholar] [CrossRef] [PubMed]
- Musa Bandowe, B.A.; Shukurov, N.; Kersten, M.; Wilcke, W. Polycyclic aromatic hydrocarbons (PAHs) and their oxygen-containing derivatives (OPAHs) in soils from the Angren industrial area, Uzbekistan. Environ. Pollut. 2010, 158, 2888–2899. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G. Polycyclic aromatic hydrocarbons in house dust and surface soil in major urban regions of Nepal, implication on source apportionment and toxicological effect. Sci. Total Environ. 2018, 616–617, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, E.; Wiegman, S.; de Voogt, P.; Kraak, M.; Leslie, H.A.; de Haas, E.; Admiraal, W. Toxicity of azaarenes. Rev. Environ. Contam. Toxicol. 2002, 173, 39–83. [Google Scholar]
- Machala, M.; Ciganek, M.; Blaha, L.; Minksova, K.; Vondrak, J. Aryl hydrocarbon receptor-mediated and estrogenic activities of oxygenated polycyclic aromatic hydrocarbons and azaarenes originally identified in extracts of river sediments. Environ. Toxicol. Chem. 2001, 20, 2736–2743. [Google Scholar]
- Xue, W.L.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage, a review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef]
- Wang, W.; Jariyasopit, N.; Schrlau, J.; Jia, Y.; Tao, S.; Yu, T.W.; Dashwood, R.H.; Zhang, W.; Wang, X.; Simonich, S.L. Concentration and photochemistry of PAHs; NPAHs; and OPAHs and toxicity of PM2.5 during the Beijing Olympic games. Environ. Sci. Technol. 2011, 45, 6887–6895. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.T.; Achten, C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl. Arom. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [Green Version]
- Chlebowski, A.C.; Garcia, G.R.; La Du, J.K.; Bisson, W.H.; Truong, L.; Massey Simonich, S.L.; Tanguay, R.L. Mechanistic investigations into the developmental toxicity of nitrated and heterocyclic PAHs. Toxicol. Sci. 2017, 157, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trine, L.S.D.; Davis, E.L.; Roper, C.; Truong, L.; Tanguay, R.L.; Simonich, S.L.M. Formation of PAH derivatives and increased developmental toxicity during steam enhanced extraction remediation of creosote contaminated superfund soil. Environ. Sci. Technol. 2019, 53, 4460–4469. [Google Scholar] [CrossRef] [PubMed]
- WHO. Selected Nitro-; and Nitro-Oxy-Polycyclic Aromatic Hydrocarbons: Environmental Health Criteria; WHO: Geneva, Switzerland, 2003; Volume 229. [Google Scholar]
- The Most Neglected Threat to Public Health in China is Toxic Soil. Economist, 13 June 2017.
- Doucette, W.; Dettenmaier, E.K.; Bugbee, B.; Mackay, D. Mass transfer from soils to plants. In Handbook of Chemical Mass Transport in the Environment; Thobodeaux, L.J., Mackay, D., Eds.; CRS Press: Boca Raton, FL, USA, 2011; pp. 389–411. [Google Scholar]
- Rengarajan, T.; Rajendran, P.; Nandakumar, N.; Lokeshkumar, B.; Rajendran, P.; Nishigaki, I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, H.; Zhang, H.; Wang, W.; Liu, Y.; Fan, Y. Smoking modify the effects of polycyclic aromatic hydrocarbons exposure on oxidative damage to DNA in coke oven workers. Int. Arch. Occup. Environ. Health 2017, 90, 423–431. [Google Scholar] [CrossRef]
- Mortamais, M.; Pujol, J.; van Drooge, B.L.; Macia, D.; Martinez-Vilavella, G.; Reynes, C.; Sabatier, R.; Rivas, I.; Grimalt, J.; Forns, J.; et al. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ. Int. 2017, 105, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, D.; Cohen, Y.; Arey, J.; Grosovsky, A.J. Multimedia analysis of PAHs and nitro- PAH daughter products in the Los Angeles Basin. Risk Anal. 2001, 21, 275–294. [Google Scholar] [CrossRef]
- Ciganek, M.; Raszyk, J.; Kohoutek, J.; Ansorgová, A.; Salava, J.; Palac, J. Polycyclic aromatic hydrocarbons (PAHs; nitro-PAHs; oxy-PAHs) polychlorinated biphenyls (PCBs) and organic chlorinated pesticides (OCPs) in the indoor and outdoor air of pig and cattle houses. Vet. Med. 2000, 43, 217–226. [Google Scholar]
- Miller-Schulze, J.P.; Paulsen, M.; Toriba, A.; Tang, N.; Hayakawa, K.; Tamura, K.; Dong, L.; Zhang, X.; Simpson, C.D. Exposures to particulate air pollution and nitro-polycyclic aromatic hydrocarbons among taxi drivers in Shenyang; China. Environ. Sci. Technol. 2010, 44, 216–221. [Google Scholar] [CrossRef] [Green Version]
- IARC. Diesel and gasoline engine exhausts and some nitroarenes. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 105, pp. 1–703. [Google Scholar]
- Zwirner-Baier, I.; Neumann, H.G. Polycyclic nitroarenes (nitro-PAHs) as biomarkers of exposure to diesel exhaust. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 141, 135–144. [Google Scholar] [CrossRef]
- Seidel, A.; Dahmann, D.; Krekeler, H.; Jacob, J. Biomonitoring of polycyclic aromatic compounds in the urine of mining workers occupationally exposed to diesel exhaust. Int. J. Hyg. Environ. Health 2002, 204, 333–338. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, A.M.P.; Gomes, J.; Nunes, T.; Duarte, M.; Bandowe, B.A.M. Polycyclic aromatic hydrocarbons (PAHs) and their derivatives (oxygenated-PAHs, nitrated-PAHs and azaarenes) in size-fractionated particles emitted in an urban road tunnel. Atmos. Res. 2016, 180, 128–137. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Liao, C.-M. Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci. Total Environ. 2006, 366, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, C.L.; Long, A.S.; Lambert, I.B.; Lundstedt, S.; Tysklind, M.; White, P.A. Cancer risk assessment of polycyclic aromatic hydrocarbon contaminated soils determined using bioassay-derived levels of benzo[a]pyrene equivalents. Environ. Sci. Technol. 2015, 49, 1797–1805. [Google Scholar] [CrossRef]
- Martins, M.; Costa, P.M.; Ferreira, A.M.; Costa, M.H. Comparative DNA damage and oxidative effects of carcinogenic and non-carcinogenic sediment-bound PAHs in the gills of a bivalve. Aquat. Toxicol. 2013, 142–143, 85–95. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons, from metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Rosenkranz, H.; Mermelstein, R. The mutagenic and carcinogenic properties of nitrated polycyclic aromatic hydrocarbons. In Nitrated Polycyclic Aromatic Hydrocarbons; White, C.M., Ed.; Huethig: Heidelberg, Germany, 1985; pp. 267–297. [Google Scholar]
- Idowu, O.; Semple, K.T.; Ramadass, K.; O’Connor, W.; Hansbro, P.; Thavamani, P. Beyond the obvious, environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019, 123, 543–557. [Google Scholar] [CrossRef]
- Takahashi, K.; Asanoma, M.; Yoshida, S.; Ning, G.; Mori, H.; Horibe, Y.; Watanabe, T.; Hirayama, T.; Nukaya, H.; Mizutani, T. Identification of 1;3;6-trinitropyrene as a major mutagen in organic extracts of surface soil from Nagoya city; Japan. Genes Environ. 2006, 28, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Takahashi, K.; Konishi, E.; Hoshino, Y.; Hasei, T.; Asanoma, M.; Hirayama, T.; Wakabayashi, K. Mutagenicity of surface soil from residential areas in Kyoto city; Japan; and identification of major mutagens. Mutat. Res. 2008, 649, 201–212. [Google Scholar] [CrossRef]
- Hasei, T.; Watanabe, T.; Endo, O.; Sugita, K.; Asanoma, M.; Goto, S.; Hirayama, T. Determination of 3;6-dinitrobenzo[e]pyrene in surface soil and airborne particles; and its possible sources; diesel particles and incinerator dusts. J. Health Sci. 2009, 55, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, M.; Watanabe, T.; Hagio, S.; Ogo, S.; Shimohara, C.; Jouchi, R.; Takayama, S.; Hasei, T.; Hirayama, T.; Oda, Y.; et al. Genotoxicity of 3;6- dinitrobenzo[e]pyrene; a novel mutagen in ambient air and surface soil; in mammalian cells in vitro and in vivo. Mutagenesis 2009, 24, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Totsuka, Y.; Hasei, T.; Watanabe, T.; Wakabayashi, K.; Kinae, N.; Masuda, S. In vivo examination of the genotoxicity of the urban air and surface soil pollutant, 3;6-dinitrobenzo[e]pyrene; with intraperitoneal and intratracheal administration. Environ. Toxicol. 2013, 28, 588–594. [Google Scholar] [CrossRef] [PubMed]
- IPCS. Selected Nitro- and Nitro-oxy-polycyclic Aromatic Hydrocarbons. Environ Health Criteria No. 229. In International Programme on Chemical Safety; IPCS: Geneva, Switzerland, 2003; pp. 1–400. [Google Scholar]
- Collins, J.; Brown, J.; Alexeeff, G.; Salmon, A. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef]
- Davie-Martin, C.L.; Stratton, K.G.; Teeguarden, J.G.; Waters, K.M.; Simonich, S.L.M. Implications of bioremediation of polycyclic aromatic hydrocarbon contaminated soils for human health and cancer risk. Environ. Sci. Technol. 2017, 51, 9458–9468. [Google Scholar] [CrossRef]
- Pedersen, D.U.; Durant, J.L.; Penman, B.W.; Crespi, C.L.; Hemond, H.F.; Lafleur, A.L. Human-cell mutagens in respirable airborne particles in the northeastern United States. 1. Mutagenicity of fractionated samples. Environ. Sci. Technol. 2004, 38, 682–689. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs), a review. Front. Microbiol. 2016, 7, 1369. [Google Scholar]
- Benigni, R.; Bossa, C. Mechanisms of chemical carcinogenicity and mutagenicity, a review with implications for predictive toxicology. Chem. Rev. 2011, 111, 2507–2536. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.C.; Chlebowski, A.C.; Truong, L.; Massey Simonich, S.L.; Anderson, K.A.; Tanguay, R.L. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Arch. Toxicol. 2018, 92, 571–586. [Google Scholar] [CrossRef]
- Wincent, E.; Jönsson, M.E.; Bottai, M.; Lundstedt, S.; Dreij, K. Aryl hydrocarbon receptor activation and developmental toxicity in zebrafish in response to soil extracts containing unsubstituted and oxygenated PAHs. Environ. Sci. Technol. 2015, 49, 3869–3877. [Google Scholar] [CrossRef]
- Dranka, B.P.; Benavides, G.A.; Diers, A.R.; Giordano, S.; Zelickson, B.R.; Reily, C.; Zou, L.; Chatham, J.C.; Hill, B.G.; Zhang, J.; et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011, 51, 1621–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knecht, A.L.; Goodale, B.C.; Truong, L.; Simonich, M.T.; Swanson, A.J.; Matzke, M.M.; Anderson, K.A.; Waters, K.M.; Tanguay, R.L. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 2013, 271, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beije, B.; Moller, L. 2-Nitrofluorene and related compounds, prevalence and biological effects. Mutat. Res. 1988, 196, 177–209. [Google Scholar] [CrossRef] [PubMed]
- El-Alawi, Y.S.; McConkey, B.J.; Dixon, D.G.; Greenberg, B.M. Measurement of short- and long-term toxicity of polycyclic aromatic hydrocarbons using luminescent bacteria. Ecotoxicol. Environ. Saf. 2002, 51, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Nakamura, J.; Richardson, S.D.; Aitken, M.D. Evaluating the effects of bioremediation on genotoxicity of polycyclic aromatic hydrocarbon contaminated soil using genetically engineered; higher eukaryotic cell lines. Environ. Sci. Technol. 2012, 46, 4607–4613. [Google Scholar] [CrossRef] [Green Version]
- Musa Bandowe, B.A.; Bigalke, M.; Kobza, J.; Wilcke, W. Sources and fate of polycyclic aromatic compounds (PAHs; oxygenated PAHs and azaarenes) in forest soil profiles opposite of an aluminium plant. Sci. Total Environ. 2018, 630, 83–95. [Google Scholar] [CrossRef]
- Aranda, E.; Godoy, P.; Reina, R.; Badia-Fabregat, M.; Rosell, M.; Marco-Urrea, E.; García- Romera, I. Isolation of Ascomycota fungi with capability to transform PAHs, insights into the biodegradation mechanisms of Penicillium oxalicum. Int. Biodeterior. Biodegrad. 2017, 122, 141–150. [Google Scholar] [CrossRef]
- Behera, B.K.; Das, A.; Sarkar, D.J.; Weerathunge, P.; Parida, P.K.; Das, B.K.; Thavamani, P.; Ramanathan, R.; Bansal, V. Polycyclic Aromatic Hydrocarbons (PAHs) in inland aquatic ecosystems, perils and remedies through biosensors and bioremediation. Environ. Pollut. 2018, 241, 212–233. [Google Scholar] [CrossRef]
- Li, Y.; Liao, X.; Huling, S.G.; Xue, T.; Liu, Q.; Cao, H.; Lin, Q. The combined effects of surfactant solubilization and chemical oxidation on the removal of polycyclic aromatic hydrocarbon from soil. Sci. Total Environ. 2019, 647, 1106–1112. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef]
- Geng, S.Y.; Xu, G.M.; Cao, W.; You, Y.; Zhu, Y.; Ding, A.Z.; Fan, F.Q.; Dou, J.F. Occurrence of polycyclic aromatic compounds and potentially toxic elements contamination and corresponding interdomain microbial community assembly in soil of an abandoned gas station. Environ. Res. 2022, 212, 113618. [Google Scholar] [CrossRef] [PubMed]
- Bourhane, Z.; Lanzén, A.; Cagnon, C.; Said, O.B.; Mahmoudi, E.; Coulon, F.; Atai, E.; Borja, A.; Cravo-Laureau, C.; Duran, R. Microbial diversity alteration reveals biomarkers of contamination in soil-river-lake continuum. J. Hazard Mater. 2022, 421, 126789. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Teng, Y.; Yao, H.Y.; Christie, P. Detection of functional microorganisms in benzene [a] pyrene-contaminated soils using DNA-SIP technology. J. Hazard Mater. 2021, 407, 124788. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Hong, Y.; Odinga, E.S.; Liu, J.; Tsang, D.C.W.; Gao, Y.Z. Bacterial community and PAH-degrading genes in paddy soil and rice grain from PAH-contaminated area. Appl. Soil Ecol. 2021, 158, 103789. [Google Scholar] [CrossRef]
- Chen, Z.S.; Hu, H.Y.; Xu, P.; Tang, H.Z. Soil bioremediation by Pseudomonas brassicacearum MPDS and its enzyme involved in degrading PAHs. Sci. Total Environ. 2022, 813, 152522. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Hu, H.Y.; Zanaroli, G.; Xu, P.; Tang, H.Z. A Pseudomonas sp. strain uniquely degrades PAHs and heterocyclic derivatives via lateral dioxygenation pathways. J. Hazard Mater. 2021, 403, 123956. [Google Scholar] [CrossRef] [PubMed]
- Brzeszcz, J.; Kaszycki, P. Aerobic bacteria degrading both n-alkanes and aromatic hydrocarbons: An undervalued strategy for metabolic diversity and flexibility. Biodegradation 2018, 29, 359–407. [Google Scholar] [CrossRef]

| Region | Land Use | ∑OPAHs (ng g−1) | # of Oxygen-PAH | ∑NPAHs (ng g−1) | # of Nitro-PAH | References |
|---|---|---|---|---|---|---|
| Gårdsjön, Sweden | background area | 108 | 8 | 21 | 9 | [51] |
| Göteborg, Sweden | urban-diffuse | 42–466 | 11–286 | |||
| Patagonian, Argentina | steppe | 2.4–38 | 15 | 0.05–124 | 4 | [42] |
| Bangkok, Thailand | urban | 12–269 | 15 | 0.1–31 | 4 | [44] |
| Xi’an, China | suburban | 854 ± 447 | 15 | 118 ± 52 | 11 | [43] |
| Kumasi, Ghana | urban | 833 (57–4202) | 10 | 73 (3–240) | 4 | [45] |
| East China | agriculture | 9 ± 8 | 4 | 50 ± 45 | 11 | [52] |
| Kathmandu, Nepal | urban | 99 (95–384) | 3 | 646 (439–3930) | 16 | [53] |
| Pokhara, Nepal | 95 (92–433) | 568 (409–2030) | ||||
| Birgunj, Nepal | 115 (93–348) | 832 (451–1750) | ||||
| Biratnagar, Nepal | 606 (227–1150) | 2100 (586–24,100) | ||||
| Newcastle, Australia | recreation | 1515 | 7 | 211 | 3 | [54] |
| industry | 3924 | 602 | ||||
| smoking area | 644 | 239 | ||||
| resident | 3649 | 391 |
| Compound | Abbrev | Concentration | References |
|---|---|---|---|
| 1-indanone | 1-INDA | 1.6–35 (industrial area), 0.05–0.54 (steppe), 32.4–59.7 (suburban), 10.5–56.1(street dust) | [42,43,45,55] |
| 1,4-naphthoquinone | 1,4-NQ | 0.3–6.7 (industrial area), 2.2–10.7 (suburban), 157.7–1443.1 (industrial heritage) | [43,54,55] |
| 1-naphthaldehyde | 1-NLD | 1.4–20.6 (industrial area), 0.16 (steppe), 7.9–35 (suburban), 2.6–37.5 (street dust) | [42,43,45,55] |
| 2-biphenylcarboxaldehyde | 2-BPCD | 0.3–25 (industrial area), 2.15 (steppe), 10.9–22.8 (suburban) | [42,43,55] |
| 9-fluorenone | 9-FLO | 14–810 (industrial area), 1.02–2.82 (steppe), 371–1243.8 (suburban), 33.2–214.4 (street dust), 0.3–10 (farmland), 42.8–1818.1 (industrial heritage) | [42,43,45,52,54,55] |
| 1,2-acenaphthylenedione | 1,2-ACQ | 2.6–14 (industrial area), 1.2 (steppe), 2.4–6.7 (suburban) | [42,43,55] |
| 9,10-anthraquinone | 9,10-ANQ | 12–959 (industrial area), 0.88–8.9 (steppe), 36.7–92.9 (suburban), 18.1–1241.7 (street dust), 0.9–26(farmland), 33.2–202.7 (industrial heritage) | [42,43,45,52,54,55] |
| 4H-cyclopenta[d,e,f]phenanthrene-4-one | CPHENone | 12.2–48 (suburban), 5.6–131.2 (street dust) | [43,45] |
| 2-methyl-9,10-anthraquinone | 2-MANQ | 2.3–18 (steppe), 7.9–27.7 (suburban), 7.1–1602.9 (street dust) | [42,43,45] |
| benzo[a]fluorenone | B(A)FLUone | 0.13–5.2 (steppe), 7.1–37 (suburban), 5.7–919.6 (street dust) | [42,43,45] |
| 7H-benz[d,e]anthracene-7-one | BANTone | 0.43–16 (steppe), 3.5–19.2 (suburban), 12.7–200.2 (street dust), 25.8–388.3 (industrial heritage) | [42,43,45,54] |
| benzo[a]anthracene-7,12-dione | 7,12-B(A)A | 20 (steppe), 12.1–58.1 (suburban), 0.2–8.4(farmland) | [42,43,52] |
| 5,12-naphthacenequinone | 5,12-NACQ | 1.5–2.4 (steppe), 2.9–28.6 (suburban) | [42,43] |
| 6H-benzo[c,d]pyrene-6-one | 6-BPYRone | 1.9–37 (steppe), 2–10.7 (suburban), 6.0–66.0 (street dust) | [42,43,45] |
| Compound | Abbrev | Concentration (ng g−1) | References |
|---|---|---|---|
| 1-Nitronaphthalene | 1-NNAPH | 0.4–1.8 (suburban), 0.2–267 (farmland), 51.9–77.6 (urban dust), 49.6–205 (industrial heritage) | [43,52,53,54] |
| 2-Nitrobiphenyl | 2-NBP | 0.4 (suburban), 0.3–66 (farmland) | [43,52] |
| 5-Nitroacenaphthene | 5-NACEN | 0.5–3.5 (suburban), 0.7–7.7 (farmland), 35.3–47.8 (urban dust) | [43,52,53] |
| 2-Nitrofluorene | 2-NFLU | 0.2–0.8 (suburban), 7.5–9.2 (farmland), 21.8–631 (urban dust), 88.7–196 (industrial heritage) | [43,52,53,54] |
| 9-Nitrophenanthrene | 9-NPHEN | 0.4–1.9 (suburban), 0.4–17 (farmland), 7.7–84.1 (urban dust) | [43,52,53] |
| 9-nitroanthracene | 9-NANT | 0.1–0.7 (suburban), 0.2–8.5 (farmland), 0.14–1.24 (urban dust), 21–271(industrial heritage) | [43,52,53,54] |
| (2 + 3)-Nitrofluoranthenes | 2 + 3-NFLA | 5.2–16.3 (suburban), 0.4–29 (farmland) | [43,52] |
| 1-Nitropyrene | 1-NPYR | 2.3–6.0 (suburban), 8.8 (farmland) | [43,52] |
| 2,7-Dinitrofluorene | 2,7-DNFLU | 13.0–39.0 (suburban), 2.0–41.0 (farmland), 28.1–44.9 (urban dust) | [43,52,53] |
| 6-Nitrochrysene | 6-NCHR | 5.7–11.4 (suburban), 26.3–67.2 (urban dust) | [43,53] |
| 6-Nitrobenzo[a]pyrene | 6-NBaP | 49.9–86.1 (suburban), 50.3–65.9 (urban dust) | [43,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Yuan, J.; Geng, S.; Zou, J.; Dou, J.; Fan, F. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons: Sources, Quantification, Incidence, Toxicity, and Fate in Soil—A Review Study. Processes 2023, 11, 52. https://doi.org/10.3390/pr11010052
Cao W, Yuan J, Geng S, Zou J, Dou J, Fan F. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons: Sources, Quantification, Incidence, Toxicity, and Fate in Soil—A Review Study. Processes. 2023; 11(1):52. https://doi.org/10.3390/pr11010052
Chicago/Turabian StyleCao, Wei, Jing Yuan, Shuying Geng, Jing Zou, Junfeng Dou, and Fuqiang Fan. 2023. "Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons: Sources, Quantification, Incidence, Toxicity, and Fate in Soil—A Review Study" Processes 11, no. 1: 52. https://doi.org/10.3390/pr11010052




