Cellulose Acetate Film Containing Bonechar for Removal of Metribuzin from Contaminated Drinking Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drinking Water Samples
2.2. Bonechar and Cellulose Acetate Film
2.3. Sorption–Desorption Metribuzin
2.4. Freundlich Model for Sorption–Desorption and Apparent Coefficient
3. Results and Discussion
3.1. Synthesis of the Acetate Film with Added Bonechar
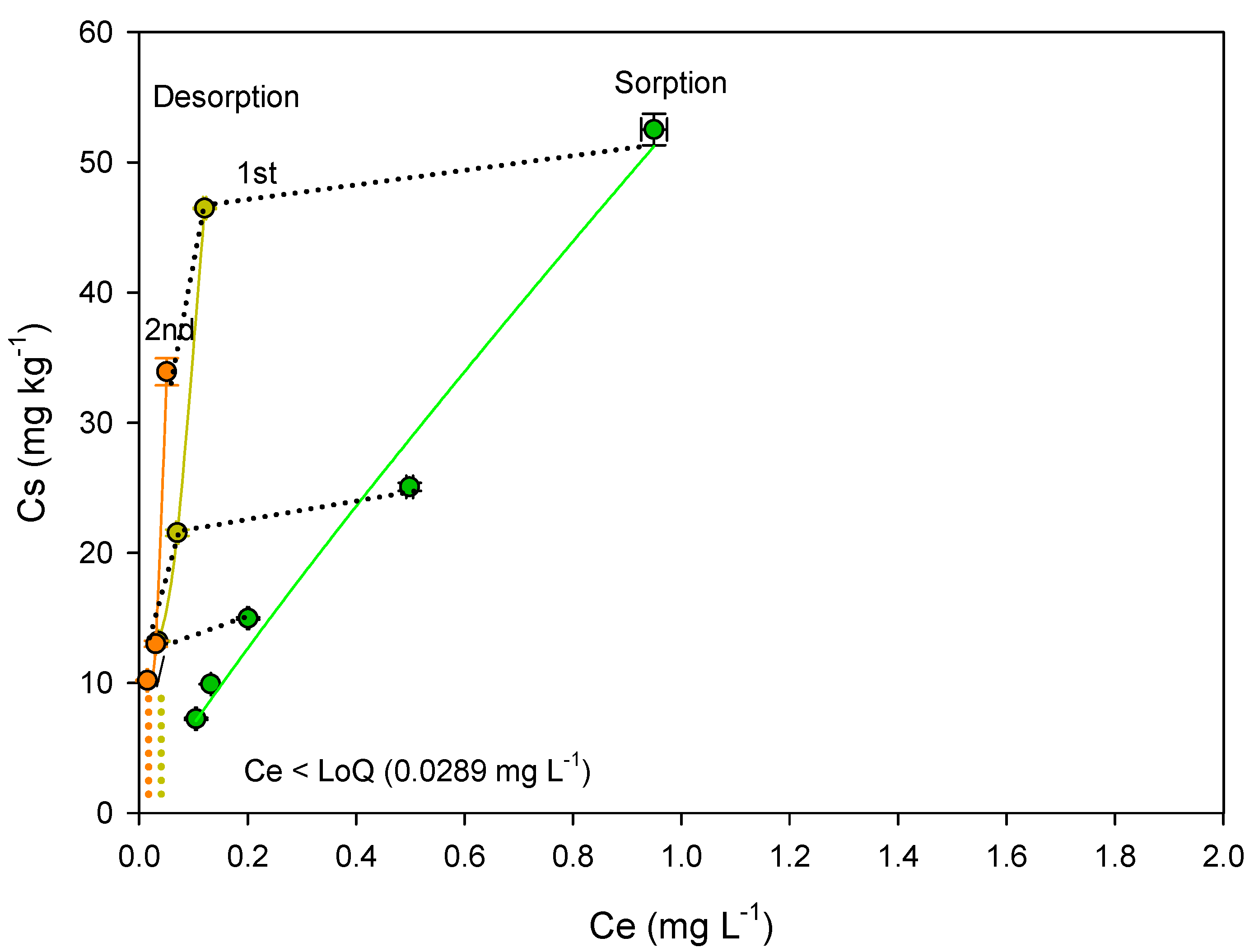
3.2. Removal (Sorption/Desorption) of Metribuzin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vryzas, Z. Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Curr. Opin. Environ. Sci. Health 2018, 4, 5–9. [Google Scholar] [CrossRef]
- Mendes, K.F.; Régo, A.P.J.; Takeshita, V.; Tornisielo, V.L. Water resource pollution by herbicide residues. In Biochemical Toxicolog—Heavy Metals and Nanomaterials; Ince, M., Ince, O.K., Ondrasek, G., Eds.; IntechOpen: London, UK, 2019; pp. 1–16. [Google Scholar]
- Peña, A.; Delgado-Moreno, L.; Rodríguez-Liébana, J.A. A review of the impact of wastewater on the fate of pesticides in soils: Effect of some soil and solution properties. Sci. Total Environ. 2020, 718, 134468. [Google Scholar] [CrossRef] [PubMed]
- SDWF-Safe Drinking Water Foundation. Pesticides and Water Pollution. 2017. Available online: https://www.safewater.org/fact-sheets-1/2017/1/23/pesticides (accessed on 6 August 2022).
- Giuliano, S.; Alletto, L.; Deswarte, C.; Perdrieux, F.; Daydé, J.; Debaeke, P. Reducing herbicide use and leaching in agronomically performant maize-based cropping systems: An 8-year study. Sci. Total Environ. 2021, 788, 147695. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.; Köck-Schulmeyer, M.; Alvarenga, P.; Ledo, L.; Barbosa, I.R.; López de Alda, M.; Barceló, D. Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci. Total Environ. 2014, 488, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lv, J.; Deng, H.; Liu, Q.; Liang, S. Occurrence and removal of triazine herbicides during wastewater treatment processes and their environmental impact on aquatic life. Int. J. Environ. Res. Public Health 2022, 19, 4557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Du, J.; Dong, X.; Huang, Y.; Xie, H.; Chen, J.; Kadokami, K. Occurrence and ecological risks of 156 pharmaceuticals and 296 pesticides in seawater from mariculture areas of Northeast China. Sci. Total Environ. 2021, 792, 148375. [Google Scholar] [CrossRef]
- Köck-Schulmeyer, M.; Ginebreda, A.; González, S.; Cortina, J.L.; De Alda, M.L.; Barceló, D. Analysis of the occurrence and risk assessment of polar pesticides in the Llobregat River Basin (NE Spain). Chemosphere 2012, 86, 8–16. [Google Scholar] [CrossRef]
- Santos, E.A.; Correia, N.M.; Silva, J.R.M.; Velini, E.D.; Passos, A.B.R.J.; Durigan, J.C. Herbicide detection in groundwater in Córrego Rico-SP watershed. Planta Daninha 2015, 33, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.S.; Duaví, W.C.; Cavalcante, R.M.; Milhome, M.A.L.; Nascimento, R.F. Estimated levels of environmental contamination and health risk assessment for herbicides and insecticides in surface water of Ceará, Brazil. Bull. Environ. Contam. Toxicol. 2016, 96, 90–95. [Google Scholar] [CrossRef]
- Pires, N.L.; Passos, C.J.S.; Morgado, M.G.; Mello, D.C.; Infante, C.M.C.; Caldas, E.D. Determination of glyphosate, AMPA and glufosinate by high performance liquid chromatography with fluorescence detection in waters of the Santarém Plateau, Brazilian Amazon. J. Environ. Sci. Health Part B 2020, 55, 794–802. [Google Scholar] [CrossRef]
- Correia, N.M.; Carbonari, C.A.; Velini, E.D. Detection of herbicides in water bodies of the Samambaia River sub-basin in the Federal District and eastern Goiás. J. Environ. Sci. Health Part B 2020, 55, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Dores, E.F.G.C.; Navickiene, S.; Cunha, M.L.; Carbo, L.; Ribeiro, M.L.; De-Lamonica-Freire, E.M. Multiresidue determination of herbicides in environmental waters from Primavera do Leste Region (Middle West of Brazil) by SPE-GC-NPD. J. Braz. Chem. Soc. 2006, 17, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Brazil Ministry of Health. Office of the Minister. Ordinance nº. 888, of May 4, 2021. Amends Annex XX of the Consolidation Ordinance GM/MS nº. 5, of September 28, 2017, to Dispose on the Procedures for Control and Surveillance of the Quality of Water for Human Consumption and Its Potability Standard. Diário Oficial da União, Brasília, DF. 4 May 2021; p. 127. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 1 November 2022).
- Saritha, J.D.; Ramprakash, T.; Rao, P.C.; Madhavi, M. Persistence of metribuzin in tomato growing soils and tomato fruits. Nat. Environ. Pollut. Technol. 2017, 16, 505. [Google Scholar]
- Guimarães, A.C.D.; Mendes, K.F.; Campion, T.F.; Christoffoleti, P.J.; Tornisielo, V.L. Leaching of herbicides commonly applied to sugarcane in five agricultural soils. Planta Daninha. 2019, 37, e019181505. [Google Scholar] [CrossRef] [Green Version]
- PPDB–Pesticide Properties Database. Footprint: Creating Tools for Pesticide Risk Assessment and Management in Europe. Developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, funded by UK National Sources and the EU-Funded FOOTPRINT project (FP6-SSP-022704). Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/469.htm (accessed on 10 March 2022).
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–13. [Google Scholar]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Li, L.; Zou, D.; Xiao, Z.; Zeng, X.; Zhang, L.; Jiang, L.; Liu, F. Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J. Cleaner Product. 2019, 210, 1324–1342. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chai, L.Y.; Shu, Y.D. Study of arsenic (V) adsorption on bone char from aqueous solution. J. Hazard. Mater. 2008, 160, 168–172. [Google Scholar] [CrossRef]
- Mendes, K.F.; Freguglia, R.M.O.; Martins, B.A.B.; Dias, R.C.; Pimpinato, R.F.; Tornisielo, V.L. Cow bonechar for pesticide removal from drinking water. J. Agric. Vet. Sci. 2017, 4, 504–512. [Google Scholar]
- Lipczynska-Kochany, E. Effect of climate change on humic substances and associated impacts on the quality of surface water and groundwater: A review. Sci. Total Environ. 2018, 640, 1548–1565. [Google Scholar] [CrossRef]
- Da Ros, S.; Aliev, A.E.; del Gaudio, I.; King, R.; Pokorska, A.; Kearney, M.; Curran, K. Characterising plasticised cellulose acetate-based historic artefacts by NMR spectroscopy: A new approach for quantifying the degree of substitution and diethyl phthalate contents. Polym. Degrad. Stab. 2021, 183, 109420. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and applications of cellulose acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Pinto, M.D.C.E.; Da Silva, D.D.; Gomes, A.L.A.; Leite, V.D.S.A.E.; Moraes, A.R.F.; De Novais, R.F.; Tronto, J.; Pinto, F.G. Film based on magnesium impregnated biochar/cellulose acetate for phosphorus adsorption from aqueous solution. RSC Advances 2019, 9, 5620–5627. [Google Scholar] [CrossRef] [PubMed]
- Vinhal, J.; Lima, C.; Cassella, R. Sorption of the herbicides diquat and difenzoquat from aqueous medium by polymeric resins in the presence of sodium dodecylsulfate: Kinetic and mechanistic study. J. Environ. Sci. Health B 2016, 51, 482–489. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, N.; Singh, S. Removal of herbicides mixture of atrazine, metribuzin, metolachlor and alachlor from water using granular carbon. Indian J. Chem. Technol. 2017, 24, 400–404. [Google Scholar]
- Zhong, B.; Wang, S.; Dong, H.; Luo, Y.; Jia, Z.; Zhou, X.; Chen, M.; Xie, D.; Jia, J. Halloysite tubes as nanocontainers for herbicide and its controlled release in biodegradable poly(vinyl alcohol)/starch film. J. Agric. Food Chem. 2017, 65, 10445–10451. [Google Scholar] [CrossRef]
- Shattar, S.; Zalzaria, N.; Foo, K. Preparation of a montmorillonite-derived adsorbent for the practical treatment of ionic and nonionic pesticides. J. Mater. Res. Technol. 2019, 8, 4713–4724. [Google Scholar] [CrossRef]
- Liu, L.; Dai, Y. Strong adsorption of metolachlor by biochar prepared from walnut shells in water. Environ. Sci. Pollut. Res. 2021, 28, 48379–48391. [Google Scholar] [CrossRef]
- Mendes, K.F.; Hall, K.E.; Takeshita, V.; Rossi, M.L.; Tornisielo, V.L. Animal bonechar increases sorption and decreases leaching potential of aminocyclopyrachlor and mesotrione in a tropical soil. Geoderma 2018, 316, 11–18. [Google Scholar] [CrossRef]
- Mendes, K.F.; de Sousa, R.N.; Takeshita, V.; Alonso, F.G.; Régo, A.P.J.; Tornisielo, V.L. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin, and quinclorac in soil. Geoderma 2019, 343, 40–49. [Google Scholar] [CrossRef]
- Nilsson, R.; Olsson, M.; Westman, G.; Matic, A.; Larsson, A. Screening of hydrogen bonds in modified cellulose acetates with alkyl chain substitutions. Carbohydr. Polym. 2022, 285, 119188. [Google Scholar] [CrossRef]
- OECD-Organisation for Economic Co-Operation and Development. Adsorption—Desorption Using A Batch Equilibrium Method; OECD: Paris, France, 2000; 44p, OECD, 106. [Google Scholar]
- Mendes, K.F.; Sousa, R.N.; Soares, M.B.; Viana, D.G.; Souza, A.J. Sorption and desorption studies of herbicides in the soil by batch equilibrium and stirred flow methods. In Radioisotopes in Weed Research; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 17–61. [Google Scholar]
- López-Piñeiro, A.; Peña, D.; Albarrán, A.; Becerra, D.; Sánchez-Llerena, J. Sorption, leaching and persistence of metribuzin in Mediterranean soils amended with olive mill waste of different degrees of organic matter maturity. J. Environ. Manag. 2013, 122, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, E.; Parlavecchia, M.; Perri, G.; Gattullo, R. Comparative assessment of metribuzin sorption efficiency of biochar, hydrochar and vermicompost. J. Environ. Sci. Health Part B 2019, 54, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Cox, L.; Spokas, K.U.R.T.; Hermosín, M.C.; Cornejo, J.; Koskinen, W.C. Influence of biochar amendments on the sorption-desorption of aminocyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Sci. Total Environ. 2014, 470, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.K.; Kim, J.Y.; Choi, Y.G. Synthesis of bone char from cattle bones and its application for fluoride removal from the contaminated water. Groundw. Sustain. Dev. 2019, 8, 324–331. [Google Scholar] [CrossRef]
- Nigri, E.M.; Bhatnagar, A.; Rocha, S.D.F. Thermal regeneration process of bone char used in the fluoride removal from aqueous solution. J. Clean Prod. 2017, 142, 3558–3570. [Google Scholar] [CrossRef]
- Mendes, K.F.; Furtado, I.F.; Sousa, R.N.D.; Lima, A.D.C.; Mielke, K.C.; Brochado, M.G.D.S. Cow bonechar decreases indaziflam pre-emergence herbicidal activity in tropical soil. J. Environ. Sci. Health Part B 2021, 56, 532–539. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Removal of methylene blue from aqueous solution by bone char. Appl. Sci. 2018, 8, 1903. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Li, F.; Tian, Q.; Nie, C.; Ma, Y.; Zhu, Z.; Liu, S. A highly porous animal bone-derived char with a superiority of promoting nZVI for Cr (VI) sequestration in agricultural soils. J. Environ. Sci. 2021, 104, 27–39. [Google Scholar] [CrossRef]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; Marchuk, A. Inorganic arsenic species removal from water using bone char: A detailed study on adsorption kinetic and isotherm models using error functions analysis. J. Hazard. Mater. 2021, 405, 124112. [Google Scholar] [CrossRef]
- Azeem, M.; Shaheen, S.M.; Ali, A.; Jeyasundar, P.G.; Latif, A.; Abdelrahman, H.; Li, R.; Almazroui, M.; Niazi, N.K.; Sarmah, A.K.; et al. Removal of potentially toxic elements from contaminated soil and water using bone char compared to plant-and bone-derived biochars: A review. J. Hazard. Mater. 2022, 427, 128131. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; El-Naas, M.H. Comparative study between adsorption and membrane technologies for the removal of mercury. Sep. Purif. Technol. 2021, 257, 117833. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Liu, J.; Wang, W.; Zhang, W.; Dong, F. Adsorption of arsenic (V) on bone char: Batch, column and modeling studies. Environ. Earth Sci. 2014, 72, 2081–2090. [Google Scholar] [CrossRef]
- Hyder, A.H.M.G.; Begum, S.A.; Egiebor, N.O. Adsorption isotherm and kinetic studies of hexavalent chromium removal from aqueous solution onto bone char. J. Environ. Chem. Eng. 2015, 3, 1329–1336. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Ara, B.; Shah, J.; Jan, M.R.; Aslam, S. Removal of metribuzin herbicide from aqueous solution using corn cob. Int. J. Sci. Environ. Technol. 2013, 2, 146–161. [Google Scholar]
- Cara, I.G.; Filip, M.; Bulgariu, L.; Raus, L.; Topa, D.; Jitareanu, G. Environmental remediation of metribuzin herbicide by mesoporous carbon rich from wheat straw. Appl. Sci. 2021, 11, 4935. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Hernandez-Montoya, V.; Moreno-Virgen, M.R.; Tovar-Gómez, R.; Montes-Morán, M.A. Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrolysis 2013, 104, 10–18. [Google Scholar] [CrossRef]
- Sousa, R.N.; Soares, M.B.; Santos, F.H.; Leite, C.N.; Mendes, K.F. Interaction mechanisms between biochar and herbicides. In Interactions of Biochar and Herbicides in the Environment; Mendes, K.F., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 80–105. [Google Scholar]
- Xiao, F.; Pignatello, J.J. π+–π Interactions between (Hetero) aromatic Amine cations and the graphitic surfaces of pyrogenic carbonaceous materials. Environ. Sci. Technol. 2015, 49, 906–914. [Google Scholar] [CrossRef]
- Essandoh, M.; Wolgemuth, D.; Pittman, C.U.; Mohan, D.; Mlsna, T. Adsorption of metribuzin from aqueous solution using magnetic and nonmagnetic sustainable low-cost biochar adsorbents. Environ. Sci. Pollut. Res. 2017, 24, 4577–4590. [Google Scholar] [CrossRef]
- Landgraf, M.D.; da Silva, S.C.; Rezende, M.O.D.O. Mechanism of metribuzin herbicide sorption by humic acid samples from peat and vermicompost. Anal. Chem. Acta. 1998, 368, 155–164. [Google Scholar] [CrossRef]
- Cerejeira, M.J.; Viana, P.; Batista, S.; Pereira, T.; Silva, E.; Valério, M.J.; Silva-Fernandes, A.M. Pesticides in Portuguese surface and ground waters. Water Res. 2003, 37, 1055–1063. [Google Scholar] [CrossRef]
- Castro, G.F.; Ferreira, J.A.; Eulálio, D.; Moraes, A.R.F.; Regina, V.; Constantino, L.; Pinto, F.G.; Novais, R.F.; Tronto, J. Organic-Inorganic Hybrid Materials: Layered Double Hydroxides and Cellulose Acetate Films as Phosphate Recovery. J. Agric. Sci. Technol. B 2018, 8, 360–374. [Google Scholar] [CrossRef] [Green Version]
- Kabiri, K.; Zohuriaan-Mehr, M. Superabsorbent hydrogel composites. Polym. Adv. Technol. 2003, 14, 438–444. [Google Scholar] [CrossRef]
- Nazir, M.S.; Tahir, Z.; Hassan, S.U.; Ali, Z.; Akhtar, M.N.; Azam, K.; Abdullah, M.A. Remediation of pesticide in water. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: New Delhi, India, 2021; pp. 271–307. [Google Scholar]
- Mphateng, T.N.; Mapossa, A.B.; Wesley-Smith, J.; Ramjee, S.; Focke, W.W. Cellulose acetate/organoclay nanocomposites as controlled release matrices for pest control applications. Cellulose 2022, 29, 3915–3933. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]

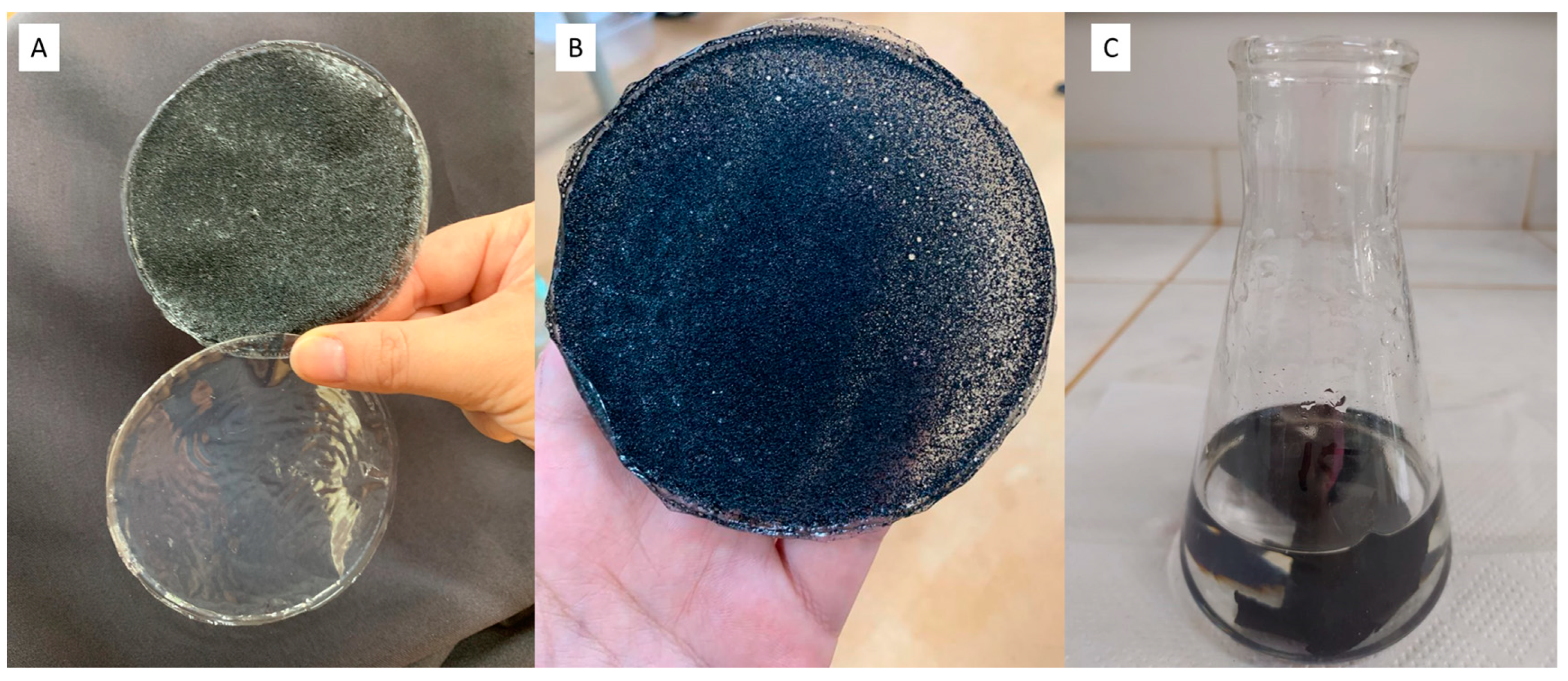


| Properties | Values |
|---|---|
| Electrical conductivity (µS cm−1) | 117.20 |
| Hardness (mg CaCO3 L−1) | 22.00 |
| Alkalinity (mg L−1) | 25.75 |
| Total residual chlorine (mg L−1) | 1.34 |
| pH | 7.25 |
| Turbidity (uT) | 0.18 |
| Temperature (°C) | 16.90 |
| Apparent color (uC) | 3.60 |
| Properties | Values |
|---|---|
| Feedstock | Cow bone |
| Production temperature (°C) | 800 |
| Total surface area (m2 g−1) | 200 |
| Carbon surface area (m2 g−1) | 50 |
| Carbon content (%) | 11 |
| pH (H2O) | 9.12 |
| Soluble ash in acid (%) | <3 |
| Insoluble ash content (%) | 0.7 |
| Tricalcium phosphate (%) | 70 |
| Calcium sulphate (%) | 0.1 |
| Iron (%) | <0.3 |
| Pore size (nm) | 7.5–60.000 |
| Pore volume (cm3 g−1) | 0.225 |
| Micropore area (m2 g−1) | 133 |
| Humidity (%) | <5 |
| Density (g cm3) | 0.65 |
| Carbonaceous Material | Kf | 1/n | R2 | Metribuzin (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.33 | 0.5 | 1.0 | 2.0 | ||||
| (mg(1−1/n) L1/n kg−1) | Kd-app (L kg−1) | |||||||
| Bonechar pure powder (2 g) | <LoQ a | - | - | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| (%) sorbed | - | - | - | 100 | 100 | 100 | 100 | 100 |
| Acetate film with bonechar (2 g) | 19.06 ± 0.07 b | 0.43 ± 0.03 | 0.995 | 52.01 ± 0.02 | 51.21 ± 0.03 | 58.21 ± 0.03 | 18.58 ± 0.04 | 14.33 ± 0.04 |
| (%) sorbed | - | - | - | 40.95 ± 0.01 | 40.08 ± 0.05 | 43.70 ± 0.02 | 19.86 ± 0.03 | 16.04 ± 0.02 |
| Acetate film with bonechar (3 g) | 53.68 ± 0.05 | 0.89 ± 0.02 | 0.992 | 78.92 ± 0.03 | 75.05 ± 0.04 | 74.43 ± 0.04 | 50.23 ± 0.01 | 55.30 ± 0.03 |
| (%) sorbed | - | - | - | 67.95 ± 0.01 | 60.01 ± 0.03 | 59.81 ± 0.03 | 50.11 ± 0.02 | 52.51 ± 0.06 |
| Treatments | Sorption | 1st Desorption (24 h) | 2nd Desorption (120 h) |
|---|---|---|---|
| Water (with herbicide) | 6.91 | 6.69 | 6.85 |
| Bonechar pure powder (2 g) | 8.72 | 8.59 | 7.87 |
| Acetate film with bonechar (2 g) | 7.14 | 7.22 | 7.39 |
| Acetate film with bonechar (3 g) | 7.72 | 7.25 | 7.02 |
| Carbonaceous Material | Metribuzin (mg L−1) | Metribuzin (mg L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.33 | 0.5 | 1.0 | 2.0 | 0.25 | 0.33 | 0.5 | 1.0 | 2.0 | |
| Kd-app (L kg−1)—1st Desorption (24 h) | Kd-app (L kg−1)—2nd Desorption (120 h) | |||||||||
| Bonechar pure powder (2 g) | <LoQ a | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| (%) desorbed | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acetate film with bonechar (2 g) | <LoQ | <LoQ | 113.65 ± 0.01 b | 111.54 ± 0.04 | 135.27 ± 0.04 | <LoQ | <LoQ | 145.85 ± 0.02 | 194.83 ± 0.01 | 230.87 ± 0.04 |
| (%) desorbed | 0 | 0 | 7.50 ± 0.08 | 7.09 ± 0.05 | 7.47 ± 0.01 | 0 | 0 | 2.50 ± 0.04 | 2.93 ± 0.04 | 2.64 ± 0.07 |
| Acetate film with bonechar (3 g) | <LoQ | <LoQ | 373.65 ± 0.04 | 304.94 ± 0.04 | 384.40 ± 0.03 | <LoQ | <LoQ | 223.25 ± 0.06 | 384.40 ± 0.06 | 859.47 ± 0.08 |
| (%) desorbed | 0 | 0 | 6.50 ± 0.07 | 7.05 ± 0.03 | 6.04 ± 0.05 | 0 | 0 | 2.20 ± 0.01 | 3.06 ± 0.04 | 2.55 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielke, K.C.; Castro, G.F.; Mendes, K.F. Cellulose Acetate Film Containing Bonechar for Removal of Metribuzin from Contaminated Drinking Water. Processes 2023, 11, 53. https://doi.org/10.3390/pr11010053
Mielke KC, Castro GF, Mendes KF. Cellulose Acetate Film Containing Bonechar for Removal of Metribuzin from Contaminated Drinking Water. Processes. 2023; 11(1):53. https://doi.org/10.3390/pr11010053
Chicago/Turabian StyleMielke, Kamila C., Gustavo F. Castro, and Kassio F. Mendes. 2023. "Cellulose Acetate Film Containing Bonechar for Removal of Metribuzin from Contaminated Drinking Water" Processes 11, no. 1: 53. https://doi.org/10.3390/pr11010053







