A Simultaneous Design and Optimization Framework for the Reaction and Distillation Sections of Methanol to Olefins Process
Abstract
1. Introduction
2. Interaction between the Reaction and Distillation Sections
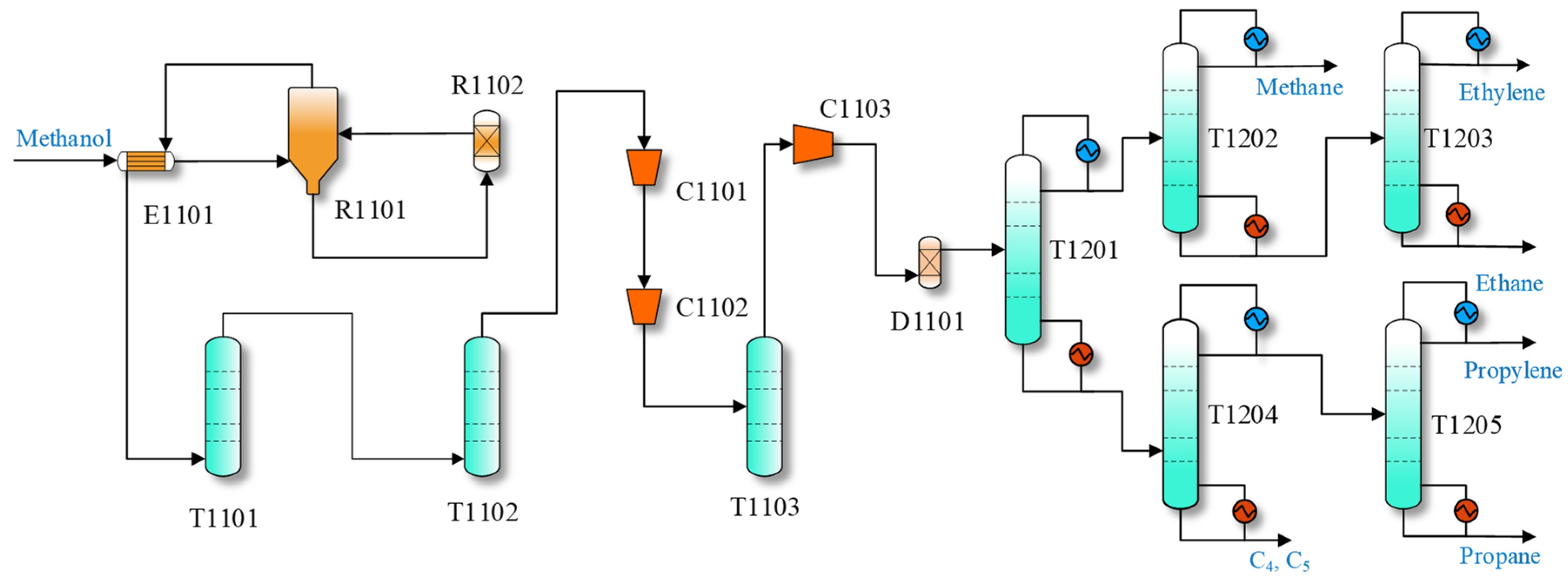
MTO Process
3. Optimization Model
3.1. Model of Physical Properties
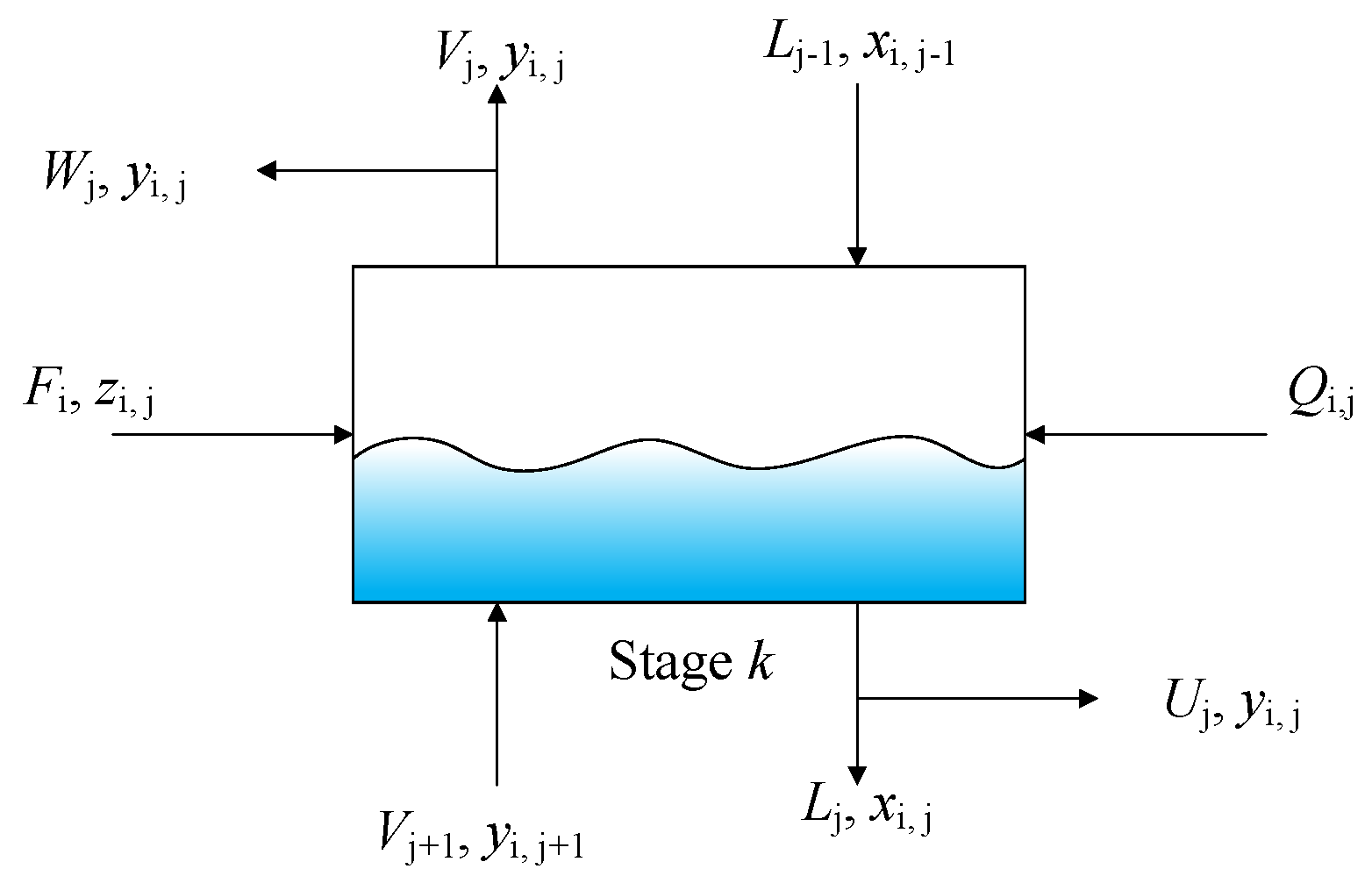
3.2. Model of Distillation
3.3. Models of Optimization
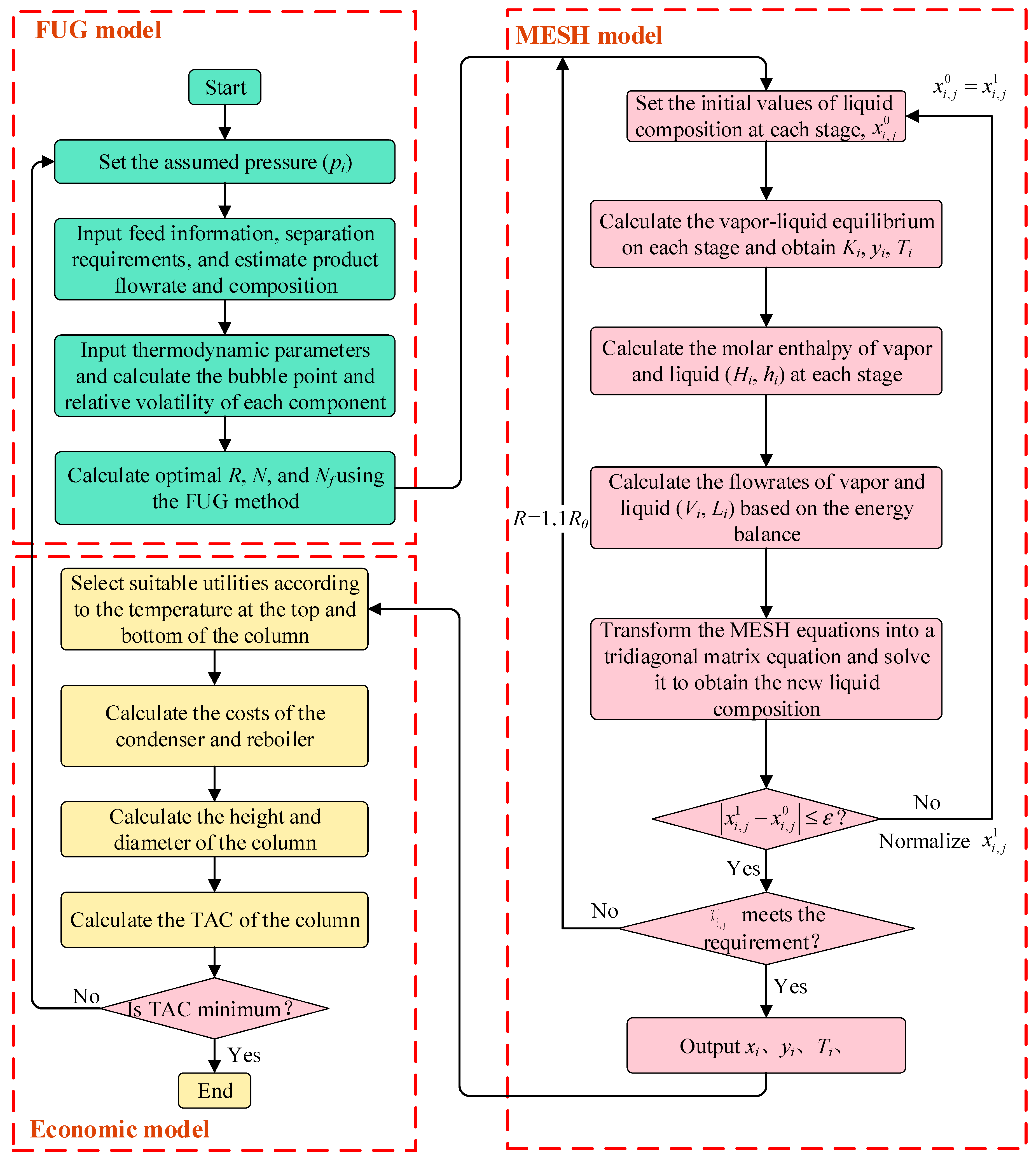
3.3.1. Evaluation of Distillation Columns
3.3.2. Optimization of Distillation Columns
3.3.3. Optimization of Reaction—Distillation System
- (1)
- Calculate the flowrate and composition of the products.
- (2)
- Estimate the flowrates and compositions of the feed and product according to the separation requirements and mass conservation.
- (3)
- Optimize columns according to the steps mentioned in Section 3.3.2, and target optimal parameters and utilities.
- (4)
- Select the units between adjacent columns and calculate their TACs.
- (5)
- Calculate the profit of the MTO process.
4. Case Study
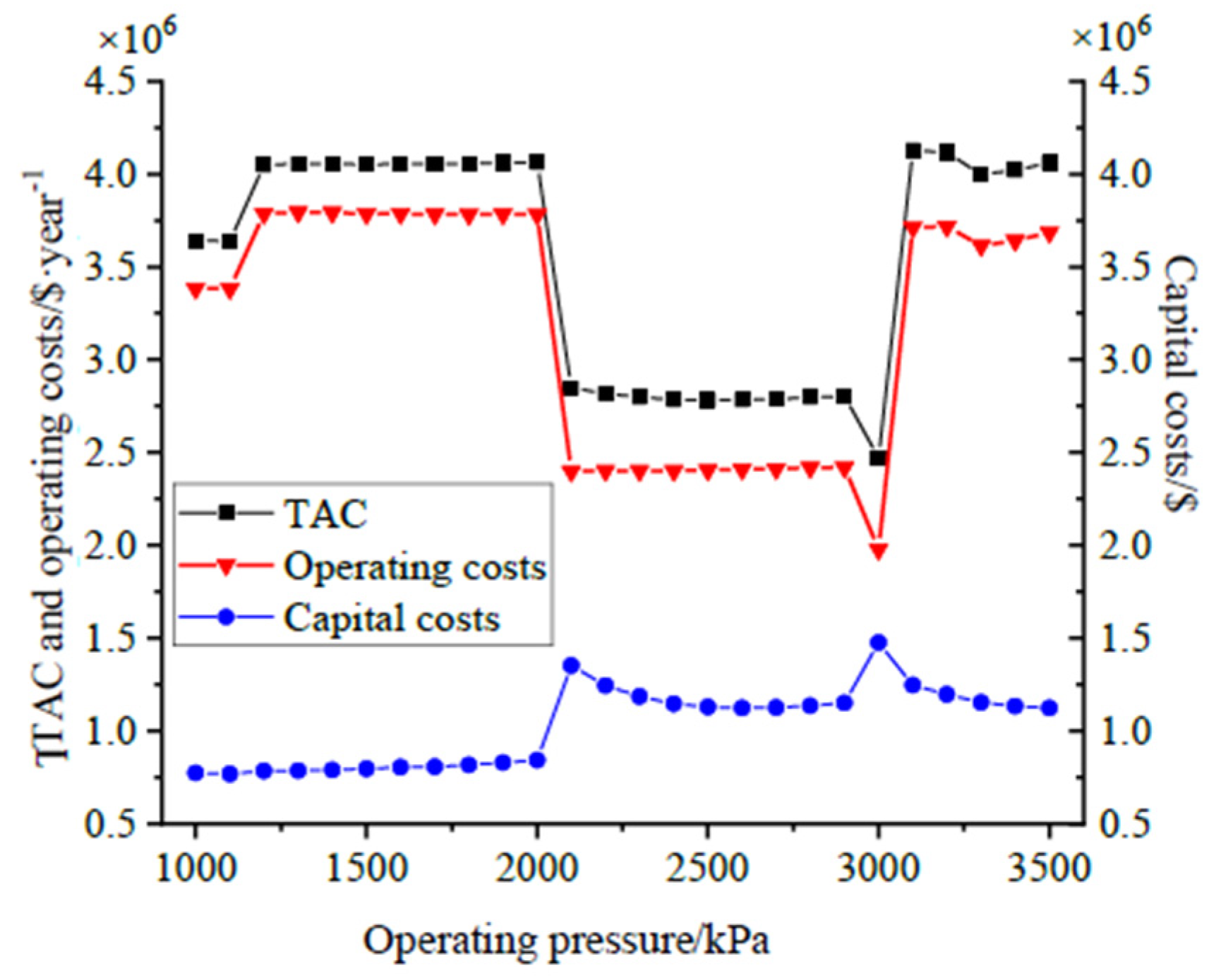
4.1. Optimization of Distillation Columns
4.2. Optimization for Reaction–Distillation System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| a, b | the relevant parameters of the component |
| am, bm | the relevant parameters of the mixture |
| Area | the heat exchange area (m2) |
| AOT | the annual operating time (h) |
| CC | coke content of the catalyst |
| Ccap | capital cost (USD/year) |
| Chex | capital costs of heat exchanger (USD/year) |
| Ci | total energy consumption (kW) |
| CMeOH | the concentration of methanol (mol∙L−1) |
| Cope | operating cost (USD/year) |
| Cplate | the expenses of plates (USD/year) |
| Cshell | the expenses of shell (USD/year) |
| Dc | the diameter of column (m) |
| Ea,I | the activation energy (J∙mol−1) |
| fugacity of liquid | |
| fugacity of vapor | |
| F | flowrate of the stream inlet into the separation section (kmol∙h−1) |
| Ffeed | the feed flowrate (kmol·h−1) |
| Fr | total flowrate of the seven lumped components (kmol∙h−1) |
| H | enthalpy (kW) |
| HR | deviation value of enthalpy (kW) |
| Hc | the height of column (m) |
| Hfeed | the enthalpy of feed |
| ki | the kinetic constant of component i |
| ki0 | kinetic constant at the reference state |
| ki,j | binary interaction parameter |
| K | gas–liquid equilibrium constant |
| L | flowrate of liquid through the plate, kmol∙h−1 |
| flowrate of liquid leaving the column, kmol∙h−1 | |
| LMTD | the logarithmic mean temperature difference, K |
| Mi | the molar mass of component i |
| N | the number of theoretical stages |
| p | pressure (kPa) |
| P | represents the annual profit (USD/year) |
| Pmaterial | the cost of methanol (USD/year) |
| Psale | the revenue of target products (USD/year) |
| PBP | the payback period (year) |
| q | feed condition |
| Q | heat load (kW) |
| Qi | the utility price (USD∙GJ−1) |
| ri | the formation rate of component I (g∙gcat−1∙min−1) |
| R | reflux ratio |
| RF | the ratio of R to Rmin |
| T | temperature (K) |
| TD | top temperature (K) |
| TAC | the total annual cost of the distillation section (USD/year) |
| U | the overall coefficient of heat transfer (kW∙°C−1∙m−2), |
| νI | the stoichiometric number |
| V | flowrate of vapor through the plate, kmol∙h−1 |
| flowrate of vapor leaving the column, kmol∙h−1 | |
| We | the annual electricity consumption (kWh) |
| the mole fractions of component i in gas and liquid, respectively. | |
| Yi | yield of component i |
| zi | the mole fractions of the stream inlet into the separation section |
| Z | compressibility factor |
| α | relative volatility |
| ∆H | enthalpy change (kW) |
| ∆T1 | temperature difference at the hot end (K) |
| ∆T2 | temperature difference at the cold end (K) |
| η | Murphree plate efficiency |
| ηpump | pump’s efficiency |
| θ | the root of the Underwood equation |
| θW | parameter describing the influence of water content in the feed |
| ν | the vapor velocity in the column (m∙s−1) |
| φ | the fugacity coefficient |
| ϕi | parameter reflecting the catalyst deactivation |
| ω | the acentric factor |
| Subscripts | |
| c | critical state |
| C | cold stream |
| H | hot stream |
| i | component |
| in | inlet stream |
| j | theoretical stage |
| out | outlet stream |
| rec | rectifying section |
| stri | stripping section |
| Superscript | |
| id | ideal state |
Appendix A
| CH4 | C2H4 | C2H6 | C3H6 | C3H8 | i-C4H8 | n-C5H10 | |
|---|---|---|---|---|---|---|---|
| CH4 | 0 | 0.01 | 0.01 | 0.021 | 0.023 | 0.0275 | 0.041 |
| C2H4 | 0.01 | 0 | 0 | 0.003 | 0.0031 | 0.004 | 0.006 |
| C2H6 | 0.01 | 0 | 0 | 0.003 | 0.0031 | 0.004 | 0.006 |
| C3H6 | 0.021 | 0.003 | 0.003 | 0 | 0 | 0.003 | 0.0045 |
| C3H8 | 0.023 | 0.0031 | 0.0031 | 0 | 0 | 0.003 | 0.0045 |
| i-C4H8 | 0.0275 | 0.004 | 0.004 | 0.003 | 0.003 | 0 | 0.0008 |
| n-C5H10 | 0.041 | 0.006 | 0.006 | 0.0045 | 0.0045 | 0.008 | 0 |
| Parameters | CH4 | C2H4 | C2H6 | C3H6 | C3H8 | i-C4H8 | n-C5H10 |
|---|---|---|---|---|---|---|---|
| Tc/K | 190.56 | 282.34 | 305.32 | 364.90 | 369.83 | 407.85 | 469.70 |
| pc/bar | 45.99 | 50.41 | 48.72 | 46.00 | 42.48 | 36.40 | 33.70 |
| ω | 0.011 | 0.087 | 0.099 | 0.142 | 0.152 | 0.186 | 0.252 |
| A | 5.964 | 6.402 | 6.107 | 6.651 | 6.809 | 6.274 | 5.969 |
| B | 438.5 | 800.9 | 720.8 | 1186 | 1348 | 1095 | 1044 |
| C | −0.9394 | 14.04 | −8.924 | 32.00 | 53.76 | −9.441 | −39.70 |
| H0/kJ∙kmol−1 | −75,402 | 51,461 | −85,110 | 18,650 | −106,481 | −137,353 | −149,685 |
| a | 4.568 | 4.221 | 4.178 | 3.834 | 3.847 | 3.351 | 7.554 |
| b × 103 | −8.975 | −8.782 | −4.227 | 3.893 | 5.131 | 17.883 | −0.368 |
| c × 105 | 3.631 | 5.795 | 5.660 | 4.688 | 6.011 | 5.477 | 11.85 |
| d × 108 | −3.047 | −6.729 | −6.651 | −6.013 | −7.893 | −8.009 | −14.94 |
| e × 1011 | 1.091 | 2.511 | 2.487 | 2.283 | 3.079 | 3.243 | 5.753 |
Appendix B
| T | CC | Profit (107$/Year) | Min TAC (107$/Year) | p1 (kPa) | p2 (kPa) | p3 (kPa) | p4 (kPa) | p5 (kPa) |
|---|---|---|---|---|---|---|---|---|
| 450 | 3.0% | −0.56 | 1.18 | 2540 | 2841 | 3345 | 916 | 1973 |
| 470 | 3.0% | 0.07 | 1.20 | 2600 | 2896 | 3051 | 1120 | 1796 |
| 490 | 3.0% | 0.52 | 1.18 | 2828 | 2890 | 3033 | 1109 | 1855 |
| 510 | 3.0% | 0.82 | 1.19 | 2888 | 2879 | 3023 | 998 | 1763 |
| 530 | 3.0% | 0.93 | 1.23 | 2832 | 3139 | 3090 | 981 | 1814 |
| 550 | 3.0% | 0.92 | 1.23 | 2780 | 2840 | 3094 | 1036 | 1790 |
| 450 | 4.0% | 0.88 | 1.19 | 2592 | 2768 | 3116 | 1056 | 1866 |
| 470 | 4.0% | 1.47 | 1.21 | 2841 | 2835 | 3053 | 998 | 1856 |
| 490 | 4.0% | 1.90 | 1.20 | 2866 | 2894 | 2972 | 1017 | 1827 |
| 510 | 4.0% | 2.15 | 1.21 | 2984 | 3072 | 3191 | 1018 | 1839 |
| 530 | 4.0% | 2.21 | 1.25 | 2967 | 3040 | 3201 | 1093 | 2013 |
| 550 | 4.0% | 2.12 | 1.26 | 3069 | 3169 | 3214 | 993 | 1778 |
| 450 | 5.0% | 2.22 | 1.21 | 2552 | 2941 | 3257 | 1129 | 1835 |
| 470 | 5.0% | 2.79 | 1.22 | 2745 | 2998 | 3035 | 1015 | 2011 |
| 490 | 5.0% | 3.16 | 1.25 | 2871 | 3021 | 2978 | 893 | 1879 |
| 510 | 5.0% | 3.38 | 1.24 | 3007 | 3079 | 3131 | 1113 | 1788 |
| 530 | 5.0% | 3.37 | 1.27 | 3056 | 3198 | 3246 | 1016 | 1918 |
| 550 | 5.0% | 3.19 | 1.28 | 3061 | 3104 | 3117 | 1045 | 1853 |
| 450 | 6.0% | 3.47 | 1.25 | 2642 | 2759 | 3238 | 901 | 1809 |
| 470 | 6.0% | 4.02 | 1.25 | 2762 | 2947 | 3086 | 987 | 1903 |
| 490 | 6.0% | 4.36 | 1.24 | 3013 | 3015 | 3159 | 1014 | 1808 |
| 510 | 6.0% | 4.50 | 1.26 | 3061 | 2996 | 3145 | 1117 | 1787 |
| 530 | 6.0% | 4.40 | 1.30 | 2966 | 3041 | 3036 | 1107 | 1769 |
| 550 | 6.0% | 4.11 | 1.35 | 3082 | 3193 | 3186 | 1033 | 1844 |
| 450 | 7.0% | 4.67 | 1.25 | 2670 | 2994 | 3049 | 1146 | 1886 |
| 470 | 7.0% | 5.16 | 1.26 | 2892 | 2951 | 3174 | 1118 | 1814 |
| 490 | 7.0% | 5.44 | 1.27 | 3007 | 2990 | 3076 | 1129 | 1793 |
| 510 | 7.0% | 5.45 | 1.32 | 2823 | 2934 | 2975 | 1050 | 1865 |
| 530 | 7.0% | 5.30 | 1.32 | 3060 | 3109 | 3146 | 1099 | 1831 |
| 550 | 7.0% | 4.89 | 1.35 | 3077 | 3303 | 3341 | 1075 | 1924 |
| 450 | 7.8% | 5.55 | 1.27 | 2827 | 2903 | 3127 | 945 | 1796 |
| 470 | 7.8% | 6.00 | 1.28 | 2960 | 3104 | 3150 | 1000 | 1934 |
| 490 | 7.8% | 6.22 | 1.29 | 2970 | 3223 | 3150 | 1100 | 1928 |
| 510 | 7.8% | 6.17 | 1.33 | 2939 | 2927 | 3075 | 1050 | 1867 |
| 530 | 7.8% | 5.95 | 1.35 | 2987 | 2927 | 3074 | 1049 | 1867 |
| 550 | 7.8% | 5.41 | 1.38 | 3127 | 3280 | 3200 | 1106 | 2113 |
References
- Gogate, M.R. Methanol-to-olefins process technology: Current status and future prospects. Pet. Sci. Technol. 2019, 37, 559–565. [Google Scholar] [CrossRef]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Chang, C. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 1977, 47, 249–259. [Google Scholar] [CrossRef]
- Chen, J.Q.; Bozzano, A.; Glover, B.; Fuglerud, T.; Kvisle, S. Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process. Catal. Today 2005, 106, 103–107. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.X.; Ye, M.; Liu, Z.M. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Ye, M.; Tian, P.; Liu, Z. DMTO: A Sustainable Methanol-to-Olefins Technology. Engineering 2021, 7, 17–21. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Mihail, R.; Straja, S.; Maria, G.; Musca, G.; Pop, G. Kinetic model for methanol conversion to olefins. Ind. Eng. Chem. Process Des. Dev. 2002, 22, 532–538. [Google Scholar] [CrossRef]
- Fatourehchi, N.; Sohrabi, M.; Royaee, S.J.; Mirarefin, S.M. Preparation of SAPO-34 catalyst and presentation of a kinetic model for methanol to olefin process (MTO). Chem. Eng. Res. Des. 2011, 89, 811–816. [Google Scholar] [CrossRef]
- Bos, A.N.R.; Tromp, P.J.J.; Akse, H.N. Conversion of Methanol to Lower Olefins-Kinetic Modeling, Reactor Simulation, and Selection. Ind. Eng. Chem. Res. 1995, 34, 3808–3816. [Google Scholar] [CrossRef]
- Ying, L.; Yuan, X.S.; Ye, M.; Cheng, Y.W.; Li, X.; Liu, Z.M. A seven lumped kinetic model for industrial catalyst in DMTO process. Chem. Eng. Res. Des. 2015, 100, 179–191. [Google Scholar] [CrossRef]
- Cui, C.T.; Yin, H.; Yang, J.; Wei, D.M.; Sun, J.S.; Guo, C.N. Selecting suitable energy-saving distillation schemes: Making quick decisions. Chem. Eng. Process.-Process Intensif. 2016, 107, 138–150. [Google Scholar] [CrossRef]
- Ye, H.T.; Zou, X.; Zhu, W.X.; Yang, Y.; Dong, H.G.; Bi, M.S. Synthesis framework for distillation sequence with sidestream columns: Application in reaction-separation-recycle system. Chem. Eng. Res. Des. 2021, 166, 172–190. [Google Scholar] [CrossRef]
- Cui, C.T.; Liu, S.Y.; Sun, J.S. Optimal selection of operating pressure for distillation columns. Chem. Eng. Res. Des. 2018, 137, 291–307. [Google Scholar] [CrossRef]
- Gómez-Castro, F.I.; Rico-Ramírez, V.; Segovia-Hernández, J.G.; Hernández-Castro, S.; González-Alatorre, G.; El-Halwagi, M.M. Simplified Methodology for the Design and Optimization of Thermally Coupled Reactive Distillation Systems. Ind. Eng. Chem. Res. 2012, 51, 11717–11730. [Google Scholar] [CrossRef]
- Fidkowski, Z.T.; Doherty, M.F.; Malone, M.F. Feasibility of Separations for Distillation of Nonideal Ternary Mixtures. AIChE J. 1993, 39, 1303–1321. [Google Scholar] [CrossRef]
- Lucia, A.; Amale, A.; Taylor, R. Distillation pinch points and more. Comput. Chem. Eng. 2008, 32, 1342–1364. [Google Scholar] [CrossRef]
- Ramapriya, G.M.; Selvarajah, A.; Cucaita, L.E.J.; Huff, J.; Tawarmalani, M.; Agrawal, R. Short-Cut Methods versus Rigorous Methods for Performance-Evaluation of Distillation Configurations. Ind. Eng. Chem. Res. 2018, 57, 7726–7731. [Google Scholar] [CrossRef]
- Kraemer, K.; Kossack, S.; Marquardt, W. Efficient Optimization-Based Design of Distillation Processes for Homogeneous Azeotropic Mixtures. Ind. Eng. Chem. Res. 2009, 48, 6749–6764. [Google Scholar] [CrossRef]
- Chen, Y.; Eslick, J.C.; Grossmann, I.E.; Miller, D.C. Simultaneous process optimization and heat integration based on rigorous process simulations. Comput. Chem. Eng. 2015, 81, 180–199. [Google Scholar] [CrossRef]
- Viswanathan, J.; Grossmann, I.E. A Combined Penalty-Function and Outer-Approximation Method for Minlp Optimization. Comput. Chem. Eng. 1990, 14, 769–782. [Google Scholar] [CrossRef]
- Dowling, A.W.; Biegler, L.T. A framework for efficient large scale equation-oriented flowsheet optimization. Comput. Chem. Eng. 2015, 72, 3–20. [Google Scholar] [CrossRef]
- Seidel, T.; Hoffmann, A.; Bortz, M.; Scherrer, A.; Burger, J.; Asprion, N.; Küfer, K.-H.; Hasse, H. A novel approach for infeasible path optimization of distillation-based flowsheets. Chem. Eng. Sci. X 2020, 7, 100063. [Google Scholar] [CrossRef]
- Yu, B.Y.; Chien, I.L. Design and Optimization of the Methanol-to-Olefin Process—Part I: Steady-State Design and Optimization. Chem. Eng. Technol. 2016, 39, 2293–2303. [Google Scholar] [CrossRef]
- Yu, B.Y.; Chien, I.L. Design and Optimization of the Methanol-to-Olefin Process—Part II: Comparison of Different Methods for Propylene/Propane Separation. Chem. Eng. Technol. 2016, 39, 2304–2311. [Google Scholar] [CrossRef]
- Dimian, A.C.; Bildea, C.S. Energy efficient methanol-to-olefins process. Chem. Eng. Res. Des. 2018, 131, 41–54. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hsieh, W.; Chang, H.; Ho, C.D. Design and economic analysis of industrial-scale methanol-to-olefins plants. J. Taiwan Inst. Chem. Eng. 2022, 130, 103893. [Google Scholar] [CrossRef]
- Yin, C.; Sun, H.; Lv, D.; Liu, G. Integrated design and optimization of reactor-distillation sequence-recycle-heat exchanger network. Energy 2022, 238, 121796. [Google Scholar] [CrossRef]
- Hentschel, B.; Peschel, A.; Freund, H.; Sundmacher, K. Simultaneous design of the optimal reaction and process concept for multiphase systems. Chem. Eng. Sci. 2014, 115, 69–87. [Google Scholar] [CrossRef]
- Kong, L.X.; Sen, S.M.; Henao, C.A.; Dumesic, J.A.; Maravelias, C.T. A superstructure-based framework for simultaneous process synthesis, heat integration, and utility plant design. Comput. Chem. Eng. 2016, 91, 68–84. [Google Scholar] [CrossRef]
- Ryu, J.; Kong, L.X.; de Lima, A.E.P.; Maravelias, C.T. A generalized superstructure-based framework for process synthesis. Comput. Chem. Eng. 2020, 133, 106653. [Google Scholar] [CrossRef]
- Trespalacios, F.; Grossmann, I.E. Review of Mixed-Integer Nonlinear and Generalized Disjunctive Programming Methods. Chem. Ing. Tech. 2014, 86, 991–1012. [Google Scholar] [CrossRef]
- Su, Y.; Jin, S.M.; Zhang, X.P.; Shen, W.F.; Eden, M.R.; Ren, J.Z. Stakeholder-oriented multi-objective process optimization based on an improved genetic algorithm. Comput. Chem. Eng. 2020, 132, 106618. [Google Scholar] [CrossRef]
- Yang, X.L.; Ward, J.D. Extractive Distillation Optimization Using Simulated Annealing and a Process Simulation Automation Server. Ind. Eng. Chem. Res. 2018, 57, 11050–11060. [Google Scholar] [CrossRef]
- Qian, X.; Jia, S.K.; Huang, K.J.; Chen, H.S.; Yuan, Y.; Zhang, L. Optimal design of Kaibel dividing wall columns based on improved particle swarm optimization methods. J. Clean. Prod. 2020, 273, 123041. [Google Scholar] [CrossRef]
- Frenkel, M.; Kabo, G.J.; Marsh, K.N.; Beganov, G.N.; Wilboit, R.C. Thermodynamics of Organic Component in the Gas State; TRC: College Station, TX, USA, 1994. [Google Scholar]
- Smith, R.; Jobson, M. Distillation; Academic Press: Oxford, UK, 2000; pp. 84–103. [Google Scholar] [CrossRef]
- Luyben, W.L. Principles and Case Studies of Simultaneous Design; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]


| Types of Fluid | U (kW∙°C−1∙m−2) |
|---|---|
| Gas—Gas | 0.17 |
| Gas—Condensing gas | 0.28 |
| Gas—Evaporating liquid | 0.28 |
| Liquid—Liquid | 0.57 |
| Liquid—Condensing gas | 0.85 |
| Liquid—Evaporating liquid | 0.85 |
| Components | CH4 | C2H4 | C2H6 | C3H6 | C3H8 | i-C4H8 | n-C5H10 |
|---|---|---|---|---|---|---|---|
| Molar fraction | 0.0685 | 0.5460 | 0.0080 | 0.2943 | 0.0170 | 0.0583 | 0.0080 |
| Utility | Inlet Temperature (°C) | Outlet Temperature (°C) | Abbreviation | Price ($∙GJ−1) |
|---|---|---|---|---|
| Refrigerant (Ethylene) | −101 (l) | −101 (g) | RE | 21 |
| Refrigerant (Propylene) | −50 (l) | −50 (g) | −50 RP | 13.11 |
| Refrigerant (Propylene) | −35 (l) | −35 (g) | −35 RP | 10.6 |
| Refrigerant (Propylene) | −20 (l) | −20 (g) | −20 RP | 8.2 |
| Chilled water | 5 | 15 | CHW | 4.43 |
| Cooling water | 25 | 35 | CW | 0.354 |
| Quench water | 120 | 90 | QW | 0.445 |
| LP-steam | 160 (g) | 160 (l) | LP | 7.78 |
| Electricity | - | - | EL | 16.8 |
| Proposed Method | Aspen Plus | Error | |
|---|---|---|---|
| Recovery of ethane | 0.99 | 0.9512 | 4.1% |
| Recovery of ethylene | 0.9999 | 0.9999 | 0 |
| Recovery of propylene | 0.999 | 0.9956 | 0.3% |
| Top temperature/°C | −31.8 | −31.3 | −1.6% |
| Bottom temperature/°C | 74.7 | 74.9 | −0.3% |
| Duty of condenser/kW | 6129 | 6491 | −5.6% |
| Duty of reboiler/kW | 8117 | 8621 | −5.8% |
| Pressure/kPa | 1000~2000 | 2100~2900 | 3000 | 3100~3500 |
|---|---|---|---|---|
| Cooling utility | RE | −50 RP | −35 RP | −35 RP |
| Heating utility | QW | QW | QW | LP |
| Parameters | T1201 | T1202 | T1203 | T1204 | T1205 |
|---|---|---|---|---|---|
| Operating pressure/kPa | 3000 | 2800 | 3100 | 1100 | 1800 |
| Top temperature/°C | −28.6 | −98.3 | −12.4 | 23.3 | 43.3 |
| Bottom temperature/°C | 78.5 | −15.6 | 10.7 | 78.6 | 53.9 |
| Reflux ratio | 0.82 | 4.91 | 4.86 | 0.95 | 14.77 |
| Number of stages | 62 | 46 | 146 | 83 | 121 |
| Feed stage | 23 | 2 | 124 | 44 | 52 |
| Duty of condenser/kW | 6137 | 1206 | 14,988 | 5872 | 38,533 |
| Duty of reboiler/kW | 8152 | 1579 | 14,975 | 5624 | 38,616 |
| Cooling utilities | −35 RP | RE | −20 RP | CHW | CW |
| Heating utilities | QW | QW | QW | QW | QW |
| Capital cost/USD 106 | 1.4739 | 1.1395 | 3.2479 | 1.6632 | 5.7811 |
| Operating cost/106 USD/year | 1.9780 | 0.7499 | 3.5112 | 0.8162 | 0.8878 |
| TAC/106 USD/year | 2.4693 | 1.1297 | 4.5939 | 1.3706 | 2.8148 |
| Parameters | Dethanizer | Demethanizer | Ethylene Column | Depropanizer | Propylene Column |
|---|---|---|---|---|---|
| Operating pressure/kPa | 3051 | 3058 | 3167 | 1134 | 1934 |
| Top temperature/°C | −29.9 | −95.6 | −11.5 | 24.5 | 46.6 |
| Bottom temperature/°C | 79.2 | −12.0 | 11.7 | 79.9 | 57.2 |
| Reflux ratio | 0.76 | 4.80 | 4.98 | 0.96 | 15.4 |
| Number of stages | 62 | 49 | 149 | 84 | 126 |
| Feed stage | 23 | 2 | 128 | 44 | 54 |
| Duty of condenser/kW | 6342 | 1311 | 15,717 | 5678 | 37,669 |
| Duty of reboiler/kW | 8390 | 1750 | 15,694 | 5456 | 37,749 |
| Cooling utilities | −35 RP | RE | −20 RP | CHW | CW |
| Heating utilities | QW | QW | QW | LP | QW |
| Capital cost/USD 106 | 1.5822 | 1.0198 | 3.2615 | 1.6485 | 5.7462 |
| Operating cost/106 USD/year | 2.0436 | 0.8154 | 3.6821 | 0.7895 | 0.8678 |
| TAC/106 USD/year | 2.5710 | 1.1554 | 4.7693 | 1.3390 | 2.7832 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zhao, L.; Li, D.; Sun, H.; Zhang, D.; Liu, G. A Simultaneous Design and Optimization Framework for the Reaction and Distillation Sections of Methanol to Olefins Process. Processes 2023, 11, 58. https://doi.org/10.3390/pr11010058
Li N, Zhao L, Li D, Sun H, Zhang D, Liu G. A Simultaneous Design and Optimization Framework for the Reaction and Distillation Sections of Methanol to Olefins Process. Processes. 2023; 11(1):58. https://doi.org/10.3390/pr11010058
Chicago/Turabian StyleLi, Ning, Liwen Zhao, Dan Li, Huifeng Sun, Di Zhang, and Guilian Liu. 2023. "A Simultaneous Design and Optimization Framework for the Reaction and Distillation Sections of Methanol to Olefins Process" Processes 11, no. 1: 58. https://doi.org/10.3390/pr11010058
APA StyleLi, N., Zhao, L., Li, D., Sun, H., Zhang, D., & Liu, G. (2023). A Simultaneous Design and Optimization Framework for the Reaction and Distillation Sections of Methanol to Olefins Process. Processes, 11(1), 58. https://doi.org/10.3390/pr11010058







