Optimization of Major Extraction Variables to Improve Recovery of Anthocyanins from Elderberry by Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Anthocyanin Extraction Procedure
2.3. Experimental Design by Response Surface Methodology
2.4. Analytical Methods
2.4.1. Total Polyphenol Content
2.4.2. Total Flavonoid Content
2.4.3. Total Anthocyanin Content
2.4.4. Ferric Reducing Antioxidant Power
2.4.5. ABTS Radical Scavenging Activity
2.4.6. DPPH Radical Scavenging Activity
3. Results and Discussion
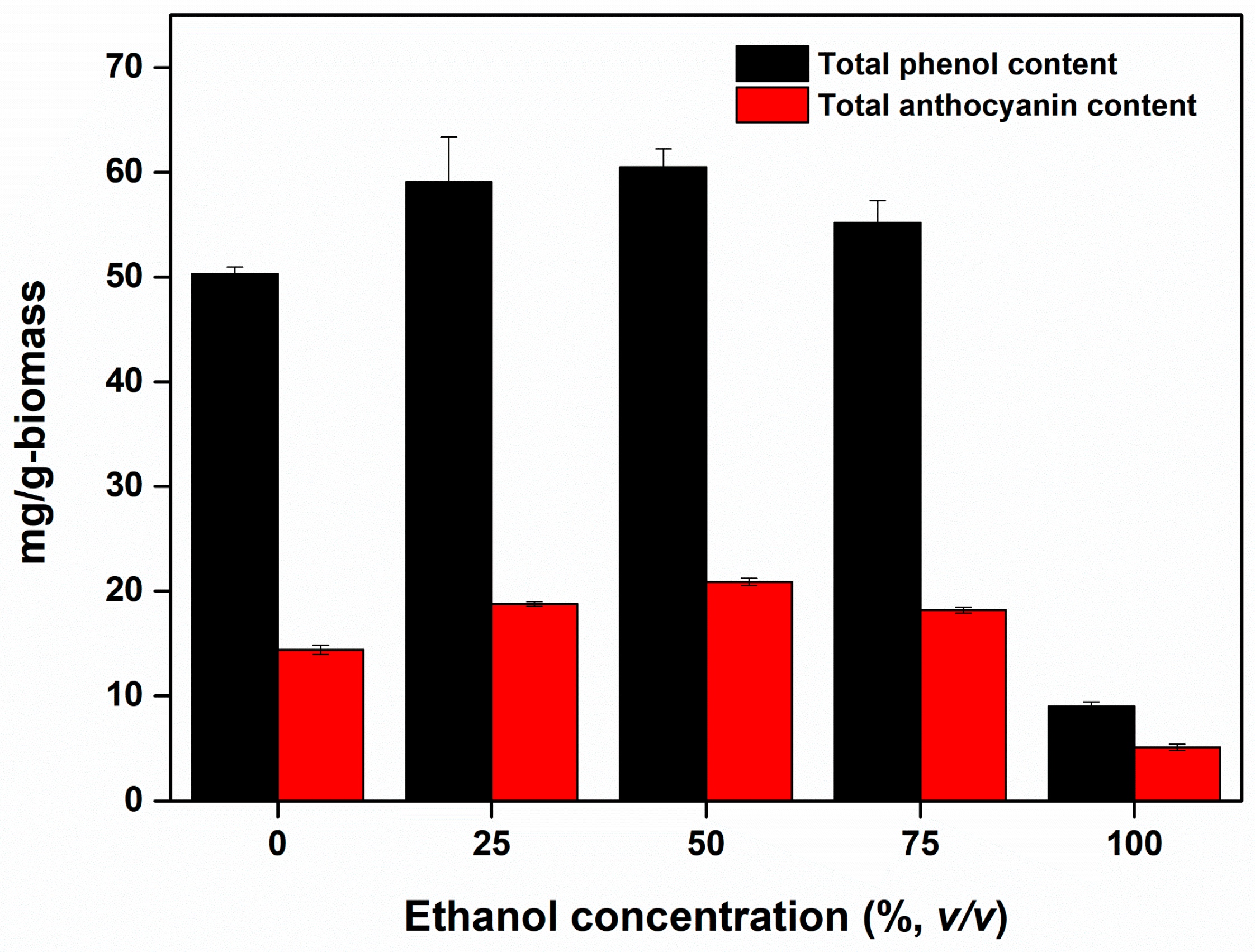
3.1. Effect of Solvent Types on the Phenolic Compound Recovery from Elderberry
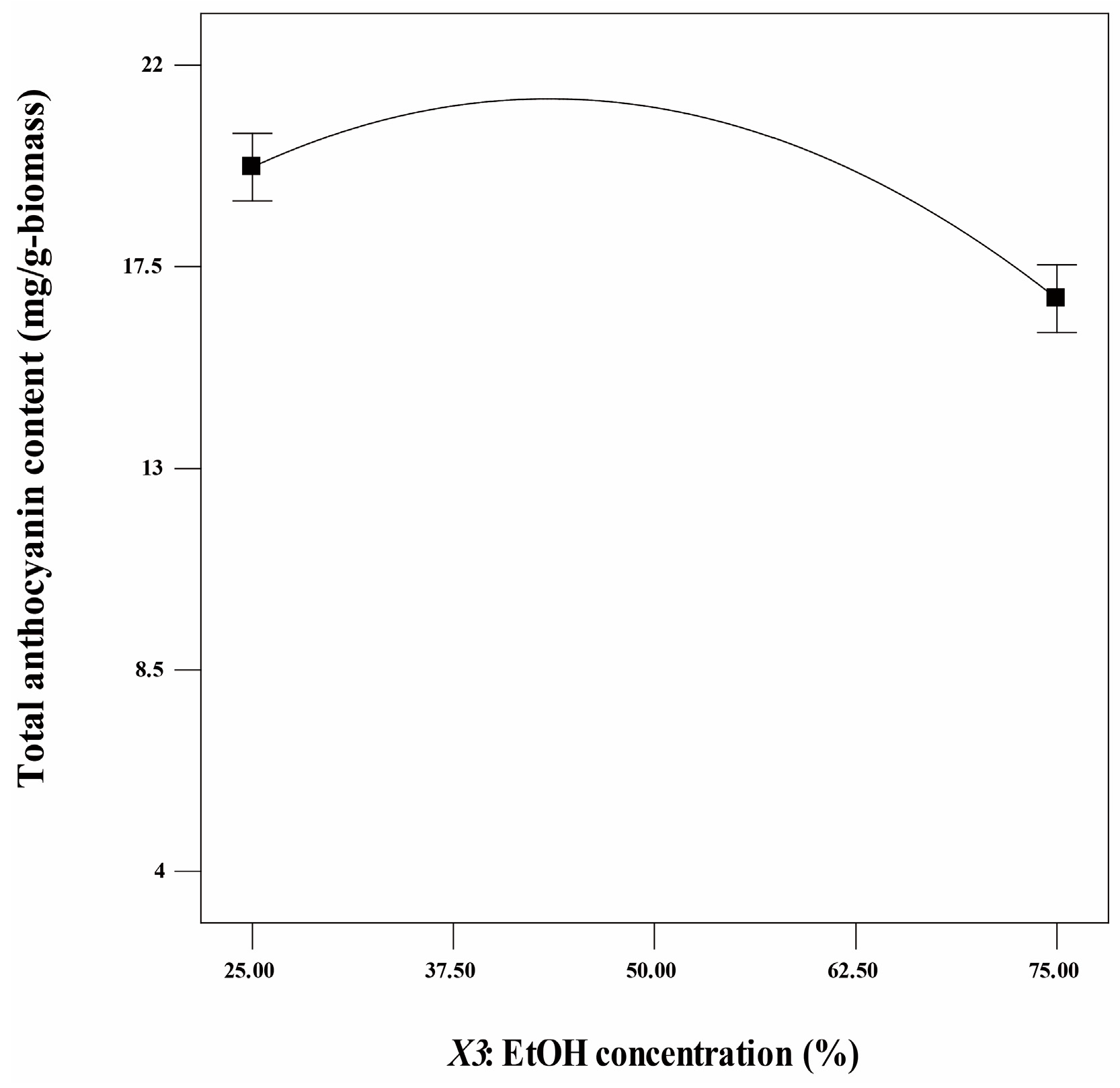
3.2. Optimization of Anthocyanin Extraction Conditions Using Response Surface Methodology
3.3. Antioxidant Activity Assessment of the Elderberry Extract
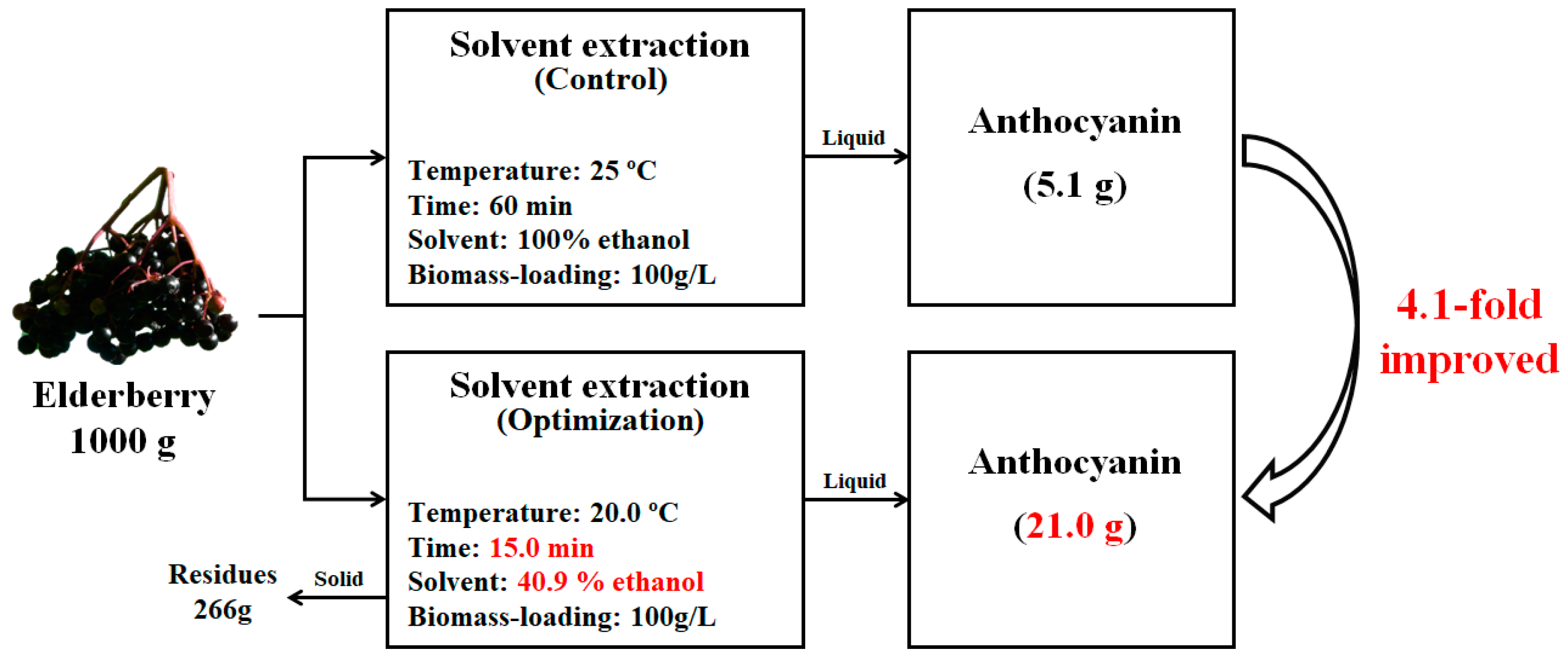
3.4. Evaluation of Overall Process for Anthocyanin Recovery from Elderberry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 258.58 | 9 | 28.73 | 10.28 | 0.0006 | significant |
| X1 | 4.03 | 1 | 4.03 | 1.44 | 0.2574 | |
| X2 | 0.019 | 1 | 0.019 | 0.00671 | 0.9363 | |
| X3 | 34.58 | 1 | 34.58 | 12.37 | 0.0056 | significant |
| X1X2 | 0.064 | 1 | 0.064 | 0.023 | 0.8827 | |
| X1X3 | 0.046 | 1 | 0.046 | 0.016 | 0.9006 | |
| X2X3 | 0.63 | 1 | 0.63 | 0.22 | 0.6462 | |
| X12 | 2.64 | 1 | 2.64 | 0.94 | 0.3542 | |
| X22 | 1.67 | 1 | 1.67 | 0.6 | 0.4568 | |
| X32 | 199.22 | 1 | 199.22 | 71.27 | <0.0001 | significant |
| Residual | 27.95 | 10 | 2.8 | |||
| Lack of fit | 23.18 | 5 | 4.64 | 4.86 | 0.0539 | not significant |
| Pure error | 4.77 | 5 | 0.95 | |||
| Total | 286.54 | 19 |
References
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, Y.G.; Giusti, M.M. Accumulation of anthocyanins and other phytochemicals in American elderberry cultivars during fruit ripening and its impact on color expression. Plants 2020, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Baldovska, S.; Kohut, L.; Sirotkin, A.V. Black Elder and Its Constituents: Molecular Mechanisms of Action Associated with Female Reproduction. Pharmaceuticals 2022, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Patinha, S.; Rudnitskaya, A.; Santos, S.A.; Silvestre, A.J.; Rocha, S.M. Sustainable Valorization of Sambucus nigra L. Berries: From Crop Biodiversity to Nutritional Value of Juice and Pomace. Foods 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Terzić, M.; Majkić, T.; Beara, I.; Zengin, G.; Miljić, U.; Đurović, S.; Mollica, A.; Radojković, M. Elderberry (Sambucus nigra L.) wine as a novel potential functional food product. Food Biosci. 2022, 50, 102047. [Google Scholar] [CrossRef]
- Rocchetti, G.; Becchi, P.P.; Lucini, L.; Cittadini, A.; Munekata, P.E.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) Encapsulated Extracts as Meat Extenders against Lipid and Protein Oxidation during the Shelf-Life of Beef Burgers. Antioxidants 2022, 11, 2130. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) fruit extract alleviates oxidative stress, insulin resistance, and inflammation in hypertrophied 3T3-L1 adipocytes and activated RAW 264.7 macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Cirlini, M.; Calani, L.; Bernini, V.; Neviani, E.; Del Rio, D.; Galaverna, G.; Lazzi, C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019, 276, 692–699. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized aqueous solutions of deep eutectic solvent (DES): A green emergent extraction of anthocyanins from a Brazilian berry processing by-product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef]
- Khadem, E.; Kharaziha, M. Red cabbage anthocyanin-functionalized tannic acid-silver nanoparticles with pH sensitivity and antibacterial properties. Mater. Chem. Phys. 2022, 291, 126689. [Google Scholar] [CrossRef]
- Jang, H.H.; Kim, H.W.; Kim, S.Y.; Kim, S.M.; Kim, J.B.; Lee, Y.M. In vitro and in vivo hypoglycemic effects of cyanidin 3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside, an anthocyanin isolated from purple-fleshed sweet potato. Food Chem. 2019, 272, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Koo, K.A.; Park, W.S.; Kang, D.M.; Kim, H.S.; Lee, B.Y.; Goo, Y.M.; Kim, J.H.; Lee, M.K.; Kwak, S.S.; et al. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef]
- Belwal, T.; Li, L.; Yanqun, X.; Cravotto, G.; Luo, Z. Ultrasonic-assisted modifications of macroporous resin to improve anthocyanin purification from a Pyrus communis var. Starkrimson extract. Ultrason. Sonochem. 2020, 62, 104853. [Google Scholar] [CrossRef] [PubMed]
- Rimpapa, Z.; Toromanovic, J.; Tahirovic, I.; Šapčanin, A.; Sofic, E. Total content of phenols and anthocyanins in edible fruits from Bosnia. Bosn. J. Basic Med. Sci. 2007, 7, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT 2020, 117, 108621. [Google Scholar] [CrossRef]
- Yang, W.; Kortesniemi, M.; Ma, X.; Zheng, J.; Yang, B. Enzymatic acylation of blackcurrant (Ribes nigrum) anthocyanins and evaluation of lipophilic properties and antioxidant capacity of derivatives. Food Chem. 2019, 281, 189–196. [Google Scholar] [CrossRef]
- Ha, T.J.; Park, J.E.; Lee, K.S.; Seo, W.D.; Song, S.B.; Lee, M.H.; Kim, S.; Kim, J.I.; Oh, E.; Pae, S.B.; et al. Identification of anthocyanin compositions in black seed coated Korean adzuki bean (Vigna angularis) by NMR and UPLC-Q-Orbitrap-MS/MS and screening for their antioxidant properties using different solvent systems. Food Chem. 2021, 346, 128882. [Google Scholar] [CrossRef]
- Singh, M.C.; Price, W.E.; Kelso, C.; Charlton, K.; Probst, Y. Impact of molar absorbance on anthocyanin content of the foods. Food Chem. 2022, 386, 132855. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total Anthocyanin Content of Strawberry and the Profile Changes by Extraction Methods and Sample Processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Kong, Y.; Ma, Y. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa extracted using an ultrasonic-microwave-assisted natural deep eutectic solvent extraction method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Melo, T.; Conde, T.A.; Moreira, A.S.; Ferreira, P.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. Food grade extraction of Chlorella vulgaris polar lipids: A comparative lipidomic study. Food Chem. 2022, 375, 131685. [Google Scholar] [CrossRef] [PubMed]
- Coklar, H.; Akbulut, M. Anthocyanins and phenolic compounds of Mahonia aquifolium berries and their contributions to antioxidant activity. J. Funct. Foods 2017, 35, 166–174. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Phytochemical properties and antioxidant activities of extracts from wild blueberries and lingonberries. Plant Foods Hum. Nutr. 2017, 72, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.N.; Borges, A.; Matias, A.A.; Bronze, M.R.; Oliveira, J. Alternative Extraction and Downstream Purification Processes for Anthocyanins. Molecules 2022, 27, 368. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.K.; Lee, J.; Kim, S.; Kim, S.W.; Park, C.; Yoo, H.Y. Energy-efficient glucose recovery from chestnut shell by optimization of NaOH pretreatment at room temperature and application to bioethanol production. Environ. Res. 2022, 208, 112710. [Google Scholar] [CrossRef]
- Pilkington, J.L.; Preston, C.; Gomes, R.L. Comparison of response surface methodology (RSM) and artificial neural networks (ANN) towards efficient extraction of artemisinin from Artemisia annua. Ind. Crops Prod. 2014, 58, 15–24. [Google Scholar] [CrossRef]
- Türker, D.A.; Doğan, M. Ultrasound-assisted natural deep eutectic solvent extraction of anthocyanin from black carrots: Optimization, cytotoxicity, in-vitro bioavailability and stability. Food Bioprod. Process. 2022, 132, 99–113. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, Y.; Wang, L.; Yin, W.; Liang, J. Antioxidant activity and subcritical water extraction of anthocyanin from raspberry process optimization by response surface methodology. Food Biosci. 2021, 44, 101394. [Google Scholar] [CrossRef]
- Ferarsa, S.; Zhang, W.; Moulai-Mostefa, N.; Ding, L.; Jaffrin, M.Y.; Grimi, N. Recovery of anthocyanins and other phenolic compounds from purple eggplant peels and pulps using ultrasonic-assisted extraction. Food Bioprod. Process. 2018, 109, 19–28. [Google Scholar] [CrossRef]
- Su, D.; Wang, Z.; Dong, L.; Huang, F.; Zhang, R.; Jia, X.; Wu, G.; Zhang, M. Impact of thermal processing and storage temperature on the phenolic profile and antioxidant activity of different varieties of lychee juice. LWT 2019, 116, 108578. [Google Scholar] [CrossRef]
- Chen, J.Y.; Du, J.; Li, M.L.; Li, C.M. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT 2020, 128, 109448. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Viskelis, P.; Viskelis, J.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Zavistanaviciute, P.; Zokaityte, E.; Starkute, V.; Ruzauskas, M. Green synthesis of silver nanoparticles using extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., physico-chemical characterization, antimicrobial and antioxidant activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Kim, H.; Ki, J.S.; Yoo, H.Y. Optimization of lutein recovery from Tetraselmis suecica by response surface methodology. Biomolecules 2021, 11, 182. [Google Scholar] [CrossRef]
- Del Pozo, C.; Bartrolí, J.; Alier, S.; Puy, N.; Fàbregas, E. Production of antioxidants and other value-added compounds from coffee silverskin via pyrolysis under a biorefinery approach. J. Waste Manag. 2020, 109, 19–27. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhang, Q.; Zeng, M.; Wu, S.; Lan, L.; Zhao, X. Novel photoactivation and solar-light-driven thermocatalysis on ε-MnO2 nanosheets lead to highly efficient catalytic abatement of ethyl acetate without acetaldehyde as unfavorable by-product. J. Mater. Chem. A 2018, 6, 14195–14206. [Google Scholar] [CrossRef]
- Soyekwo, F.; Liu, C.; Hu, Y. Crosslinked copolystyrenes based membranes bearing alkylcarboxylated and alkylsulfonated side chains for organic solvent nanofiltration. Sep. Purif. Technol. 2021, 274, 119028. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of temperature, solvent, and pH on the selective extraction of phenolic compounds from tiger nuts by-products: Triple-TOF-LC-MS-MS characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Hu, S.; Huang, H.; He, H.; Guo, Y.; Liu, H.; Han, J.; Wang, P. Solubility Behavior of N-Carbobenzoxy-l-2-phenylglycine in 11 Pure and a Binary Ethanol+Water Solvent Systems at 283.15–323.15 K. J. Chem. Eng. Data 2021, 66, 2856–2864. [Google Scholar] [CrossRef]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod. Process. 2019, 115, 47–58. [Google Scholar] [CrossRef]
- Brahmi, F.; Mateos-Aparicio, I.; Garcia-Alonso, A.; Abaci, N.; Saoudi, S.; Smail-Benazzouz, L.; Guemghar-Haddadi, H.; Madani, K.; Boulekbache-Makhlouf, L. Optimization of Conventional Extraction Parameters for Recovering Phenolic Compounds from Potato (Solanum tuberosum L.) Peels and Their Application as an Antioxidant in Yogurt Formulation. Antioxidants 2022, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, S.; Feng, M.; Dai, H.; Pan, Y.; Cheng, X. Organic solvent-saving preparation of water glass based aerogel granules under ambient pressure drying. J. Non-Cryst. Solids 2019, 521, 119507. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Fan, L. Simultaneous optimization of the acidified water extraction for total anthocyanin content, total phenolic content, and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L.) using response surface methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process. Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Nabet, N.; Gilbert-López, B.; Madani, K.; Herrero, M.; Ibáñez, E.; Mendiola, J.A. Optimization of microwave assisted extraction recovery of bioactive compounds from Origanum glandulosum and Thymus fontanesii. Ind. Crops Prod. 2019, 129, 395–404. [Google Scholar] [CrossRef]
- Huerta, R.R.; Saldaña, M.D.A. Pressurized fluid treatment of barley and canola straws to obtain carbohydrates and phenolics. J. Supercrit. Fluids 2018, 141, 12–20. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- Orozco-Flores, L.A.; Salas, E.; González-Sánchez, G.; Chávez-Flores, D.; Ramírez-García, R.A.; Rocha-Gutiérrez, B.A.; Rocha-Gutiérrez, B.A.; Peralta-Pérez, M.D.R.; Ballinas-Casarrubias, M.D.L. Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace. Processes 2022, 10, 1112. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Zhou, C.; Wen, Z.; Dong, X. An improved microwave-assisted extraction of anthocyanins from purple sweet potato in favor of subsequent comprehensive utilization of pomace. Food Bioprod. Process. 2019, 115, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhang, R.; Yang, X.; Sun, Y.; Shi, L.; Xue, P. Optimization of ultrasound-assisted extraction by response surface methodology, antioxidant capacity, and tyrosinase inhibitory activity of anthocyanins from red rice bran. Food Sci. Nutr. 2020, 8, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Khan, A.; Abdi, A.; Ghadiri, M. Combination of RSM and NSGA-II algorithm for optimization and prediction of thermal conductivity and viscosity of bioglycol/water mixture containing SiO2 nanoparticles. Arab. J. Chem. 2021, 14, 103204. [Google Scholar] [CrossRef]
- Peng, Y.; Khaled, U.; Al-Rashed, A.A.; Meer, R.; Goodarzi, M.; Sarafraz, M. Potential application of Response Surface Methodology (RSM) for the prediction and optimization of thermal conductivity of aqueous CuO (II) nanofluid: A statistical approach and experimental validation. Phys. A Stat. Mech. Appl. 2020, 554, 124353. [Google Scholar] [CrossRef]
- Jang, Y.W.; Lee, K.H.; Yoo, H.Y. Improved sugar recovery from orange peel by statistical optimization of thermo-alkaline pretreatment. Processes 2021, 9, 409. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical optimization of alkali pretreatment to improve sugars recovery from spent coffee grounds and utilization in lactic acid fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Radio frequency-assisted enzymatic extraction of anthocyanins from Akebia trifoliata (Thunb.) Koidz. flowers: Process optimization, structure, and bioactivity determination. Ind. Crop. Prod. 2020, 149, 112327. [Google Scholar] [CrossRef]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Suktham, T.; Jones, A.; Soliven, A.; Dennis, G.R.; Shalliker, R.A. A comparison of the performance of the cupric reducing antioxidant potential assay and the ferric reducing antioxidant power assay for the analysis of antioxidants using reaction flow chromatography. Microchem. J. 2019, 149, 104046. [Google Scholar] [CrossRef]
- Alam, N.; Hossain, M.; Mottalib, M.A.; Sulaiman, S.A.; Gan, S.H.; Khalil, M.I. Methanolic extracts of Withania somnifera leaves, fruits and roots possess antioxidant properties and antibacterial activities. BMC Complement. Altern. Med. 2012, 12, 175. [Google Scholar] [CrossRef]
- Kim, J.G.; Lim, J.J.; You, J.S.; Kwon, H.J.; Lim, H.B. Comparative study of bioactivity and safety evaluation of ethanolic extracts of Zanthoxylum schinifolium fruit and pericarp. Molecules 2021, 26, 5919. [Google Scholar] [CrossRef] [PubMed]
- ALNasser, M.N.; Mellor, I.R.; Carter, W.G. A Preliminary Assessment of the Nutraceutical Potential of Acai Berry (Euterpe sp.) as a Potential Natural Treatment for Alzheimer’s Disease. Molecules 2022, 27, 4891. [Google Scholar] [CrossRef] [PubMed]
- Veloso, M.I.; Coelho, E.; Trabulo, O.; Coimbra, M.A. Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Appl. Sci. 2022, 12, 9463. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef]
- Jiao, G. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Kerbstadt, S.; Eliasson, L.; Mustafa, A.; Ahrné, L. Effect of novel drying techniques on the extraction of anthocyanins from bilberry press cake using supercritical carbon dioxide. Innov. Food Sci. Emerg. 2015, 29, 209–214. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of Natural Products Using Supercritical Fluids and Pressurized Liquids Assisted by Ultrasound: Current Status and Trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Hutabarat, R.P.; Xiao, Y.D.; Wu, H.; Wang, J.; Li, D.J.; Huang, W.Y. Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. J. Food Qual. 2019, 2019, 6806970. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- López, C.J.; Caleja, C.; Prieto, M.; Barreiro, M.F.; Barros, L.; Ferreira, I.C. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. Fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef]
| Variables | Unit | Symbol | Coded Level | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| Temperature | °C | X1 | 20 | 30 | 40 | 50 | 60 |
| Time | min | X2 | 15 | 30 | 45 | 60 | 75 |
| EtOH concentration | % | X3 | 0 | 25 | 50 | 75 | 100 |
| Run | Coded Factor Levels | Response | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Predicted TAC (mg/g-Biomass) | Experimental TAC (mg/g-Biomass) | |
| 1 | −1 | −1 | −1 | 20.1 | 20.1 |
| 2 | 1 | −1 | −1 | 18.7 | 18.7 |
| 3 | −1 | 1 | −1 | 20.5 | 20.3 |
| 4 | 1 | 1 | −1 | 19.5 | 19.0 |
| 5 | −1 | −1 | 1 | 17.5 | 19.6 |
| 6 | 1 | −1 | 1 | 16.5 | 18.2 |
| 7 | −1 | 1 | 1 | 16.9 | 18.5 |
| 8 | 1 | 1 | 1 | 16.2 | 17.6 |
| 9 | −2 | 0 | 0 | 20.8 | 19.8 |
| 10 | 2 | 0 | 0 | 18.8 | 18.3 |
| 11 | 0 | −2 | 0 | 22.1 | 21.0 |
| 12 | 0 | 2 | 0 | 22.2 | 21.8 |
| 13 | 0 | 0 | −2 | 12.8 | 13.9 |
| 14 | 0 | 0 | 2 | 6.9 | 4.3 |
| 15 | 0 | 0 | 0 | 21.1 | 21.4 |
| 16 | 0 | 0 | 0 | 21.1 | 20.1 |
| 17 | 0 | 0 | 0 | 21.1 | 21.9 |
| 18 | 0 | 0 | 0 | 21.1 | 21.9 |
| 19 | 0 | 0 | 0 | 21.1 | 19.9 |
| 20 | 0 | 0 | 0 | 21.1 | 20.0 |
| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 248.33 | 2 | 124.17 | 55.25 | <0.0001 | significant |
| X3 | 34.58 | 1 | 34.58 | 15.39 | 0.0011 | significant |
| X32 | 213.75 | 1 | 213.75 | 95.11 | <0.0001 | significant |
| Residual | 38.20 | 17 | 2.25 | |||
| Lack of fit | 33.43 | 12 | 2.79 | 2.92 | 0.1227 | not significant |
| Pure error | 4.77 | 5 | 0.95 | |||
| Total | 286.54 | 19 |
| Variables | Coded Values | Actual Values |
| Temperature | −2.0 | 20.0 °C |
| Time | −2.0 | 15.0 min |
| EtOH concentration | −0.363 | 40.9% |
| Response | Predicted | Experimental |
| TAC (mg/g-biomass) | 21.2 | 21.0 |
| Content (mg/g-Biomass) | |
|---|---|
| Total polyphenol | 67.4 |
| Total flavonoid | 43.8 |
| Total anthocyanin | 21.0 |
| Ascorbic Acid | Elderberry Extract | |
|---|---|---|
| FRAP value (mmol/L) | 63.3 | 80.2 |
| ABTS IC50 (mg/mL) | 0.5 | 0.4 |
| DPPH IC50 (mg/mL) | 0.8 | 0.9 |
| Biomass | Extraction Method | Conditions | TAC (mg/g-Biomass) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Solvent | Temp. (°C) | Time (min) | S/L Ratio (g/L) | ||||
| Bilberry | Supercritical carbon-dioxide extraction | 50% EtOH (with 0.1% HCl) | 50 | 50 | 100 | 13.7 | [66] |
| Blackberry | Microwave-assisted extraction | 52% EtOH | – | 4 | 25 | 2.2 | [72] |
| Blue honeysuckle berry | Maceration | 0.35% HCl | 42 | 30 | 20 | 24.0 | [45] |
| Blueberry | Ultrasound-assisted extraction | 72.5% EtOH (with 0.02% HCl) | 30 | 1,440 | 50 | 16.2 | [73] |
| Elderberry | Maceration | 100% MeOH (with 1% HCl) | – | 20 | 50 | 8.1 | [20] |
| Elderberry | Maceration | 100% MeOH (with 1% HCl) | – | 20 | 50 | 9.5 | [71] |
| Elderberry | Ultrasound-assisted extraction | 100% MeOH (with 0.1% HCl) | 24 | 90 | 50 | 0.7 | [74] |
| Strawberry | Ultrasound-assisted extraction | 80% EtOH | 90 | 5 | – | 0.4 | [75] |
| Elderberry | Maceration | 40.9% EtOH | 20.0 | 15.0 | 100 | 21.0 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Son, H.; Pang, S.Y.; Yang, J.J.; Lee, J.; Lee, K.H.; Lee, J.H.; Park, C.; Yoo, H.Y. Optimization of Major Extraction Variables to Improve Recovery of Anthocyanins from Elderberry by Response Surface Methodology. Processes 2023, 11, 72. https://doi.org/10.3390/pr11010072
Kim S, Son H, Pang SY, Yang JJ, Lee J, Lee KH, Lee JH, Park C, Yoo HY. Optimization of Major Extraction Variables to Improve Recovery of Anthocyanins from Elderberry by Response Surface Methodology. Processes. 2023; 11(1):72. https://doi.org/10.3390/pr11010072
Chicago/Turabian StyleKim, Seunghee, Hyerim Son, So Young Pang, Jin Ju Yang, Jeongho Lee, Kang Hyun Lee, Ja Hyun Lee, Chulhwan Park, and Hah Young Yoo. 2023. "Optimization of Major Extraction Variables to Improve Recovery of Anthocyanins from Elderberry by Response Surface Methodology" Processes 11, no. 1: 72. https://doi.org/10.3390/pr11010072







