Modeling of CO2 Adsorption on Surface-Functionalized Rubber-Seed Shell Activated Carbon: Isotherm and Kinetic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbent
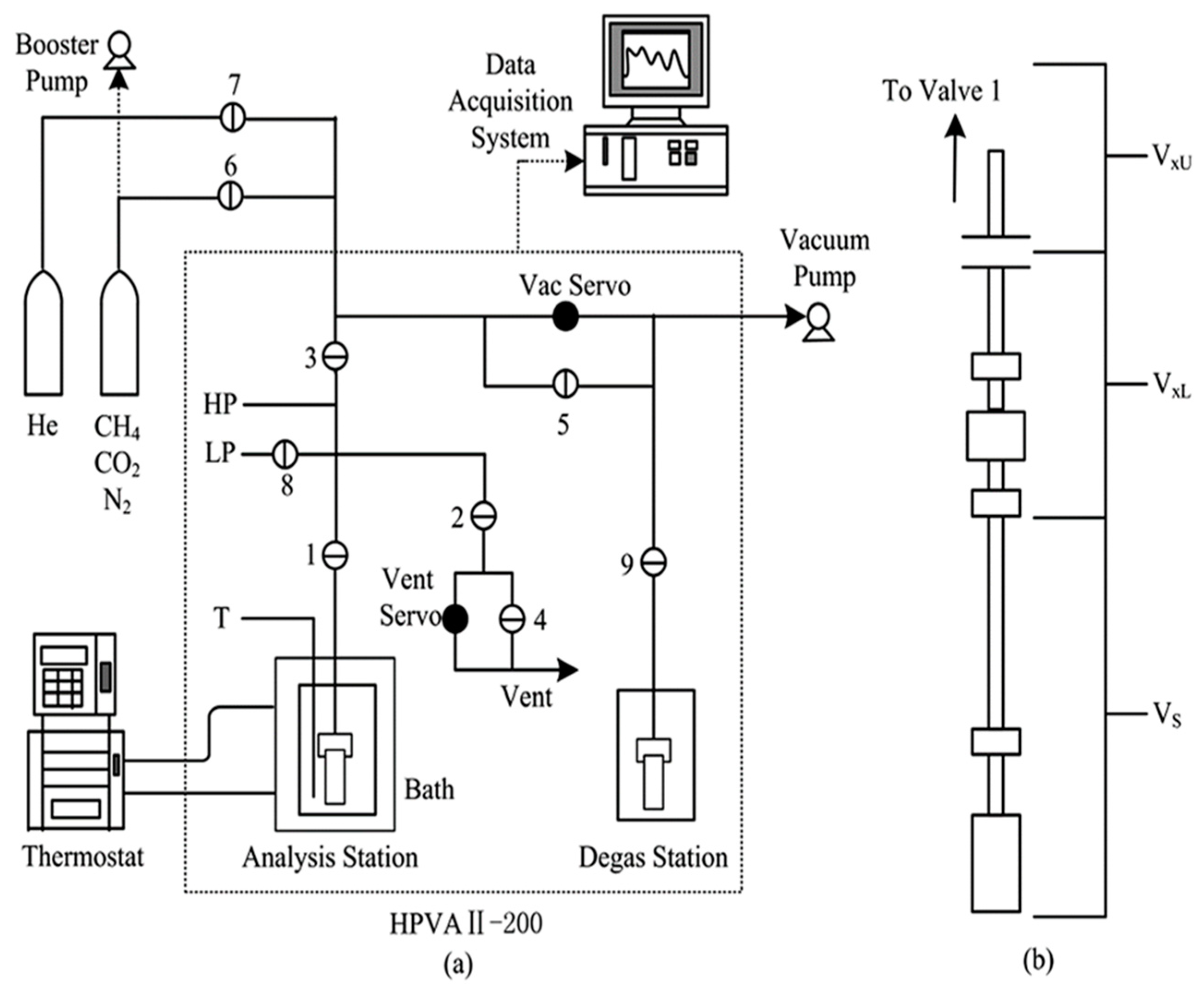
2.2. CO2 Adsorption Study
2.3. Adsorption Isotherm
2.3.1. Langmuir Isotherm Model
- The number of localized active sites is fixed.
- Each adsorption site can accommodate only one adsorbate molecule, and once adsorbed, adsorbate molecules lose the freedom to migrate to other active sites.
- All adsorption sites are energetically equal, indicating a homogeneous surface.
- Adsorbate molecules adsorbed on neighboring active sites do not interact with each other.
- The adsorption process is in dynamic equilibrium, and the rate of adsorption and desorption are equal at equilibrium.
2.3.2. Freundlich Isotherm Model
2.3.3. Temkin Isotherm Model
2.4. Adsorption Kinetics
2.4.1. Rate of Adsorption
2.4.2. Pseudo-First Order Model
2.4.3. Pseudo-Second Order Model
2.4.4. Elovich Kinetic Model
2.4.5. Avrami’s Kinetic Model
2.5. Validity of Kinetic Model
2.6. Rate-Limiting Kinetic Models
2.6.1. Intra-Particle Diffusion Model
2.6.2. Boyd’s Film Diffusion Model
3. Results and Discussion
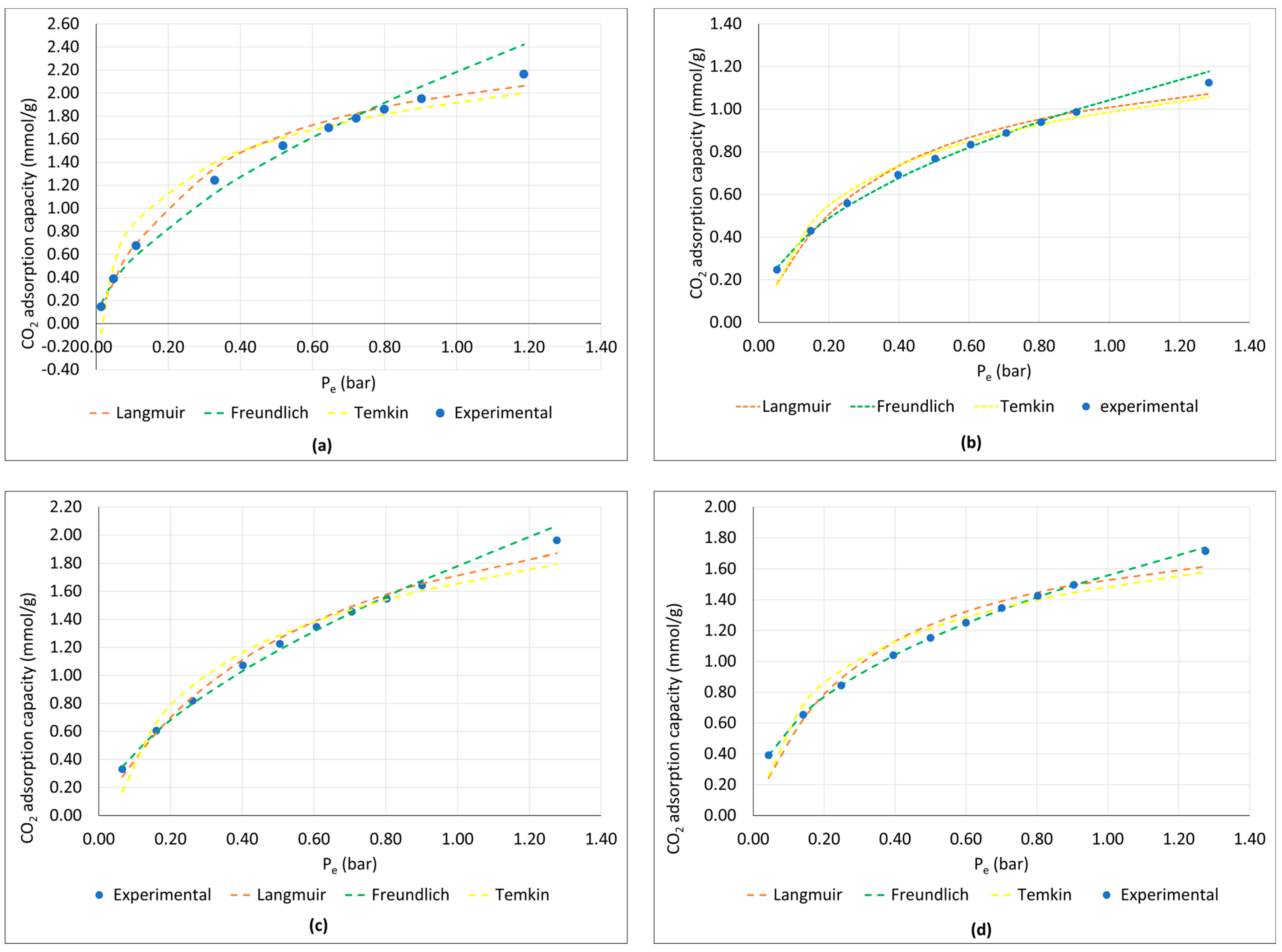
3.1. Adsorption Isotherm Analysis
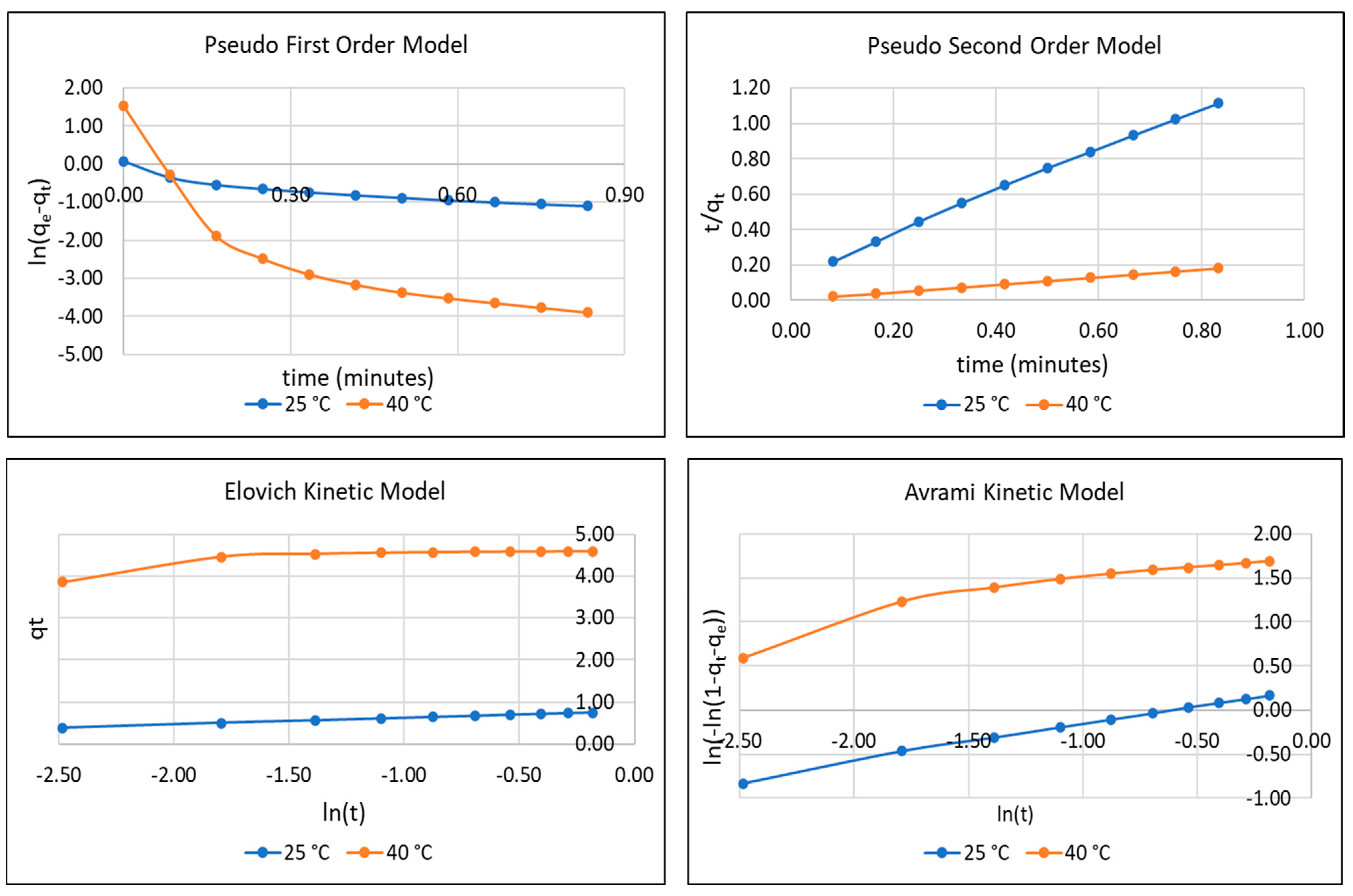
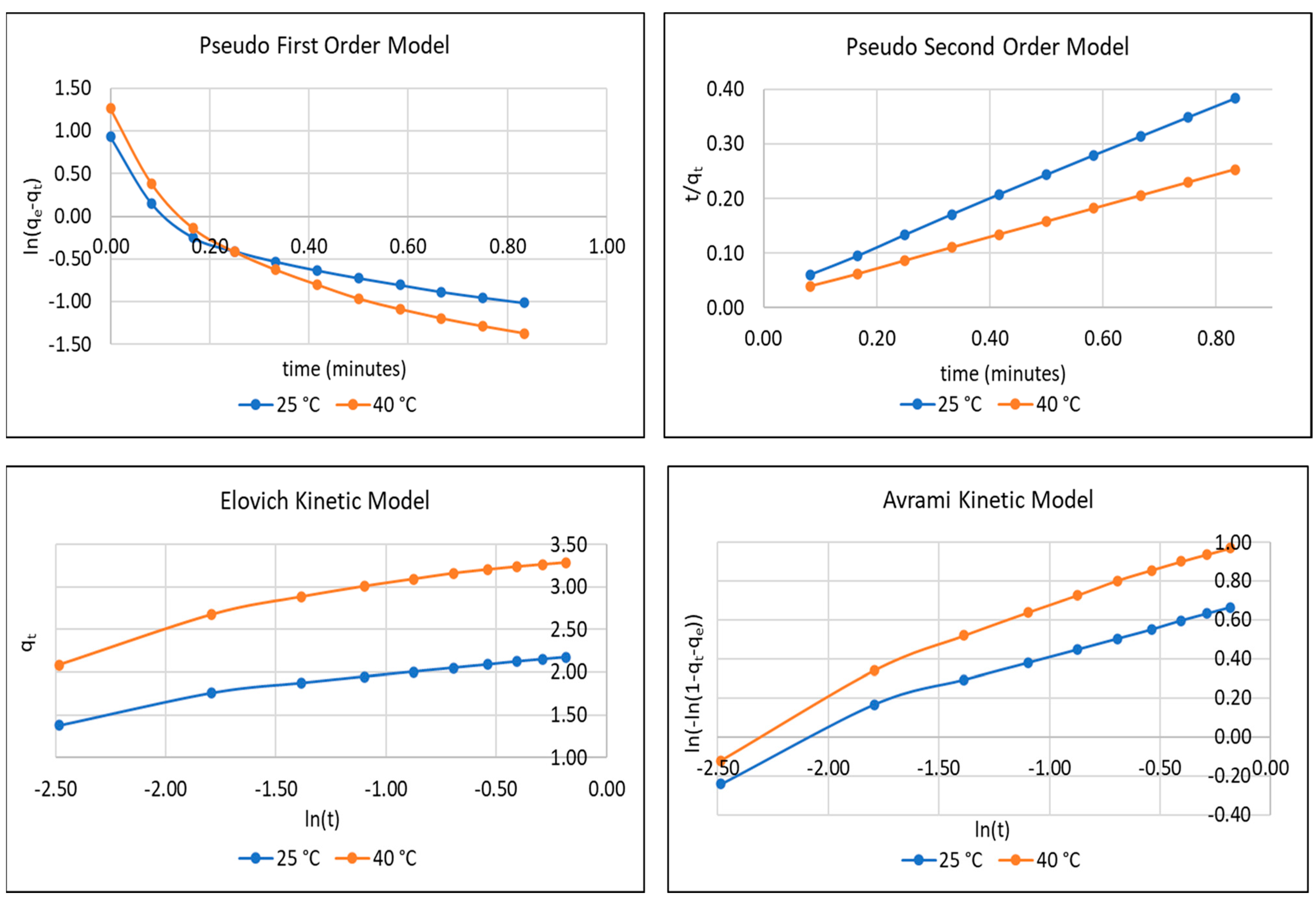
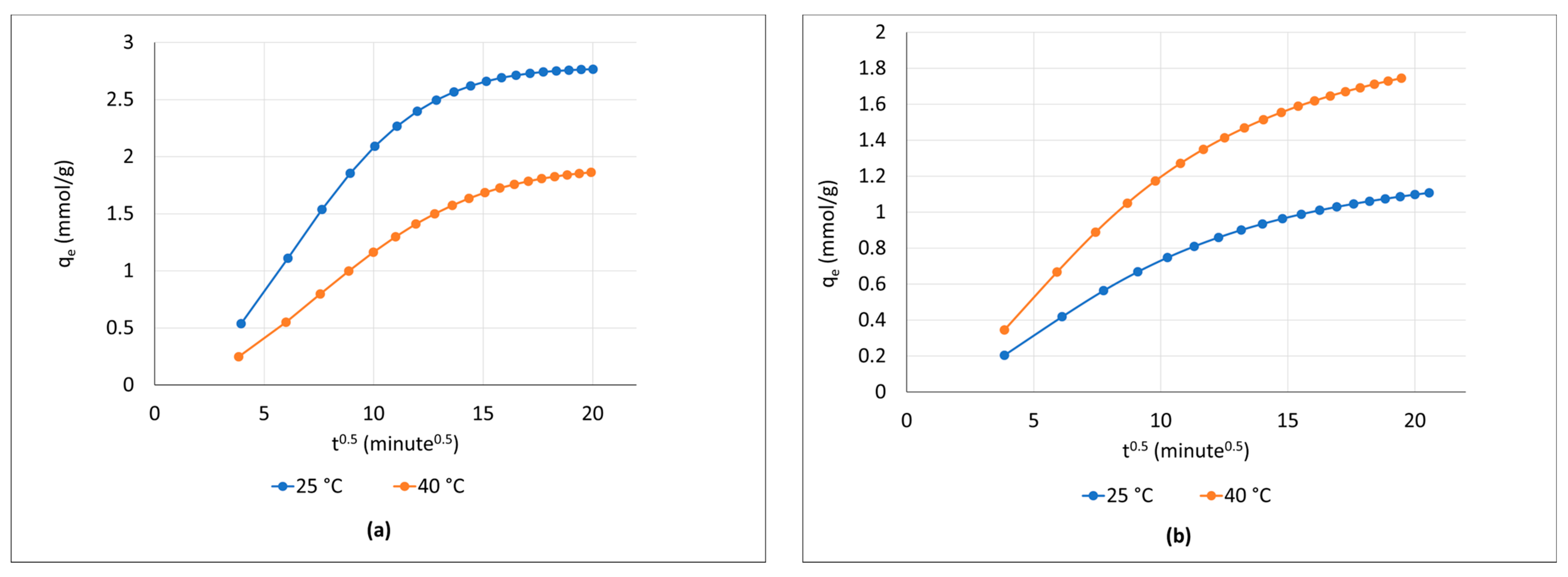
3.2. Adsorption Kinetic Analysis
3.3. CO2 Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Diez, N.; Alvarez, P.; Granda, M.; Blanco, C.; Santamaria, R.; Menendez, R. CO2 adsorption capacity and kinetics in nitrogen-enriched activated carbon fibers prepared by different methods. Chem. Eng. J. 2015, 281, 704–712. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, T.; Wu, X.; Mu, D.; Zhang, Z.; Wang, G.G. Mass and heat transfer characteristic in MEA absorption of CO2 improved by meso-scale method. Int. J. Greenh. Gas Control 2016, 47, 310–321. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Zhu, F.; Chen, X.; Tong, M.; Kang, W.; Zhou, Y.; Lu, J. Experimental study of a hybrid solvent MEA-Methanol for post-combustion CO2 absorption in an absorber packed with three different packing: Sulzer BX500, Mellapale Y500, Pall rings 16 × 16. Sep. Purif. Technol. 2016, 163, 23–29. [Google Scholar] [CrossRef]
- Zhou, Z.; Mei, L.; Ma, C.; Xu, F.; Xiao, J.; Xia, Q.; Li, Z. A novel bimetallic MIL-101(Cr, Mg) with high CO2 adsorption capacity and CO2/N2 selectivity. Chem. Eng. Sci. 2016, 147, 109–117. [Google Scholar] [CrossRef]
- Xiao, G.; Singh, R.; Chaffee, A.; Webley, P. Advanced adsorbents based on MgO and K2CO3 for capture of CO2 at elevated temperatures. Int. J. Greenh. Gas Control 2011, 5, 634–639. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Wang, S.; Lu, M.; Li, H.; Ma, W.; Keener, T.C. Ammonia-treated porous carbon derived from ZIF-8 for enhanced CO2 adsorption. Appl. Surf. Sci. 2016, 369, 390–397. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Oliveira, R.S.; Vieira, R.S.; Cavalcante, C.L., Jr. Adsorption of CO2 on nitrogen-enriched activated carbon and zeolite 13X. Adsorption 2011, 17, 235–246. [Google Scholar] [CrossRef]
- Jiang, Q.; Rentschler, J.; Sethia, G.; Weinman, S.; Perrone, R.; Liu, K. Synthesis of T-type zeolite nanoparticles for the separation of CO2/N2 and CO2/CH4 by adsorption process. Chem. Eng. J. 2013, 230, 380–388. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of acetate-based ionic liquids into a zeolite imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]
- Abutaleb, A.; Eldoma, M.A.; Imran, M.; Taha, K.K.; Bakather, O.Y.; Zouli, N.; Hegazi, S.E.F.; Hassan, M. Active adsorption performance of planetary ball milled Saudi Arabian bentonite clay for the removal of copper ions from aqueous solution. Europhys. Lett. 2021, 135, 30005. [Google Scholar] [CrossRef]
- Bai, B.C.; Cho, S.; Yu, H.-R.; Yi, K.B.; Kim, K.-D.; Lee, Y.-S. Effects of aminated carbon molecular sieves on breakthrough curve behavior in CO2/CH4 separation. J. Ind. Eng. Chem. 2013, 19, 776–783. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, Y.; Duan, J.; Wang, C.; Wen, L.; Zhao, J.; Li, D. A microporous Zn(II)-MOF with open metal sites: Structure and selective adsorption properties. Dalton Trans. 2014, 43, 8311–8317. [Google Scholar] [CrossRef]
- Barcia, P.S.; Bastin, L.; Hurtado, E.J.; Silva, J.A.C.; Rodrigues, A.E.; Chen, B. Single and multicomponent sorption of CO2, CH4 and N2 in a microporous metal-organic framework. Sep. Sci. Technol. 2008, 43, 3494–3521. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Wang, Q.; Tao, M.; He, Y.; Shi, Y. Carbon dioxide capture with polyethylenimine-functionalized industrial-grade mutiwalled carbon nanotubes. Ind. Eng. Chem. Res. 2014, 53, 17468–17475. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chen, C.F. Characterization of bias-controlled carbon nanotubes. Diam. Relat. Mater. 2018, 12, 1615–1620. [Google Scholar] [CrossRef]
- Abutaleb, A.; Imran, M.; Zouli, N.; Khan, A.H.; Hussain, S.; Ali, M.A.; Bakather, O.; Gondal, M.A.; Khan, N.A.; Panchal, H.; et al. Fe3O4-multiwalled carbon nanotubes-bentonite as adsorbent for removal of methylene blue from aqueous solutions. Chemosphere 2023, 316, 137824. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef]
- Aroua, M.K.; Daud, W.M.; Yin, C.Y.; Adinata, D. Adsorption capacities of carbon dioxide, oxygen, nitrogen and methane on carbon molecular basket derived from polyethyleneimine impregnation on microporous palm shell activated carbon. Sep. Purif. Technol. 2008, 62, 609–613. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chiarakorn, S.; Laosiripojana, N.; Towprayoon, S.; Chidthaisong, A. Synthesis of activated carbon and MCM-41 from bagasse and rice husk and their carbon dioxide adsorption. J. Sustain. Energy Environ. 2011, 2, 77–81. [Google Scholar]
- Sanz-Perez, E.S.; Arencibia, A.; Calleja, G.; Sanz, R. Tuning the textural properties of HMS mesoporous silica. Functionalization towards CO2 adsorption. Microporous Mesoporous Mater. 2018, 260, 235–244. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Fermoso, J.; Rubiera, F.; Pis, J.J. A comparison of two methods for producing CO2 capture adsorbents. Energy Procedia 2009, 1, 1107–1113. [Google Scholar] [CrossRef]
- Malaysia, D.o.S. Monthly Rubber Statistics Malaysia, January 2020; Department of Statistics Malaysia: Putrajaya, Malaysia, 2020. [Google Scholar]
- Erto, A.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodriguez-Reinoso, F.; Balsamo, M.; Lancia, A.; Fabio, M. Carbon-supported ionic liquids as innovative adsorbents for CO2 separation from synthetic flue-gas. J. Colloid Interface Sci. 2015, 448, 41–50. [Google Scholar] [CrossRef]
- Yusuf, N.Y.; Masdar, M.S.; Isahak, W.N.R.W.; Nordin, D.; Husaini, T.; Majlan, E.H.; Rejab, S.A.M.; Chew, C.L. Ionic-liquid impregnated activated carbon for biohydrogen purification in an adsorption unit. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012071. [Google Scholar] [CrossRef]
- Arenillas, A.; Smith, K.M.; Drage, T.C.; Snape, C.E. CO2 capture using some fly ash-derived carbon materials. Fuel 2005, 84, 2204–2210. [Google Scholar] [CrossRef]
- Fatima, S.S.; Borhan, A.; Faheem, M. A comparison of two methods for the development of low-cost carbonaceous adsorbent from rubber seed shell (RSS). In Springer Proceedings in Complexity, Proceedings of the 6th international Conference on Fundamental and Applied Sciences: ICFAS 2020, Singapore, 14–16 July 2020; Springer: Singapore, 2021; pp. 107–116. [Google Scholar]
- Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. CO2 adsorption performance on surface-functionalized activated carbon impregnated with pyrrolidinium-based ionic liquid. Processes 2022, 10, 2372. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Chirone, R. CO2 adsorption on a fine activated carbon in a sound assisted fluidized bed: Thermodynamics and kinetics. Chem. Eng. J. 2017, 322, 302–313. [Google Scholar] [CrossRef]
- Zhu, J.; Xin, F.; Huang, J.; Dong, X.; Liu, H. Adsorption and diffusivity of CO2 in phosphonium ionic liquid modified silica. Chem. Eng. J. 2014, 246, 79–87. [Google Scholar] [CrossRef]
- Zhu, J.; He, B.; Huang, J.; Li, C.; Ren, T. Effect of immobilization methods and the pore structure on CO2 separation performance in silica-supported ionic liquids. Microporous Mesoporous Mater. 2018, 260, 190–200. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 355–471. [Google Scholar]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Ngila, J.C.; Kim, D.G.; Jo, Y.M. Isotherm, thermodynamic and kinetic studies of selective CO2 adsorption on chemically modified carbon surfaces. Aerosol Air Qual. Res. 2016, 16, 3312–3329. [Google Scholar] [CrossRef]
- Monazam, E.R.; Spenik, J.; Shadle, L.J. Fluid bed adsorption of carbon dioxide on immobilized polyethylenimine (PEI): Kinetic analysis and breakthrough behavior. Chem. Eng. J. 2013, 223, 795–805. [Google Scholar] [CrossRef]
- Stevens, L.; Williams, K.; Han, W.Y.; Drage, T.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem. Eng. J. 2013, 215–216, 699–708. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Edubilli, S.; Mishra, A. CO2 adsoprtion kinetics on mesoporous silica under wide range of pressure and temperature. Chem. Eng. J. 2014, 256, 1–8. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Sayari, A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves. Chem. Eng. J. 2010, 161, 182–190. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumar, E.A. Comparative studies on CO2 adsorption kinetics by solid adsorbents. Energy Procedia 2016, 90, 316–325. [Google Scholar] [CrossRef]
- Lopes, E.C.N.; dos Anjos, F.S.C.; Vieira, E.F.S.; Cestari, A.R. An alternative avrami equation to evaluate kinetic parameters of the interaction of Hg(II) with thin chitosan membranes. J. Colloid Interface Sci. 2003, 263, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Benedict, J.B.; Coppens, P. Kinetics of the single-crystal to single-crystal two-photon photodimerization of α-trans-Cinnamic Acid to α-Truxillic Acid. J. Phys. Chem. A 2009, 113, 3116–3120. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhu, X.; Chen, R.; Liao, Q.; Ding, Y.-D.; Chen, L. An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem. Eng. J. 2016, 283, 175–183. [Google Scholar] [CrossRef]
- Tsibranska, I.; Hristova, E. Comparison of different kinetic models for adsorption of heavy metals onto activated carbon from apricot stones. Bulg. Chem. Commun. 2011, 43, 370–377. [Google Scholar]
- Garces-Polo, S.I.; Villarroel-Rocha, J.; Sapag, K.; Korili, S.A.; Gil, A. A comparative study of CO2 diffusion from adsorption kinetic measurements on microporous materials at low pressures and temperatures. Chem. Eng. J. 2016, 302, 278–286. [Google Scholar] [CrossRef]
- Lunhong, A.; Li, M.; Li, L. Adsorption of methylene blue from aqueous solution with activated carbon/cobalt ferrite/alginate composite beads: Kinetics, isotherms, and thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Xu, C.; Chen, D.-L.; Zhu, W.; Krishna, R. Utilizing transient breakthroughs for evaluating the potential of kureha carbon for CO2 capture. Chem. Eng. J. 2015, 269, 135–147. [Google Scholar] [CrossRef]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Sharma, P.; Das, M.R. Removal of a cationic dye from aqueous solution using grephene oxide nanosheets: Investigation of adsorption parameters. J. Chem. Eng. Data 2013, 58, 151–158. [Google Scholar] [CrossRef]
- Perez, N.; Sanchez, M.; Rincon, G.; Delgado, L. Study of the behavior of metal adsorption on acid solutions on lignin using a comparison of different adsorption isotherms. Lat. Am. Appl. Res. 2015, 37, 157–162. [Google Scholar]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chem. Eng. Res. Des. 2018, 134, 540–552. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S.; Borhan, A. Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia Eng. 2016, 148, 630–637. [Google Scholar] [CrossRef]
- Borah, J.M.; Sarma, J.; Mahiuddin, S. Adsorption comparison at the α-alumina/water interface: 3,4-Dihydroxybenzoic acid vs catechol Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 50–56. [Google Scholar] [CrossRef]
- Mengqi, W.; Qingbo, Y.; Wenjun, D.; Limin, H.; Kaijie, L.; Qin, Q.; Sihong, L.; Jinjie, D. Equilibrium and kinetics analysis of CO2 adsorption on waste ion-exchange resin-based activated carbon. J. Taiwan Inst. Chem. Eng. 2017, 77, 161–167. [Google Scholar] [CrossRef]

| Sample | AC-Blank | AC-Functionalized | ||
|---|---|---|---|---|
| Temperature (°C) | 25 | 40 | 25 | 40 |
| Langmuir isotherm | ||||
| qmax (mmol/g) | 2.5907 | 2.7196 | 1.3535 | 2.0117 |
| kL (1/bar) | 3.2991 | 1.7239 | 2.9635 | 3.1927 |
| R2 | 0.9871 | 0.9789 | 0.9815 | 0.9708 |
| Freundlich isotherm | ||||
| n | 1.6821 | 1.6681 | 2.1079 | 2.2681 |
| 1/n | 0.5945 | 0.4409 | 0.4744 | 0.4409 |
| kF (mmol/g·bar) | 2.1871 | 1.5619 | 1.0454 | 1.5619 |
| R2 | 0.9889 | 0.9964 | 0.9968 | 0.9998 |
| Temkin isotherm | ||||
| Bt (J/mol) | 130.5117 | 103.1559 | 222.0873 | 144.0238 |
| kT (mol/g·bar) | 59.8707 | 20.8912 | 36.1641 | 44.6218 |
| R2 | 0.9608 | 0.9626 | 0.9738 | 0.9536 |
| Units | AC-Blank | AC-Functionalized | ||||
|---|---|---|---|---|---|---|
| Temperature | °C | 25 | 40 | 25 | 40 | |
| qe,exp | cm3/g | 1.0802 | 4.6064 | 2.5351 | 3.5421 | |
| Two parameter models | ||||||
| Pseudo-second order model | qe,cal | cm3/g | 0.8450 | 4.6512 | 2.3115 | 3.5044 |
| k2 | g·min/cm3 | 9.8422 | 21.9141 | 7.3616 | 5.2338 | |
| R2 | - | 0.9981 | 0.9998 | 0.9997 | 1.000 | |
| Δq | % | 23.45 | 5.88 | 4.98 | 5.06 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, S.S.; Borhan, A.; Ayoub, M.; Ghani, N.A. Modeling of CO2 Adsorption on Surface-Functionalized Rubber-Seed Shell Activated Carbon: Isotherm and Kinetic Analysis. Processes 2023, 11, 2833. https://doi.org/10.3390/pr11102833
Fatima SS, Borhan A, Ayoub M, Ghani NA. Modeling of CO2 Adsorption on Surface-Functionalized Rubber-Seed Shell Activated Carbon: Isotherm and Kinetic Analysis. Processes. 2023; 11(10):2833. https://doi.org/10.3390/pr11102833
Chicago/Turabian StyleFatima, Syeda Saba, Azry Borhan, Muhammad Ayoub, and Noraini Abd Ghani. 2023. "Modeling of CO2 Adsorption on Surface-Functionalized Rubber-Seed Shell Activated Carbon: Isotherm and Kinetic Analysis" Processes 11, no. 10: 2833. https://doi.org/10.3390/pr11102833








