Actinic Radiation, Viruses, Bacteria, the Open Air Factor (OAF) and Indoor Sterilization with UV-C Radiation
Abstract
:1. Introduction
2. Naturally Selective Possibilities
3. Mechanisms and Tests
3.1. Photodissociation of Nucleic Acids and Proteins
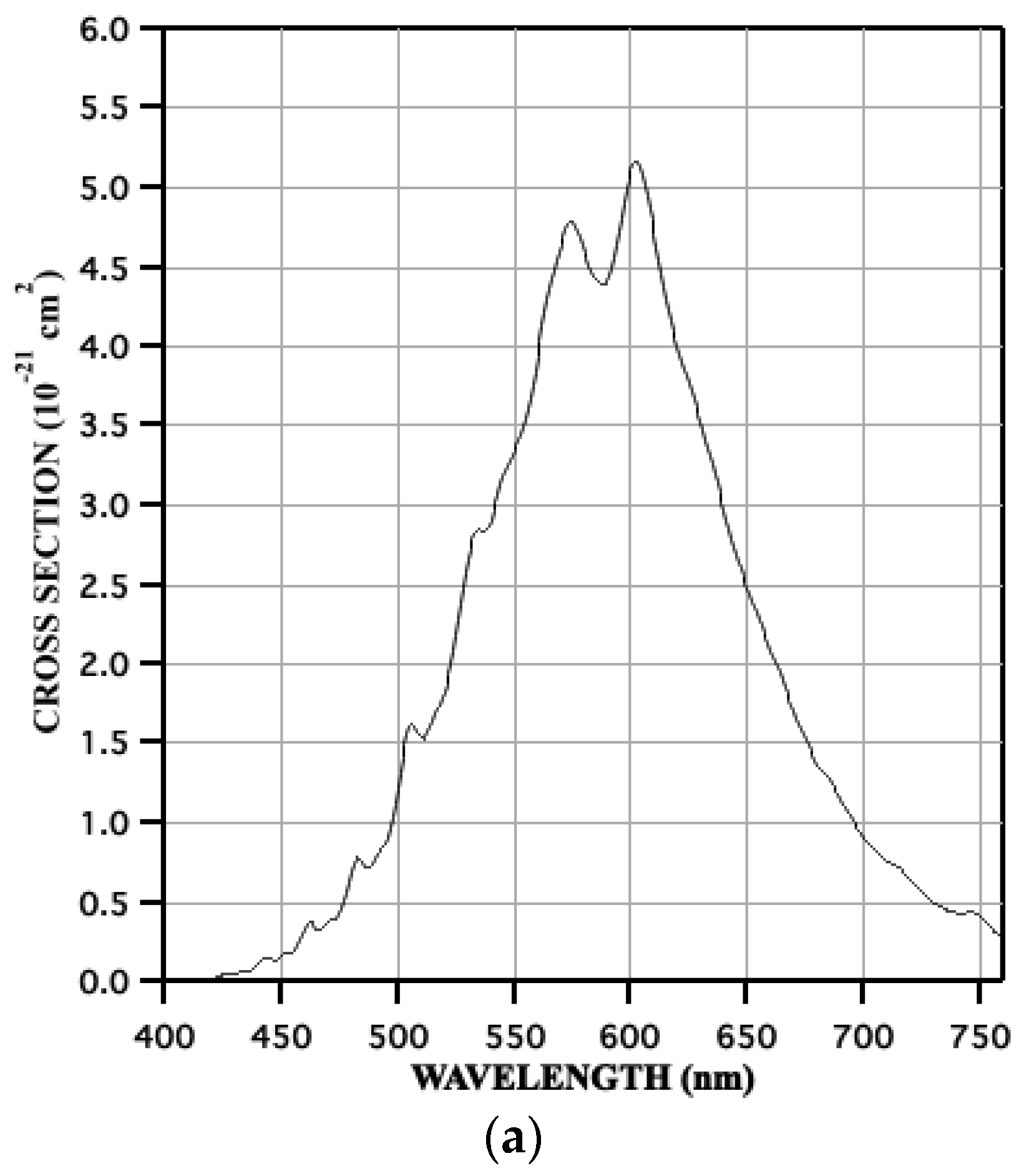
3.2. Reactions of O(3P) and O2(a1Δg) with Nucleic Acids and Proteins
| O3 + hυ | → O(3P) + O2(X3Σg−) | 101 kJ mol−1 | 1180 nm |
| → O(3P) + O2(1Δg) | 195 kJ mol−1 | 612 nm | |
| → O(3P) + O2(b1Σg+) | 258 kJ mol−1 | 463 nm | |
| → O(1D) + O2(X3Σg−) | 291 kJ mol−1 | 411 nm | |
| → O(1D) + O2(1Δg) | 386 kJ mol−1 | 310 nm | |
| → O(1D) + O2(b1Σg+) | 448 kJ mol−1 | 267 nm | |
| → 3O(3P) | 595 kJ mol−1 | 201 nm | |
| → O(1S) + O2(1Δg) | 610 kJ mol−1 | 196 nm |
3.3. Aerosols, Airborne Microbes
3.4. Experimental Tests
3.4.1. Laboratory Experiments
3.4.2. Indoor Tests
3.4.3. Open AirTests
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, R.A.; Ammann, M.; Crowley, J.N.; Griffiths, P.T.; Herrmann, H.; Hoffmann, E.H.; Jenkin, M.E.; McNeill, V.F.; Mellouki, A.; Penkett, C.J.; et al. Opinion: The germicidal effect of ambient air (open-air factor) revisited. Atmos. Chem. Phys. 2021, 21, 13011–13018. [Google Scholar] [CrossRef]
- Prather, K.A.; Marr, L.C.; Schooley, R.T.; McDiarmid, M.A.; Wilson, M.E.; Milton, D.K. Airborne transmission of SARS-CoV-2. Science 2020, 370, 303–304. [Google Scholar]
- Tuck, A.F. Air temperature intermittency and photofragment excitation. Meteorology 2023, 1. submitted. [Google Scholar]
- Logan, J.A.; McElroy, M.B. Distribution functions for energetic oxygen atoms in the earth’s lower atmosphere. Planet. Space Sci. 1977, 25, 117–122. [Google Scholar] [CrossRef]
- Deal, A.M.; Vaida, V. Oxygen effect on the ultraviolet-C photochemistry of lactic acid. J. Phys. Chem. A 2023, 127, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Pereverzev, A.Y.; Kopysov, V.N.; Boyarkin, O.V. Peptide bond ultraviolet absorption enables vibrational cold-ion spectroscopy of nonaromatic peptides. J. Phys. Chem. Lett. 2018, 9, 5262–5266. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Mandal, I.; Singh, S.; Paul, A.; Mandal, B.; Venkatramani, R.; Swaminathan, R. Near UV-Visible electronic absorption originating from charged amino acids in a monomeric protein. Chem. Sci. 2017, 8, 5415–5433. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh, M.; Adeli, B. A critical review on ultraviolet disinfection systems against COVID-19 outbreak: Applicability, validation, and safety considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Hengst, M.; Kurte, L.; Dorador, C.; Jeffrey, W.H.; Wattiez, R.; Molina, V.; Matallana-Surget, S. Bacterial Survival under Extreme UV Radiation: A Comparative Proteomics Study of Rhodobacter sp., Isolated from High Altitude Wetlands in Chile. Front. Microbiol. 2017, 8, 1173. [Google Scholar] [CrossRef]
- Paulino-Lima, I.G.; Fujishima, K.; Navarette, J.U.; Galante, D.; Rodrigues, F.; Azua-Bustos, A.; Rothschild, L.J. Extremely high UV-C radiation resistant microorganisms, from desert environments with different manganese concentrations. Photochem. Photobiol. B Biol. 2016, 163, 327–336. [Google Scholar] [CrossRef]
- Burrows, S.M.; Elbert, W.; Lawrence, M.G.; Pöschl, U. Bacteria in the global atmosphere-Part 1: Review and synthesis literature data for different ecosystems. Atmos. Chem. Phys. 2009, 9, 9263–9280. [Google Scholar] [CrossRef]
- Smith, D.J.; Jaffe, D.A.; Birmele, M.N.; Griffin, D.W.; Schnerzer, A.C.; Hee, J.; Roberts, M.S. Free tropospheric transport from Asia to North America. Microb. Ecol. 2012, 64, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.M.; Thomson, D.S.; Mahoney, M.J. In situ measurements of organics, meteoritical material, mercury and other elements in aerosols at 5 to 19 kilometers. Science 1998, 282, 1664–1669. [Google Scholar] [CrossRef]
- Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric processing of organic aerosols. J. Geophys. Res. D 1999, 104, 11633–11641. [Google Scholar] [CrossRef]
- Griffith, E.C.; Tuck, A.F.; Vaida, V. Ocean-atmosphere interactions in the emergence of complexity in simple chemical systems. Accs. Chem. Res. 2012, 45, 2106–2113. [Google Scholar] [CrossRef]
- Yamano, M.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaka-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term effects of 222-nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Graeffe, F.; Luo, Y.; Guo, Y.; Ehn, M. Unwanted indoor air quality effects from using ultraviolet C lamps for disinfection. Environ. Sci. Technol. Lett. 2023, 10, 172–178. [Google Scholar] [CrossRef]
- Peng, Z.; Miller, S.L.; Jimenez, J.L. Model evaluation of secondary chemistry due to disinfection of indoor air by germicidal ultraviolet lamps. Environ. Sci. Technol. Lett. 2023, 10, 6. [Google Scholar] [CrossRef]
- Ong, Q.; Wee, W.; Dela Cruz, J.; Teo, W.R.; Han, W. 222-nm far UV-C exposure results in DNA damage and transcriptional changes to mammalian cells. bioRxiv 2022. [Google Scholar] [CrossRef]
- Parrish, D.D.; Derwent, R.G.; Steinbrecht, W.; Stübi, R.; Van Malderen, R.; Steinbacher, M.; Trickl, T.; Ries, L.; Xu, X. Zonal similarity of long-term changes and seasonal cycles of baseline ozone at northern midlatitudes. J. Geophys. Res.-Atmos. 2020, 125, e2019JD031908. [Google Scholar] [CrossRef]
- Fowler, D.; Brimblecombe, P.; Burrows, J.; Heal, M.R.; Grennfelt, P.; Stevenson, D.S.; Jowett, A.; Nemitz, E.; Coyle, M.; Liu, X.; et al. A chronology of global air quality. Phil. Trans. R. Soc. A 2020, 378, 20190314. [Google Scholar] [CrossRef]
- You, B.; Zhou, W.; Li, J.; Li, Z.; Sun, Y. A review of indoor air gaseous organic compounds and human chemical exposure: Insights from real-time measurements. Env. Intnl. 2022, 170, 107611. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.P.V.; Xavier, C.S.F.; Cobrado, L. Disinfection methods against SARS-CoV-2: A systematic review. J. Hosp. Infect. 2022, 119, 84–117. [Google Scholar] [CrossRef]
- Rapf, R.J.; Dooley, M.R.; Kappes, K.; Perkins, R.J.; Vaida, V. pH dependence of aqueous photochemistry of α-keto acids. J. Phys. Chem. A 2017, 121, 8368–8379. [Google Scholar] [CrossRef] [PubMed]
- Pereverzev, A.Y.; Koczor-Benda, Z.; Saparbaev, E.; Kopysov, V.N.; Rosta, E.; Boyarkin, O.V. Spectroscopic Evidence for Peptide-Bond-Selective Ultraviolet Photodissociation. J. Phys. Chem. Lett. 2020, 11, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.M.; Wang, Z.-C.; Lineberger, W.C.; Bierbaum, V.M. Gas-Phase Reactions of Deprotonated Nucleobases with H, N, and O Atoms. J. Phys. Chem. Lett. 2019, 10, 4863–4867. [Google Scholar] [CrossRef]
- Tuck, A.F. Gibbs free energy and reaction rate acceleration in and on microdroplets. Entropy 2019, 21, 1044. [Google Scholar] [CrossRef]
- Kang, H.; Lee, K.F.; Jung, B.; Ko, Y.J.; Kim, S.K. Intrinsic lifetimes of the excited states of DNA and RNA oligonucleotides. J. Am. Chem. Soc. 2002, 124, 12958–12959. [Google Scholar] [CrossRef]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef]
- Tavakoli, A.; Min, J.-H. Photochemical modifications for DNA/RNA nucleotides. RSC Adv. 2022, 12, 6484–6507. [Google Scholar] [CrossRef]
- Rapf, R.J.; Vaida, V. Sunlight as an energetic driver in the synthesis of molecules necessary for life. Phys. Chem. Chem. Phys. 2016, 18, 20067–20084. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.L.; Arnbjerg, J.; Ogilby, P.R. Reaction of singlet oxygen with tryptophan in proteins: A pronounced effect of the local environment on the reaction rate. J. Am. Chem. Soc. 2012, 134, 9820–9826. [Google Scholar] [CrossRef] [PubMed]
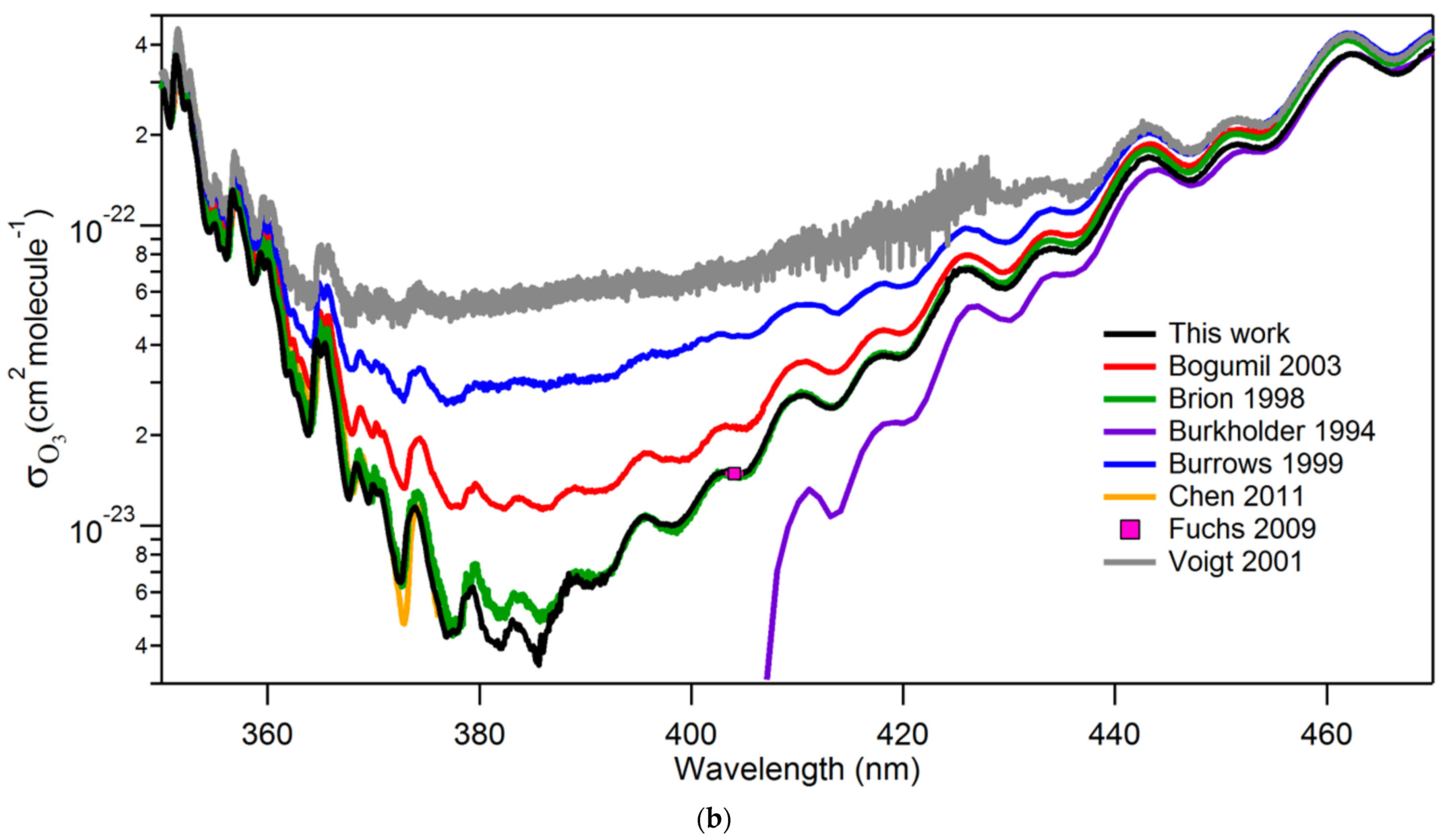
- Axson, J.L.; Washenfelder, R.A.; Kahan, T.F.; Young, C.J.; Vaida, V.; Brown, S.S. Absolute ozone absorption cross-sections in the Huggins Chappuis minimum (350–470 nm) at 296 K. Atmos. Chem. Phys. 2011, 11, 11581–11590. [Google Scholar] [CrossRef]
- Tervahattu, H.; Hartonen, K.; Kerminen, V.-M.; Kupiainen, K.; Aarnio, P.; Koskentalo, K.; Tuck, A.F.; Vaida, V. New evidence of an organic layer on marine aerosols. J. Geophys. Res. Atmos. 2002, 107, AAC1–AAC8. [Google Scholar] [CrossRef]
- Tervahattu, H.; Juhanoja, J.; Vaida, V.; Tuck, A.F.; Niemi, J.V.; Kupiainen, K.; Kulmala, M.; Vehkamäki, H. Fatty acids on continental sulfate aerosol particles. J. Geophys. Res. Atmos. 2005, 110, D06207. [Google Scholar] [CrossRef]
- Yu, W.; Thurston, G.D. An interrupted time series analysis of the cardiovascular health benefits of a coal coking operation closure. Env. Res. Health 2023, 1, 045002. [Google Scholar] [CrossRef]
- Oswin, H.P.; Haddrell, A.E.; Otero-Fernandez, M.; Mann, J.F.; Cogan, T.A.; Hilditch, T.G.; Tian, J.; Hardy, D.A.; Hill, D.J.; Finn, A.; et al. The dynamics of SARS-COV-2 infectivity with changes in aerosol microenvironment. Proc. Natl. Acad. Sci. USA 2022, 119, e2200109119. [Google Scholar] [CrossRef] [PubMed]
- Needleman, D.; Shelley, M. The stormy fluid dynamics of the living cell. Phys. Today 2019, 72, 32–39. [Google Scholar] [CrossRef]
- Krapf, D.; Metzler, R. Strange interfacial molecular dynamics. Phys. Today 2019, 72, 48–55. [Google Scholar] [CrossRef]
- NOAA SABRE Mission. Available online: https://csl.noaa.gov/projects/sabre/wb57/payload.html (accessed on 1 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuck, A.F. Actinic Radiation, Viruses, Bacteria, the Open Air Factor (OAF) and Indoor Sterilization with UV-C Radiation. Processes 2023, 11, 2882. https://doi.org/10.3390/pr11102882
Tuck AF. Actinic Radiation, Viruses, Bacteria, the Open Air Factor (OAF) and Indoor Sterilization with UV-C Radiation. Processes. 2023; 11(10):2882. https://doi.org/10.3390/pr11102882
Chicago/Turabian StyleTuck, Adrian F. 2023. "Actinic Radiation, Viruses, Bacteria, the Open Air Factor (OAF) and Indoor Sterilization with UV-C Radiation" Processes 11, no. 10: 2882. https://doi.org/10.3390/pr11102882
APA StyleTuck, A. F. (2023). Actinic Radiation, Viruses, Bacteria, the Open Air Factor (OAF) and Indoor Sterilization with UV-C Radiation. Processes, 11(10), 2882. https://doi.org/10.3390/pr11102882






