The Preparation of Mn-Modified CeO2 Nanosphere Catalysts and Their Catalytic Performance for Soot Combustion
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.1.1. Preparation of CeO2 Nanospheres
2.1.2. Preparation of MnOx/CeO2 Nanosphere Catalysts
2.1.3. Preparation of MnOx Catalysts
2.2. Catalyst Characterization
2.3. Catalytic Activity Measurements
3. Result and Discussion
3.1. Structural Features of the Synthesized Catalysts
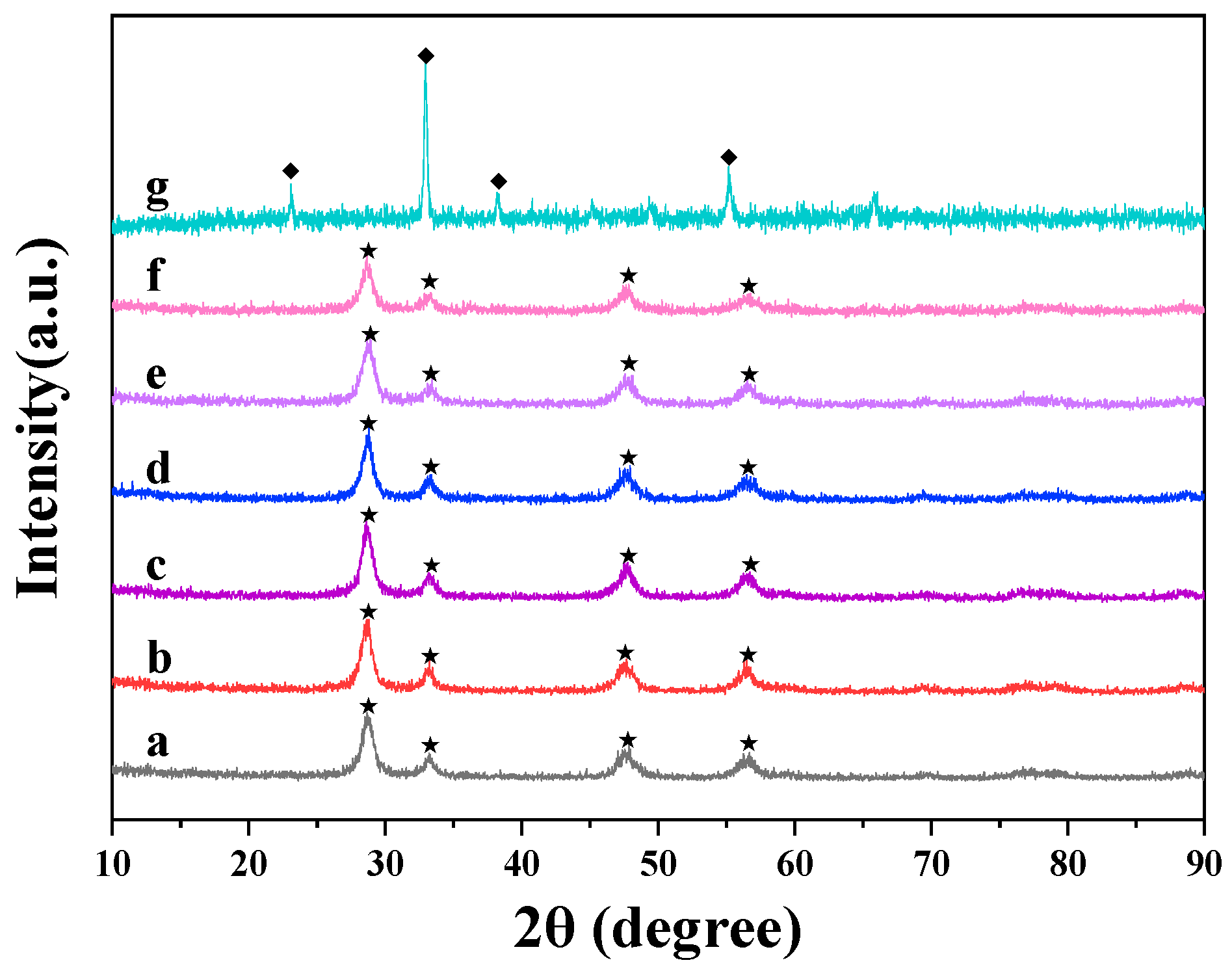
3.1.1. XRD Analysis
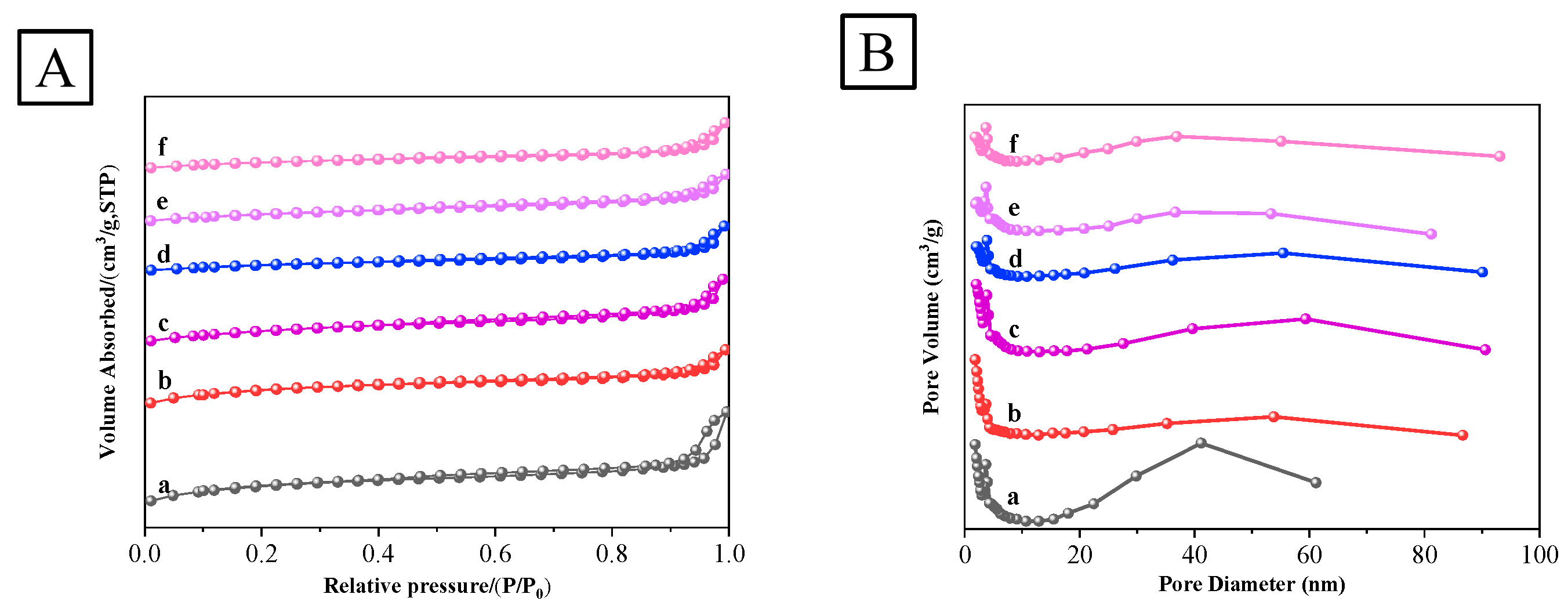
3.1.2. N2 Physisorption Analysis
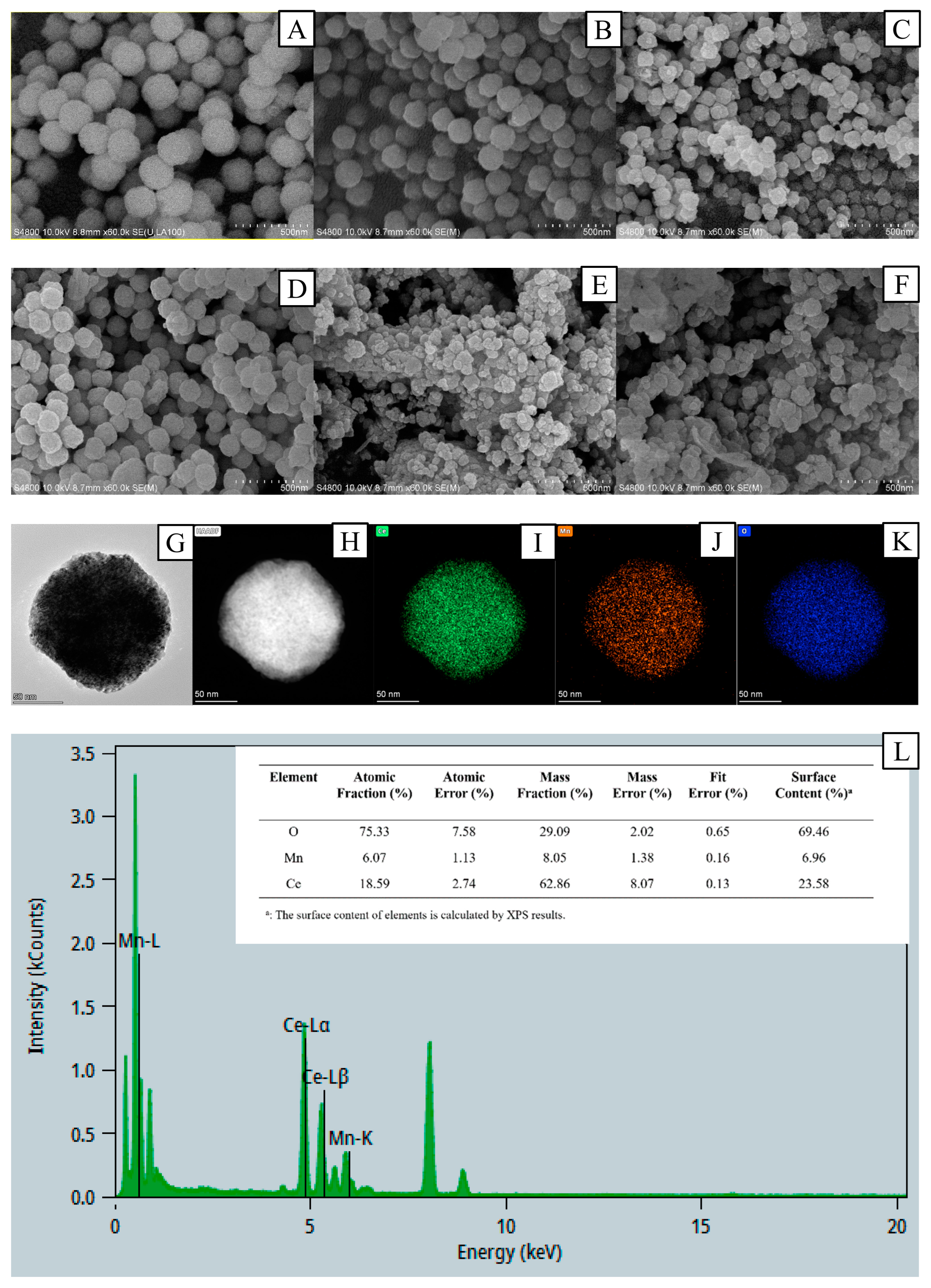
3.1.3. Morphological Characterization of the Prepared Catalysts
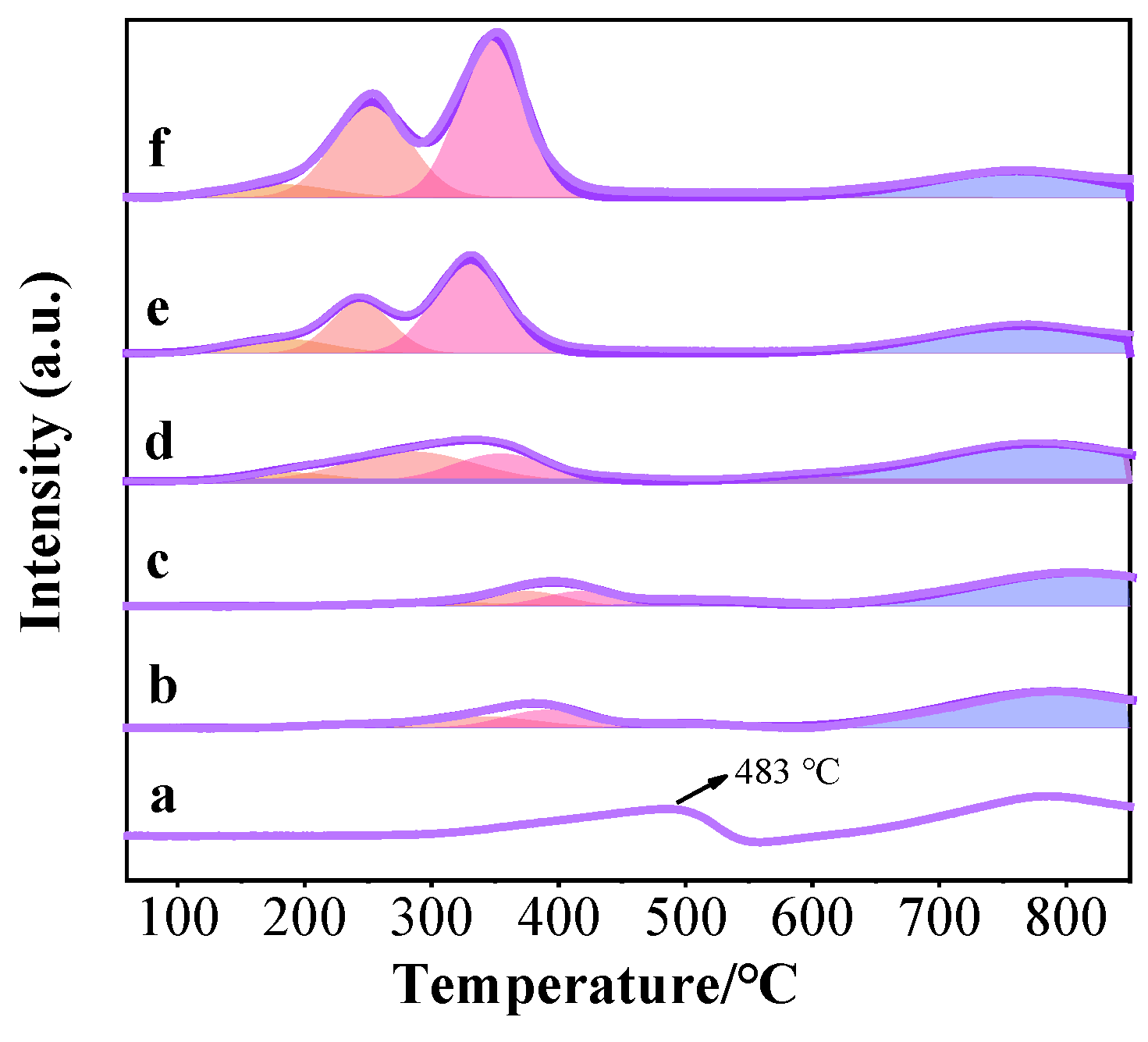
3.1.4. H2-TPR Analysis
3.1.5. O2-TPD Analysis
3.1.6. XPS Analysis
3.1.7. Soot-TPR Analysis
3.1.8. NO-TPO Analysis
3.1.9. In situ DRIFTS Analysis
3.2. Catalytic Performance in Soot Combustion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Wang, L.; Zhao, Z.; Fan, X.; Chen, M.; Wei, Y.; Liu, J. 3DOM SiO2-Supported Different Alkali Metals-Modified MnOx Catalysts: Preparation and Catalytic Performance for Soot combustion. ChemistrySelect 2017, 2, 10176–10185. [Google Scholar] [CrossRef]
- Frank, B.; Schuster, M.E.; Schlögl, R.; Su, D.S. Emission of Highly Activated Soot Particulate—The Other Side of the Coin with Modern Diesel Engines. Angew. Chem. Int. Ed. 2013, 52, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lan, X.; Zhang, C.; Cao, C.; Yu, Y. Recent advances in soot combustion catalysts with designed micro-structures. Chin. Chem. Lett. 2022, 33, 1763–1771. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Ran, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar] [CrossRef]
- Yeste, M.P.; Cauqui, M.Á.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; Muñoz, M.Á.; García-García, A. Catalytic activity of Cu and Co supported on ceria-yttria-zirconia oxides for the diesel soot combustion reaction in the presence of NOx. Chem. Eng. J. 2020, 380, 122370. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Lan, L.; Chen, S.; Li, H.; Wang, J.; Li, D.; Chen, Y. Optimized synthesis of highly thermal stable CeO2-ZrO2/Al2O3 composite for improved Pd-only three-way catalyst. Mater. Des. 2018, 147, 191–199. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Liu, J.; Xu, C.; Jiang, G.; Duan, A. Design and Synthesis of 3D Ordered Macroporous CeO2-Supported Pt@CeO2-δ Core–Shell Nanoparticle Materials for Enhanced Catalytic Activity of Soot Oxidation. Small 2013, 9, 3957–3963. [Google Scholar] [CrossRef]
- Mukherjee, D.; Reddy, B.M. Noble metal-free CeO2-based mixed oxides for CO and soot oxidation. Catal. Today 2018, 309, 227–235. [Google Scholar] [CrossRef]
- Liu, T.; Li, Q.; Xin, Y.; Zhang, Z.; Tang, X.; Zheng, L.; Gao, P.-X. Quasi free K cations confined in hollandite-type tunnels for catalytic solid (catalyst)-solid (reactant) oxidation reactions. Appl. Catal. B 2018, 232, 108–116. [Google Scholar] [CrossRef]
- Neeft, J.P.A.; Makkee, M.; Moulijn, J.A. Metal oxides as catalysts for the oxidation of soot. Chem. Eng. J. Biochem. Eng. 1996, 64, 295–302. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, X.; Yuan, Z.; Wu, M.; Zhou, H. Oxygen defect-stabilized heterogeneous single atom catalysts: Preparation, properties and catalytic application. J. Mater. Chem. A 2021, 9, 3855–3879. [Google Scholar] [CrossRef]
- Ji, W.; Wang, N.; Li, Q.; Zhu, H.; Lin, K.; Deng, J.; Chen, J.; Zhang, H.; Xing, X. Oxygen vacancy distributions and electron localization in a CeO2(100) nanocube. Inorg. Chem. Front. 2022, 9, 275–283. [Google Scholar] [CrossRef]
- Xu, B.; Xia, L.; Zhou, F.; Zhao, R.; Chen, H.; Wang, T.; Zhou, Q.; Liu, Q.; Cui, G.; Xiong, X.; et al. Enhancing Electrocatalytic N2 Reduction to NH3 by CeO2 Nanorod with Oxygen Vacancies. ACS Sustain. Chem. Eng. 2019, 7, 2889–2893. [Google Scholar] [CrossRef]
- Machida, M.; Ueno, M.; Omura, T.; Kurusu, S.; Hinokuma, S.; Nanba, T.; Shinozaki, O.; Furutani, H. CeO2-Grafted Mn–Fe Oxide Composites as Alternative Oxygen-Storage Materials for Three-Way Catalysts: Laboratory and Chassis Dynamometer Tests. Ind. Eng. Chem. Res. 2017, 56, 3184–3193. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Sun, P.; Ye, S. Study on the simultaneous reduction of diesel engine soot and NO with nano-CeO2 catalysts. RSC Adv. 2016, 6, 102028–102034. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, D.Y.; Choung, J.W.; Kim, C.H.; Ham, H.C.; Lee, K.-Y. Roles of noble metals (M = Ag, Au, Pd, Pt and Rh) on CeO2 in enhancing activity toward soot oxidation: Active oxygen species and DFT calculations. J. Hazard. Mater. 2021, 403, 124085. [Google Scholar] [CrossRef]
- Wang, H.; Jin, B.; Wang, H.; Ma, N.; Liu, W.; Weng, D.; Wu, X.; Liu, S. Study of Ag promoted Fe2O3@CeO2 as superior soot oxidation catalysts: The role of Fe2O3 crystal plane and tandem oxygen delivery. Appl. Catal. B 2018, 237, 251–262. [Google Scholar] [CrossRef]
- Shan, W.; Yang, L.; Ma, N.; Yang, J. Catalytic Activity and Stability of K/CeO2 Catalysts for Diesel Soot Oxidation. Chin. J. Catal. 2012, 33, 970–976. [Google Scholar] [CrossRef]
- Małecka, M.A.; Kępiński, L.; Miśta, W. Structure evolution of nanocrystalline CeO2 and CeLnOx mixed oxides (Ln = Pr, Tb, Lu) in O2 and H2 atmosphere and their catalytic activity in soot combustion. Appl. Catal. B 2007, 74, 290–298. [Google Scholar] [CrossRef]
- Cheng, L.; Men, Y.; Wang, J.; Wang, H.; An, W.; Wang, Y.; Duan, Z.; Liu, J. Crystal facet-dependent reactivity of α-Mn2O3 microcrystalline catalyst for soot combustion. Appl. Catal. B 2017, 204, 374–384. [Google Scholar] [CrossRef]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous Heterogeneous Catalytic Reactions over Mn-Based Oxides for Environmental Applications: A Critical Review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, L.; Ye, F.; Diao, G.; Yu, Q.; Hao, Z.; Zheng, Y.; Yuan, L. Transition metal doped cryptomelane-type manganese oxide for low-temperature catalytic combustion of dimethyl ether. Chem. Eng. J. 2013, 220, 320–327. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Sun, H.; Qiu, J.; Men, Y. Highly improved soot combustion performance over synergetic MnxCe1−xO2 solid solutions within mesoporous nanosheets. J. Colloid Interface Sci. 2020, 577, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, D.; Peng, C.; Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Research progress on preparation of 3DOM-based oxide catalysts and their catalytic performances for the combustion of diesel soot particles. Appl. Catal. B 2022, 319, 121946. [Google Scholar] [CrossRef]
- Zhang, W.; Niu, X.; Chen, L.; Yuan, F.; Zhu, Y. Soot Combustion over Nanostructured Ceria with Different Morphologies. Sci. Rep. 2016, 6, 29062. [Google Scholar] [CrossRef]
- Yang, Q.; Gu, F.; Tang, Y.; Zhang, H.; Liu, Q.; Zhong, Z.; Su, F. A Co3O4–CeO2 functionalized SBA-15 monolith with a three-dimensional framework improves NOx-assisted soot combustion. RSC Adv. 2015, 5, 26815–26822. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Liu, J.; Jiang, G.; Duan, A.; Zheng, J.; Chen, S.; Zhou, R. Three dimensionally ordered macroporous Ce1−xZrxO2 solid solutions for diesel soot combustion. ChemComm 2010, 46, 457–459. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Xu, J.; Zheng, J.; Liu, J.; Jiang, G.; Duan, A.; He, H. Comparative study on the preparation, characterization and catalytic performances of 3DOM Ce-based materials for the combustion of diesel soot. Appl. Catal. B 2011, 107, 302–315. [Google Scholar] [CrossRef]
- Su, Z.; Yang, W.; Wang, C.; Xiong, S.; Cao, X.; Peng, Y.; Si, W.; Weng, Y.; Xue, M.; Li, J. Roles of Oxygen Vacancies in the Bulk and Surface of CeO2 for Toluene Catalytic Combustion. Environ. Sci. Technol. 2020, 54, 12684–12692. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Zhu, Z.; Zhao, P.; Wang, L.; Wan, H.; Guan, G. Facile fabrication of trepang-like CeO2@MnO2 nanocomposite with high catalytic activity for soot removal. Appl. Surf. Sci. 2020, 515, 146013. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Yu, X.; Wang, L.; Chen, M.; Fan, X.; Zhao, Z.; Cheng, K.; Chen, Y.; Sojka, Z.; Wei, Y.; Liu, J. Enhanced activity and sulfur resistance for soot combustion on three-dimensionally ordered macroporous-mesoporous MnxCe1-xOδ/SiO2 catalysts. Appl. Catal. B 2019, 254, 246–259. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yu, D.; Ren, Y.; Yu, X.; Fan, X.; Wang, L.; Wang, R.; Zhao, Z.; Cheng, K.; Chen, Y.; Sojka, Z.; et al. Facile synthesis of birnessite-type K2Mn4O8 and cryptomelane-type K2-xMn8O16 catalysts and their excellent catalytic performance for soot combustion with high resistance to H2O and SO2. Appl. Catal. B 2021, 285, 119779. [Google Scholar] [CrossRef]
- Chen, D.; He, D.; Lu, J.; Zhong, L.; Liu, F.; Liu, J.; Yu, J.; Wan, G.; He, S.; Luo, Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B 2017, 218, 249–259. [Google Scholar] [CrossRef]
- Yu, D.; Wang, L.; Zhang, C.; Peng, C.; Yu, X.; Fan, X.; Liu, B.; Li, K.; Li, Z.; Wei, Y.; et al. Alkali Metals and Cerium-Modified La–Co-Based Perovskite Catalysts: Facile Synthesis, Excellent Catalytic Performance, and Reaction Mechanisms for Soot Combustion. ACS Catal. 2022, 12, 15056–15075. [Google Scholar] [CrossRef]
- Yu, X.; Ren, Y.; Yu, D.; Chen, M.; Wang, L.; Wang, R.; Fan, X.; Zhao, Z.; Cheng, K.; Chen, Y.; et al. Hierarchical Porous K-OMS-2/3DOM-m Ti0.7Si0.3O2 Catalysts for Soot Combustion: Easy Preparation, High Catalytic Activity, and Good Resistance to H2O and SO2. ACS Catal. 2021, 11, 5554–5571. [Google Scholar] [CrossRef]
- Cui, B.; Zhou, L.; Li, K.; Liu, Y.-Q.; Wang, D.; Ye, Y.; Li, S. Holey Co-Ce oxide nanosheets as a highly efficient catalyst for diesel soot combustion. Appl. Catal. B 2020, 267, 118670. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Q.; Kong, M.; Shi, W.; Huang, J.; Tang, J.; Zhao, X. Coupling Oxygen Ion Conduction to Photocatalysis in Mesoporous Nanorod-like Ceria Significantly Improves Photocatalytic Efficiency. J. Phys. Chem. C 2011, 115, 14050–14057. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Deng, J.; Dai, H.; He, H. Controlled Synthesis, Characterization, and Morphology-Dependent Reducibility of Ceria−Zirconia−Yttria Solid Solutions with Nanorod-like, Microspherical, Microbowknot-like, and Micro-octahedral Shapes. Inorg. Chem. 2009, 48, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Zhang, D.; Shi, L.; He, X.; Li, H.; Mai, H.; Yan, T. Facile synthesis, characterization and low-temperature catalytic performance of Au/CeO2 nanorods. Mater. Lett. 2009, 63, 2132–2135. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Demeter, M.; Bartkowski, S.; Neumann, M.; Ovechkina, N.A.; Kurmaev, E.Z.; Lobachevskaya, N.I.; Mukovskii, Y.M.; Mitchell, J.; Ederer, D.L. Mn 3s exchange splitting in mixed-valence manganites. Phys. Rev. B 2002, 65, 113102. [Google Scholar] [CrossRef]
- Yu, D.; Yu, X.; Zhang, C.; Wang, L.; Fan, X.; Zhao, Z.; Wei, Y.; Liu, J.; Gryboś, J.; Leszczyński, B.; et al. Layered Na2Mn3O7 decorated by Cerium as the robust catalysts for efficient low temperature soot combustion. Appl. Catal. B 2023, 338, 123022. [Google Scholar] [CrossRef]
- Hou, J.; Li, Y.; Mao, M.; Ren, L.; Zhao, X. Tremendous Effect of the Morphology of Birnessite-Type Manganese Oxide Nanostructures on Catalytic Activity. ACS Appl. Mater. Interfaces 2014, 6, 14981–14987. [Google Scholar] [CrossRef]
- Chen, H.; Sayari, A.; Adnot, A.; Larachi, F.ç. Composition–activity effects of Mn–Ce–O composites on phenol catalytic wet oxidation. Appl. Catal. B 2001, 32, 195–204. [Google Scholar] [CrossRef]
- Cao, C.; Xing, L.; Yang, Y.; Tian, Y.; Ding, T.; Zhang, J.; Hu, T.; Zheng, L.; Li, X. Diesel soot elimination over potassium-promoted Co3O4 nanowires monolithic catalysts under gravitation contact mode. Appl. Catal. B 2017, 218, 32–45. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, D.; Gao, Z.; Tian, Y.; Ding, T.; Zhang, J.; Jiang, Z.; Li, X. Interface interaction induced oxygen activation of cactus-like Co3O4/OMS-2 nanorod catalysts in situ grown on monolithic cordierite for diesel soot combustion. Appl. Catal. B 2021, 286, 119932. [Google Scholar] [CrossRef]
- Weiman, L.; Haidi, L.; Yunfa, C. Mesoporous MnOx–CeO2 composites for NH3-SCR: The effect of preparation methods and a third dopant. RSC Adv. 2019, 9, 11912–11921. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, M.; Han, L.; Hou, Y.; Bao, W.; Zhang, C.; Feng, G.; Chang, L.; Huang, Z.; Wang, J. Improved Activity and SO2 Resistance by Sm-Modulated Redox of MnCeSmTiOx Mesoporous Amorphous Oxides for Low-Temperature NH3-SCR of NO. ACS Catal. 2020, 10, 9034–9045. [Google Scholar] [CrossRef]
- Wang, L.; Ren, Y.; Yu, X.; Yu, D.; Peng, C.; Zhou, Q.; Hou, J.; Zhong, C.; Yin, C.; Fan, X.; et al. Facile preparation, catalytic performance and reaction mechanism of MnxCo1−xOδ/3DOM-m Ti0.7Si0.2W0.1Oy catalysts for the simultaneous removal of soot and NOx. Catal. Sci. Technol. 2022, 12, 1950–1967. [Google Scholar] [CrossRef]
- Sellers-Antón, B.; Bailón-García, E.; Cardenas-Arenas, A.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A. Enhancement of the Generation and Transfer of Active Oxygen in Ni/CeO2 Catalysts for Soot Combustion by Controlling the Ni–Ceria Contact and the Three-Dimensional Structure. Environ. Sci. Technol. 2020, 54, 2439–2447. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Yang, Y.; Ren, W.; Xing, L.; Zhao, D.; Tian, Y.; Ding, T.; Li, X. Kinetic analysis of morphologies and crystal planes of nanostructured CeO2 catalysts on soot oxidation. Chem. Eng. Sci. 2020, 226, 115891. [Google Scholar] [CrossRef]
- Zhai, G.; Wang, J.; Chen, Z.; Yang, S.; Men, Y. Highly enhanced soot oxidation activity over 3DOM Co3O4-CeO2 catalysts by synergistic promoting effect. J. Hazard. Mater. 2019, 363, 214–226. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx–CeO2–Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef]
- Yu, X.; Li, J.; Wei, Y.; Zhao, Z.; Liu, J.; Jin, B.; Duan, A.; Jiang, G. Three-Dimensionally Ordered Macroporous MnxCe1–xOδ and Pt/Mn0.5Ce0.5Oδ Catalysts: Synthesis and Catalytic Performance for Soot Oxidation. Ind. Eng. Chem. Res. 2014, 53, 9653–9664. [Google Scholar] [CrossRef]

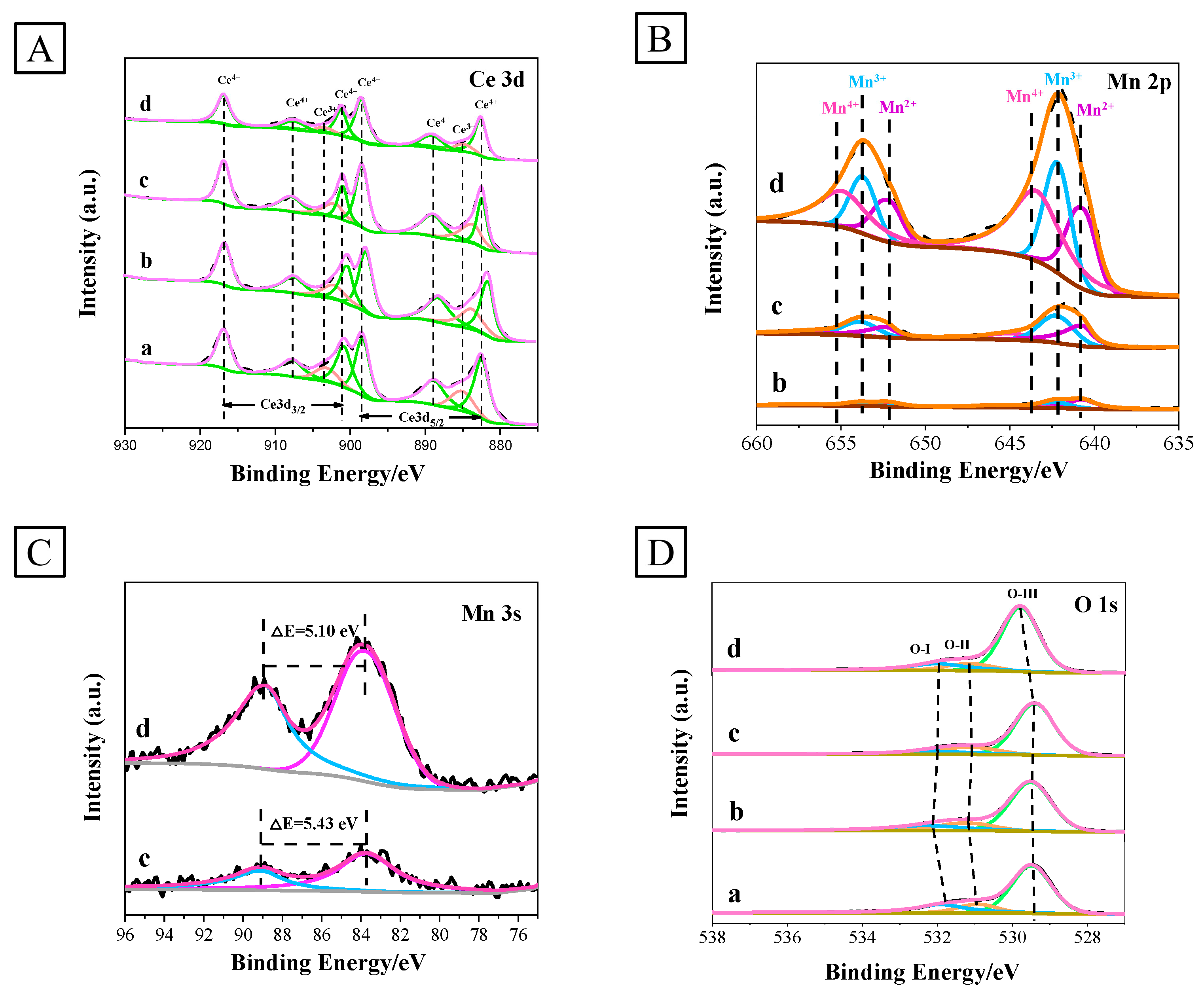
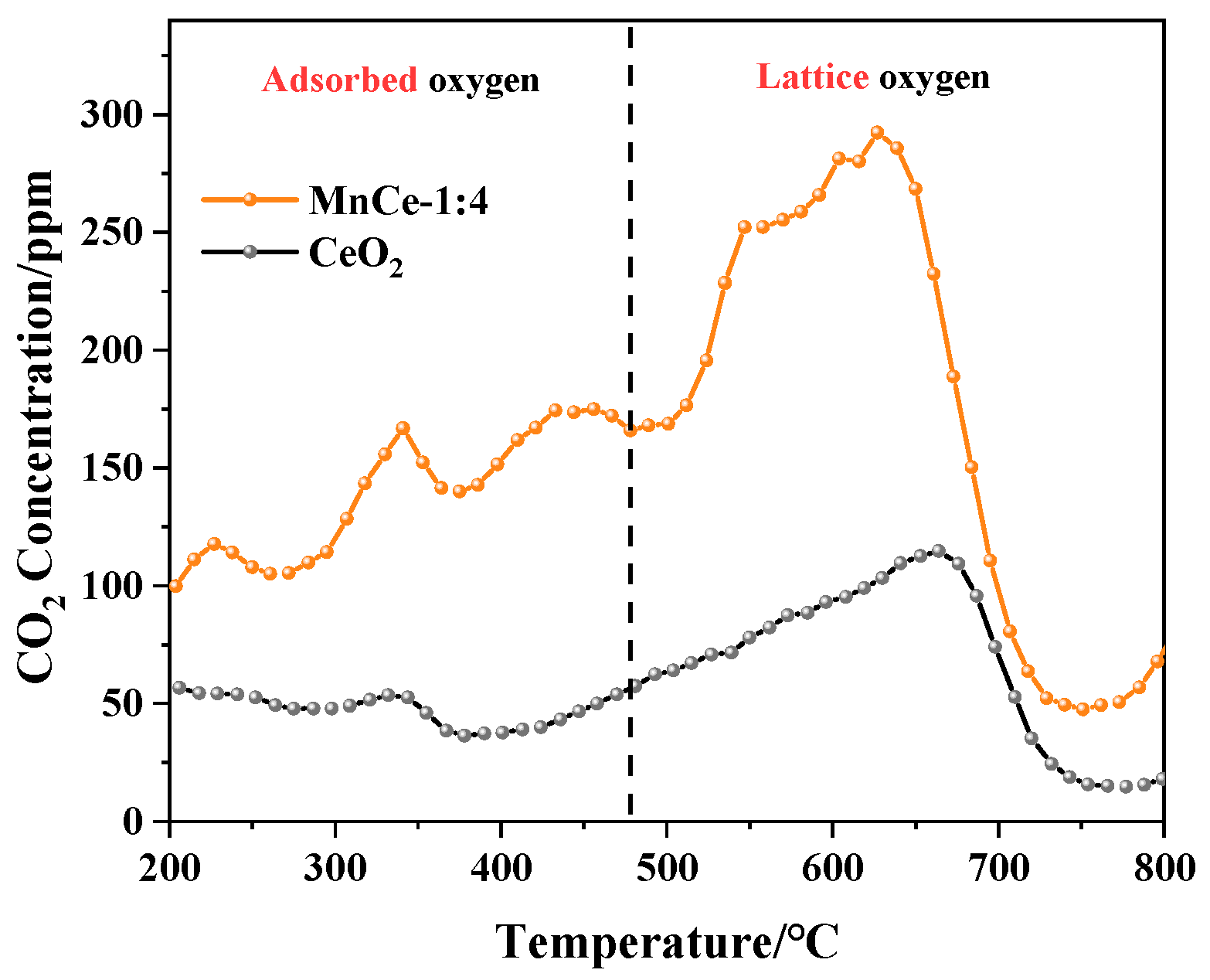
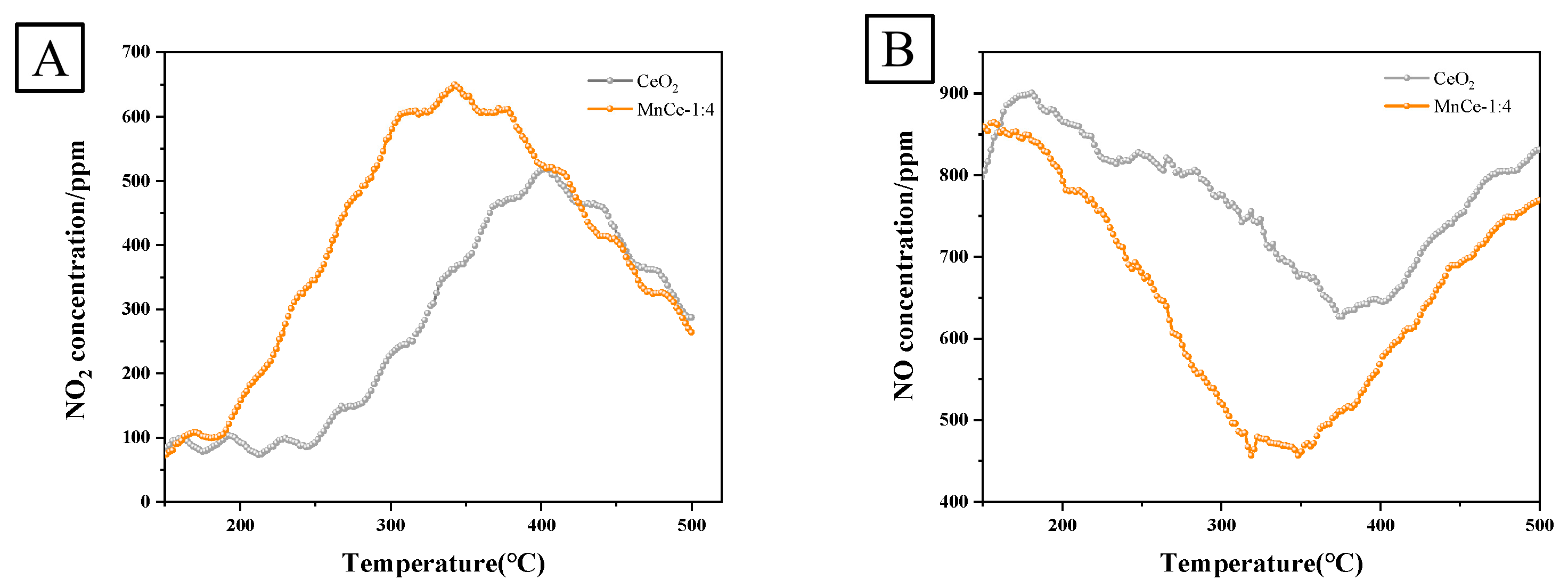
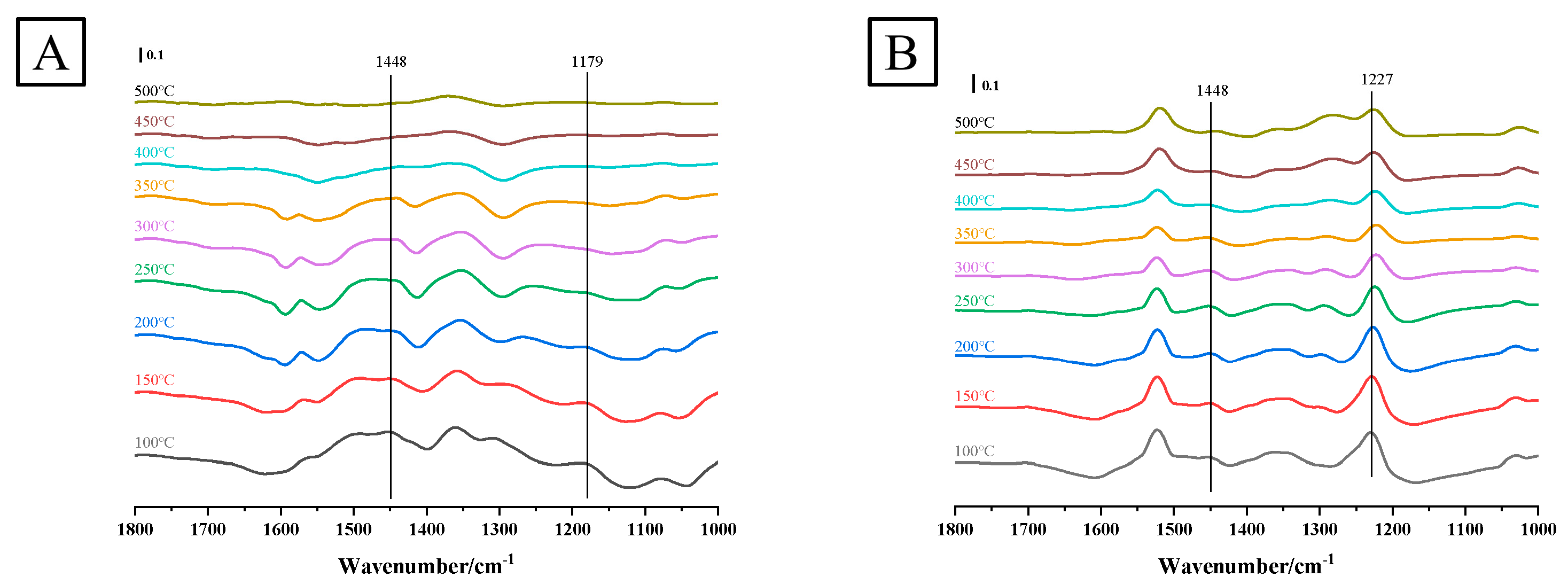
| Catalysts | Ce | Mn |
|---|---|---|
| MnCe-1:8 | 76.0% | 3.8% |
| MnCe-1:4 | 72.1% | 6.8% |
| MnCe-1:1 | 53.5% | 21.4% |
| Catalysts | Surface Area (m2/g) a | Total Pore Volume (cm3/g) b | Pore Size (nm) c |
|---|---|---|---|
| CeO2 | 110.9 | 0.119 | 5.9 |
| MnCe-1:16 | 95.5 | 0.083 | 4.7 |
| MnCe-1:8 | 70.7 | 0.103 | 5.6 |
| MnCe-1:4 | 38.4 | 0.070 | 6.9 |
| MnCe-1:2 | 46.9 | 0.074 | 5.9 |
| MnCe-1:1 | 43.2 | 0.067 | 7.2 |
| Sample | Molar Fraction | |||||||
|---|---|---|---|---|---|---|---|---|
| Manganese | Cerium | Oxygen | ||||||
| Mn4+ | Mn3+ | Mn2+ | Ce4+ | Ce3+ | Olatt | Oads | Osur | |
| CeO2 | 85.11% | 14.89% | 68.16% | 11.71% | 20.13% | |||
| MnCe-1:16 | 17.34% | 26.21% | 56.45% | 81.94% | 18.06% | 69.94% | 14.15% | 15.91% |
| MnCe-1:4 | 16.78% | 47.04% | 36.18% | 81.41% | 18.59% | 74.04% | 14.70% | 11.26% |
| MnCe-1:1 | 43.95% | 30.56% | 25.48% | 92.19% | 7.81% | 74.07% | 9.97% | 15.96% |
| Catalysts | T10/°C | T50/°C | T90/°C | SCO2m/% |
|---|---|---|---|---|
| Soot | 461 | 552 | 594 | 38.5% |
| CeO2 | 318 | 363 | 402 | 96.5% |
| Mn2O3 | 294 | 357 | 395 | 99.6% |
| Mn2O3 + CeO2 (mechanical mixing) | 294 | 350 | 384 | 99.5% |
| MnCe-1:16 | 294 | 350 | 384 | 99.5% |
| MnCe-1:8 | 292 | 344 | 375 | 99.3% |
| MnCe-1:4 | 289 | 340 | 373 | 99.3% |
| MnCe-1:4 (under tight contact mode) | 278 | 327 | 353 | 99.6% |
| MnCe-1:2 | 286 | 347 | 382 | 99.5% |
| MnCe-1:1 | 286 | 346 | 383 | 99.5% |
| Catalysts a | T10/°C | δ b | T50/°C | δ | T90/°C | δ | SCO2m/% | δ |
|---|---|---|---|---|---|---|---|---|
| MnCe-1:4-1 | 291 | 0.69% | 340 | 0% | 379 | 1.6% | 99.5% | 0.20% |
| MnCe-1:4-2 | 292 | 1.04% | 342 | 0.59% | 374 | 0.27% | 99.6% | 0.30% |
| MnCe-1:4-3 | 290 | 0.35% | 337 | 0.88% | 373 | 0% | 99.6% | 0.30% |
| Catalysts | Reaction Conditions | Soot/ Catalysts | T10/Ti (°C) | T50/Tm (°C) | T90/Tf (°C) | References |
|---|---|---|---|---|---|---|
| CeO2@MnO2 | 500 ppm NO + 5% O2 + N2 balance | 1:9 | 312 | 373 | 423 | [32] |
| (NiO-CeO2)-3DOM | 500 ppm NO + 5% O2 + N2 balance | 1:4 | 530 | [54] | ||
| CeO2 nanocube | 10 vol.% O2 + N2 balance | 1:10 | 459 | 554 | [55] | |
| 3DOM Co3O4-CeO2 | 0.25% NO + 5% O2 + N2 balance | 1:10 | 406 | [56] | ||
| MnOx-CeO2-Al2O3 | 1000 ppm NO + 10% O2 + N2 balance | 1:10 | 455 | [57] | ||
| 3DOM Mn0.5Ce0.5Oδ | 0.2% NO + 10% O2 + Ar balance | 1:10 | 297 | 358 | 396 | [58] |
| MnCe-1:4 | 2000 ppm NO + 10% O2 + Ar balance | 1:10 | 289 | 340 | 373 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Yu, D.; Zhou, S.; Zhang, C.; Wang, L.; Fan, X.; Yu, X.; Zhao, Z. The Preparation of Mn-Modified CeO2 Nanosphere Catalysts and Their Catalytic Performance for Soot Combustion. Processes 2023, 11, 2902. https://doi.org/10.3390/pr11102902
Gao S, Yu D, Zhou S, Zhang C, Wang L, Fan X, Yu X, Zhao Z. The Preparation of Mn-Modified CeO2 Nanosphere Catalysts and Their Catalytic Performance for Soot Combustion. Processes. 2023; 11(10):2902. https://doi.org/10.3390/pr11102902
Chicago/Turabian StyleGao, Siyu, Di Yu, Shengran Zhou, Chunlei Zhang, Lanyi Wang, Xiaoqiang Fan, Xuehua Yu, and Zhen Zhao. 2023. "The Preparation of Mn-Modified CeO2 Nanosphere Catalysts and Their Catalytic Performance for Soot Combustion" Processes 11, no. 10: 2902. https://doi.org/10.3390/pr11102902






