Abstract
Yucatan, Mexico, is renowned for its rich plant diversity, with ~40% melliferous plants. Yucatan bee honey (BH) constitutes ~15.83% of Mexico’s annual BH production, giving high international value. Major melliferous families in Yucatan include Fabaceae, with Piscidia piscipula (“Jabin”) as an example, and Polygonaceae, with Gymnopodium floribundum (“Dzidzilche”), crucial for BH production. This study aimed to profile the metabolome of Jabin and Dzidzilche flowers and their associated BH to identify metabolites for each flower coming from two regions (Tahdziu and Acanceh) of Yucatán. Liquid chromatography–tandem mass spectrometry (LC–MS2), total polyphenol content (TPC), and antioxidant capacity (AC) were implemented. As many as 101 metabolites (69 in flowers, 55 in BH) were tentatively identified using spectral libraries and in silico predictions, predominantly flavonoids, which accounted for 50.7% of the total identified metabolites in flower and 16.4% in BH. Samples exhibited variations in TPC, AC, secondary metabolites, and chemical classes depending on geography and botanical origin. Dzidzilche flowers from Acanceh displayed the highest total polyphenol content (TPC, 1431.24 ± 15.38 mg GAE/100 g dry matter) and antioxidant capacity (AC, 93.63% inhibition). Among the metabolites detected in flowers (Piscidia piscipula, Gymnopodium floribundum), 50.7% were found to be part of the flavonoid chemical class, whereas in their respective honey samples, only 16.4% of the identified metabolites were categorized as flavonoids. Vanillin and vitexin were tentatively identified as potential markers for the botanical origin identification of honey from Piscidia piscipula and Gymnopodium floribundum, respectively. Recognizing botanical and geographic BH origin is important for product authentication, identification, and traceability. This study offers chemical insights that can be valuable and complementary to melissopalynology, aiding in determining the origin and quality of Yucatan BH.
1. Introduction
The Yucatán Peninsula in México has a diverse and abundant flora, with approximately 2329 reported taxa at the species level grouped in 956 genera and 161 native or wild families. In the case of Yucatán, there is a record of 1402 reported plant species, distributed in 120 families and 652 genera, of which 11 species are endemic. As part of the extensive range of documented plant species, the classification of melliferous flora has critical importance because it constitutes 40% of the reported species in Yucatan, representing about 849 species that serve as sources of nectar and pollen for bees. Bees, in turn, transform these resources into various products, like propolis, wax, and, primarily, honey [1]. The production of honey is one of the most important activities in Yucatan due to the abundance of a large quantity of plants (mentioned earlier). The honey production in Yucatan contributes 15.83% of Mexico’s national production (9180 tons per year) [2]. The quality of Yucatecan BH is highly preferred in the international market; a total of 95% of the BH produced in Yucatán is destined for the markets of Germany, Switzerland, and England [3]. The quality of the BH is determined by the available floral composition for the foraging of Apis mellifera; in this sense, it is essential for apiaries to be located in areas with a high availability of nectar and pollen plant supply. It is reported that bees can fly between 2 and 5 km searching for floral resources [4].
According to melissopalynology studies characterizing honey in relation to pollen, it has been determined that certain taxonomic families exhibit a predominant presence in BH. Notably, among these families are Fabaceae and Polygonaceae [5]. Additionally, the presence of polyphenols in Yucatán’s plants has been extensively studied. The scientific literature highlights the abundant presence of polyphenols in Yucatán’s flora, showcasing significant antioxidant properties [6,7]. Alongside their antioxidant attributes, these plants yield a diverse range of secondary metabolites, carrying medicinal and therapeutic potential that has been harnessed by local cultures since ancient times. The metabolites present in the BH flora of Yucatan include flavonoids, terpenes, phenolic acids, and alkaloids, among others. This results in plants containing antioxidants, anti-inflammatory, antimicrobial, and analgesic characteristics. These plants have a history of use in traditional medicine for addressing a range of issues, including headaches, inflammation, infections, and gastrointestinal disorders. [8,9]. A study on the floristic knowledge of the Yucatan Peninsula reported that Fabaceae, commonly known as legumes, is one of the most diverse families in the region, with 230 species reported [10]. In these species, flavonoids, isoflavonoids, and quinones are reported as the prominent classes of secondary metabolites [11]. An important tree of Fabaceae species is the Piscidia piscipula, commonly known as “Jabin”, a well-distributed tree within the Yucatán Peninsula, Mexico, that can reach up to 20 m in height, and its flowering occurs during the dry months of March and April. The inflorescences are generated on the branches, where up to 50 flowers develop. These flowers represent a food source for bees and many other insects [12] and play a significant ecological and socio-cultural role due to their use as a firewood (fuel) source or as poles, and it is considered by locals as a “godmother” plant or “mother of fire” [13]. The Piscidia piscipula honey has been classified as a potential source of monofloral honey [14] and contains several types of polyphenols, including flavonoids, such as genistein and quercetin, and phenolic acids, such as caffeic acid and chlorogenic acid; these metabolites have also been found in Jabin bark, with significant antioxidant anti-inflammatory properties [15,16]. Another plant family reported to be presented in Yucatecan BH is Polygonaceae, a diverse group of plants that include herbs, shrubs, trees, and vines; this family has alternate leaves, small flowers, and inflorescences with cymes form. A phytochemical study revealed the presence of tannins, saponins, flavonoids, alkaloids, and sesquiterpenes [17]. Gymnopodium floribundum, a prominent member of the Polygonaceae family in Yucatan, holds significant importance. Referred to as ‘Dzidzilche’ in the Mayan language, this species plays a vital role within the Yucatecan region, as it is utilized in commercial honey production [18] and traditional medicine for its anti-inflammatory and analgesic properties due to the high content of polyphenols in its leaves [19,20] as well as its tannins [21].
Speaking exclusively about flowers, these are a source of bioactive compounds of interest [22]; an example is the melliferous flora of Yucatán, which is important not only for honey production but also for its medicinal and therapeutic properties due to terpenes, polyphenols, and alkaloids, three metabolites present in these plants.
The polyphenol group is the most widely distributed and diverse category in plants from all around the world [23]. Polyphenols contribute to taste, color, odor, astringency, oxidative stability, bitterness, and chemical defense; they are synthesized by the phenylpropanoid and/or polyketide metabolic pathways derived from shikimic acid, and phenylalanine is the main compound needed for the synthesis of other polyphenols, such as flavonoids, phenolic acids, stilbenes, and lignans [15]. All the reported metabolites in Gymnopodium floribundum and Piscidia piscipula species from Yucatan are the result of biotic and abiotic stress in the region, mainly environmental conditions, such as climate and humidity. In this sense, the total content of polyphenols in flowers varies according to factors such as species, plant age, geographic origin, and environmental conditions [15,24].
A behavior similar to that in flower metabolites is observed in the chemical composition of honey, which is highly variable and mainly depends on the geographical and floral origin. Derived from this, honey is classified according to two characteristics: the region of origin and the flora of that region [25,26]. In a study conducted by Peilin et al. [27], the effects of different floral origins of honey (Schisandra chinensis, Vicia dichroant, Astragalus sinicus, Robinia pseudoacacia, and Ziziphus jujuba) on alcohol metabolism and their correlation with chemical composition were found to have significant differences in the polyphenol profile, especially in protocatechuic acid, p-Hydroxybenzoic acid, kaempferol, apigenin, and caffeic acid. Generally, it is recognized that evaluating the patterns of phenolic acids, phenolic esters, and aromatic carbonyl compounds can provide a good indication of the botanical origin of honey [28].
Some phenolic acids (e.g., ellagic acid) are used as floral markers of brezo honey (Calluna vulgaris), while the hydroxycinnamates (caffeic, p-coumaric, and ferulic acids) are used as floral markers of castaño honey (Castanea sativa) [29]. On the other hand, pinocembrin, pinobanksin, and chrysin are characteristic flavonoids found in most European honey [30], while abscisic acid was detected in rapeseed (Brassica napus), lime (Citrus sp.), and acacia (Acacia sp.) tropical honey [31].
Hence, the importance and interest in understanding the effect of geographical origin and different species of nectar-producing flowers are evident. Derived products, such as honey produced by Apis mellifera (hereinafter honeybee), have the potential to contain both the metabolites and bioactive properties that can be advantageous for human health. In this study, our objective was to investigate the impact of geographical location and flower species on metabolites identified using LC–MS2. This approach represents a novel and innovative process seeking to establish potential correlations between these factors and the honey produced by Apis mellifera from these local (Yucatán) floral species.
2. Materials and Methods
2.1. Collection of Plant Material
The collection of Dzidzilche (Gymnopodium floribundum) and Jabin (Piscidia piscipula) flowers was carried out in February, March, and April 2023 in two different apiaries in two locations in Yucatán, Mexico: Tahdziu and Acanceh. The selected sampling locations for plant material were near the apiaries (500 m approximately).
Flower collection consisted of taking a fragment of the complete terminal branch where flowering was present. Subsequently, the flowers were stored in resealable plastic bags in a Styrofoam container with ice for transportation (<4 h) to the Research and Technology Assistance Center of the State of Jalisco (CIATEJ), southeast unit.
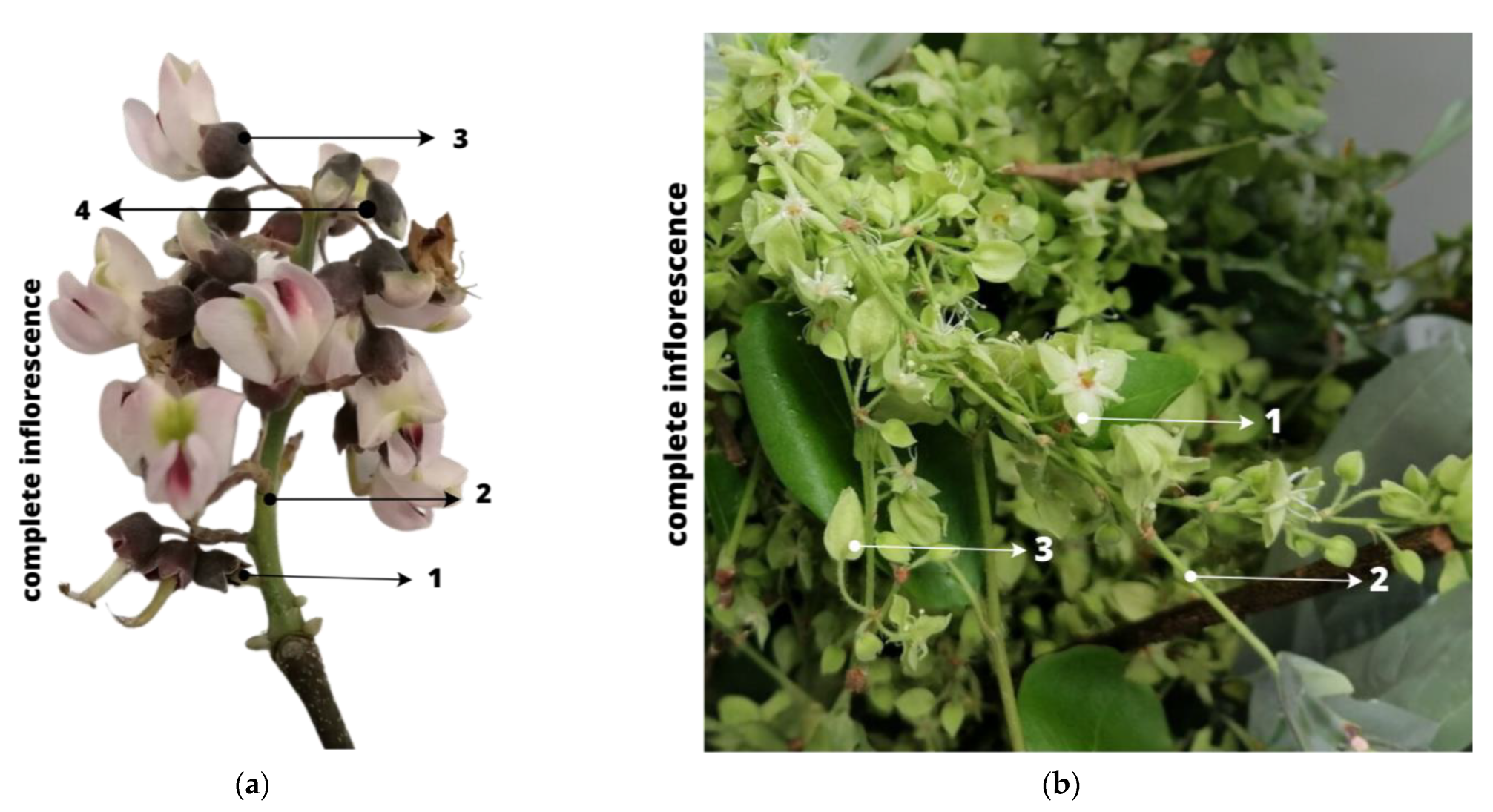
The collected samples consisted of complete inflorescences of each species; however, only floral structures were exclusively utilized for the analyses. For the Jabin species (Figure 1a), the open flowers with the pedicel (3) and closed flowers with the pedicel (4) were employed, while the rachis (2) and developing fruits (1) were excluded. In the case of the Dzidzilche species (Figure 1b), we utilized the open flowers with the pedicel (1) and closed flowers with the pedicel (3); the rachis (2) was discarded.

Figure 1.
(a) Floral structures of Piscidia piscipula (Jabin). (b) Floral structures of Gymnopodium floribundum (Dzidzilche). Figure (a) numeration: 1 = developing fruit; 2 = rachis; 3 = open flowers with pedicel; 4 = closed flowers with pedicel. Figure (b) numeration: 1 = open flower with pedicel; 2 = rachis; 3 = closed flower with pedicel.
2.2. Selection and Drying of Flowers
The selection of flower structures took place at the CIATEJ facilities for subsequent freeze-drying. Freeze-drying was performed according to Oney-Montalvo et al. [32] with some modifications. Before freeze-drying, the selected flowers were ultra-frozen (−32 °C, 24 h) in resealable plastic bags. Then, the frozen flowers were freeze-dried for 72 h at a temperature of −50 °C and a pressure of 240 mBar using the Freezone lyophilizer, LABCONCO® Brand, C to obtain a moisture content of ≤5%.
The Jabin flower sample obtained from the location of Tahdziu was used as a conventional dry control. It was dried according to Chel-Guerrero et al. [33], with some modifications, in a FELISA oven (model FE-292) at 50 °C for 48 h to obtain a moisture content of ≤5%.
Grinding and Sieving
Freeze-dried and oven-dried flowers were first ground using a coffee grinder (MasterChef®) until homogeneous powders (flower flours) were obtained. The flower flours were sieved using a No. 35 mesh (500 µm particle size). The final product was stored in resealable bags, labeled, and lined with aluminum foil at room temperature until use.
2.3. Determination of Moisture in Flowers and Flower Flours
The fresh flowers selected were subjected to moisture determination prior to the drying process (freeze-drying or oven-drying) according to the methodology reported by Avilés-Betanzos et al., 2023 with some modifications [34]. With an OHAUS® thermobalance (model MB90, NJ, USA), the moisture content of the samples was determined by placing 0.5 g of sample in an aluminum plate; the samples were kept in the equipment at a constant temperature of 105 °C until a constant weight was reached (weight loss < 1 mg in 60 s). The same procedure was followed with both the fresh flower and the flower flours. Moisture was the difference between the initial and final weights of the sample and was reported in percentage and in triplicate.
2.4. Extraction of Metabolites in Flower Flours
2.4.1. Obtainment of Total Polyphenol Content (TPC) and Antioxidant Capacity (AC)
The extraction process was carried out according to the methodology reported by Avilés-Betanzos et al. [34] with some modifications. A sample of flower flour was weighed (0.5 g); then, 2.5 mL of a methanol: water solution (80:20) was added. The solution was mixed with a vortex until a homogeneous solution was obtained. The solution was sonicated for 30 min at a frequency of 42 kHz using a BRANSON® sonicator (model 3510). After sonication, the samples were centrifuged at 4700× g rpm for 30 min at 4 °C. The supernatants were recovered and filtered using a 0.22 μm nylon filter. Finally, the sample was placed in chromatographic vials and refrigerated (5 °C) until use (<48 h).
2.4.2. Flower Extraction for LC–MS2
We followed the methodology previously described by Contreras-Angulo et al. [35] with minor modifications. To 5 mg of freeze-dried flowers, 500 µL of a solvent mixture of methanol: acetonitrile (ACN): ethyl acetate (1:1:1) was added, and the mixture was vortexed for 1 min and sonicated for 30 min in ice-cold water. Then, the samples were centrifuged at 14,000× g rpm for 10 min at 4 °C, and 400 µL of the supernatant (top layer) was recovered, transferred to an Eppendorf tube, and dried in a SpeedVac system at room temperature. The dried extract was resuspended with 200 µL of a mixture of water: ACN (80:20), vortexed again for 1 min, and sonicated for 30 min in ice-cold water. Finally, extracts were centrifuged at 14,000 rpm for 10 min at 4 °C to obtain a particle-free supernatant suitable for LC–MS2 data acquisition.
2.5. Extraction of Metabolites in Honey
2.5.1. Honey Dilution for Determination of CTP and AC
The dilution was carried out according to Al-Mamary et al. [36] with some modifications. A sample of honey (1 g) was weighed, and distilled water was added until a total weight of 10 g was reached. The sample was then vortexed to make a homogeneous solution to be used later to assess CTP and AC.
2.5.2. Honey Extraction for LC–MS2 Data
A sample of 100 mg of freeze-dried honey was extracted with 500 µL of methanol: ACN: ethyl acetate (1:1:1) solution under sonication for 30 min in ice-cold water. Thereafter, the same steps described in Section 2.4.2 were followed. However, after the evaporation of solvents, the extracts were resuspended at 500 mg/µL using a mixture of water: ACN (80:20).
Then the same methodology as reported in Section 2.4.2 was subsequently followed.
2.6. Experimental Design
To evaluate the effects of the geographical zone on the total polyphenol content and antioxidant capacity in Dzidzilche and Jabin flowers and honey, a 23 factorial design was established. The levels of the main factors (botanical origin, geographical zone, and raw material) were presented at two levels (−1 for Tahdziu, Jabin, and flower and 1 for Acanceh, Dzidzilche, and honey), as shown in Table 1. The raw material factor (honey and flower) was also individually analyzed considering the factors of botanical origin and geographical origin.

Table 1.
Experimental 23 design employed for the response variables total polyphenol content and antioxidant capacity.
2.6.1. Determination of TPC in Dzidzilche and Jabin Flower Flours and Honey
The polyphenols contained in the extracts were evaluated using the Folin–Ciocalteu method with some modifications, following Singleton et al. [37]. A total of 25 μL of the sample (flower flour extract or honey dilution) was taken and diluted with distilled water (25 μL). Then, 3 mL of distilled water and 250 μL of Folin–Ciocalteu reagent (St. Louis, MO, USA) were added. After 5 min, 750 μL of 20% sodium carbonate (Na2CO3, 20%, St. Louis, MO, USA) and 950 μL of distilled water were added, and the mixture was incubated for 30 min. Finally, the samples were read at 765 nm using a quartz cell and UV–Vis spectrophotometer (THERMO SCIENTIFIC® Genesys 140 (Waltham, MA, USA)) to determine absorbance. A calibration curve (0.005−0.1 mg of gallic acid/mL) was prepared prior to sample analysis (flower and honey), obtaining an R2 = 0.999 for both curves (the graphs can be observed in Figure S1).
2.6.2. Determination of AC in Dzidzilche and Jabin Flower Flours and Honey
The antioxidant capacity of the extracts was evaluated using the DPPH method, according to Brand et al. [38]. The 2,2-diphenyl-1- picrylhydrazyl (DPPH) reagent from Sigma Aldrich® (D9132-1G, St. Louis, MO, USA) was weighted (3.3 mg); then, reagent-grade methanol was added (100 mL). The obtained DPPH solution was adjusted (absorbance 0.700 ± 0.002) with a UV–Vis spectrophotometer (THERMO SCIENTIFIC® Genesys 140). To establish the antioxidant capacity, a sample volume of 100 µL (extract of Dzidzilche and Jabin flower flours and honey) was taken, and then a volume of 3.9 mL of DPPH-adjusted solution was added. The mixed solution was stirred and incubated for 30 min. Finally, the samples were read at 515 nm. The results of the extract were presented as the percentage of inhibition (%), according to Equation (1):
DPPHadj = Absorbance of adjusted DPPH solution.
DPPHsam = Absorbance of sample (extracts of Dzidzilche and Jabin flower flours and honey).
2.7. Untargeted LC–MS2 Data Acquisition
We followed the instrumentation and protocol previously described [35] with some modifications. We loaded 4 μL of the resuspended metabolite solutions into an Agilent 1260 Infinity LC (Agilent Technologies, Inc., Santa Clara, CA, USA). For the separation of molecules, we employed a ProtID-Chip-43 II column (C18, 43 mm × 75 μm, 300 Å, 5 μm particle size, equipped with a 40 nL enrichment column). The mobile phases were H2O with 0.1% formic acid (FA) as solution A and ACN with 0.1% FA as solution B. The gradient started at 5% B, was increased linearly to 40% B in 20 min and then to 100% B for 5 min, was held at 100% B for 5 min, returned to 5% B within 1 min, and was finally maintained at 5% B for 9 min before the next sample to ensure column re-equilibration. The total analysis time was 40 min at a flow rate of 300 nL/min. To minimize potential carryover, two blank samples (5 μL of mobile phases A and B at an 80:20 ratio) were run between experimental samples. The eluate was delivered to a 6530 Accurate-Mass Q-TOF mass spectrometer (Agilent Technologies, Inc., Santa Clara, CA, USA) using an HPLC-Chip Cube MS interface. Nanospray ionization under positive mode was utilized. Data-dependent acquisition was employed over the mass range 110–2000 m/z with a scan speed of 4 spectra/s. MS2 data were retrieved from the top 5 most intense precursor ions per cycle, reaching 150 cps over the mass range 50–2000 m/z at a rate of 3 spectra/s. The active exclusion option was enabled, set to 2 spectra, and disabled after 0.25 min. A ramped collision energy (CE) was used with slope and offset values of 6 and 4, respectively. Before data acquisition and every 24 h, the instrument was externally calibrated using an ESI-L low mix concentration tuning solution (Agilent Technologies, Inc., Santa Clara, CA, USA). The samples were randomly allocated in the autosampler for data acquisition.
LC–MS2 Data Processing
The methodology followed was based on that reported previously by our group [35] with some modifications. The LC–MS2 datasets were analyzed using a workflow that consisted of open-source software packages and free-access online platforms. First, datasets with Agilent, Santa Clara, CA, USA, proprietary format were converted to open-format files (.mzXML) using the MSConvert tool (version 3) from ProteoWizard [39]. The mzXML files were uploaded to the Global Natural Products Social Molecular Networking (GNPS) online platform (https://gnps.ucsd.edu (accessed on 25 august 2023)) [40] to perform classical molecular networking (CMN) and automated structural annotation (against GNPS spectral libraries) (Metabolomics Standards Initiative (MSI) classification level 2 [41]). The principal parameters used within GNPS were 0.02 Da for the mass tolerance of precursor and product ions, a minimum cosine score of 0.6, and at least 4 peaks matched at the MS2 level. To enhance the putative identification of metabolites (MSI, level 3), we utilized MolDiscovery [42] and DEREPLICATOR+ (version 1.0.0) [43] in silico tools integrated into the GNPS platform. The clustered .mgf file generated by CMN was used for MolDiscovery analysis, and the DEREPLICATOR+ analysis was run through the “Annotate with DEREPLICATOR+” option on the results page of CMN. The precursor ion and product ion mass tolerances were set to 0.02 Da, while all other parameters were left as default. As an additional annotation tool, the CSI: FingerID algorithm from the SIRIUS software (version 4.9.12) [44] was used to assign structures found in the BioDatabases (MSI, level 3). First, a molecular formula was assigned with SIRIUS without databases [45] and reranked with the ZODIAC algorithm [46]. The chemical classes of the identified metabolites were assigned on the basis of ClassyFire chemical ontology using the web application http://classyfire.wishartlab.com (accessed on 25 August 2023) [47]. Spectra found in blank samples and annotations with >10 ppm mass accuracy and >5% FDR were filtered out from the results.
2.8. Statistical Analysis
The data presented are reported as means ± standard deviations. Data analysis for a factorial 23 design was employed to assess the total polyphenol content and antioxidant capacity of the factors (1) botanical origin (Dzidzilche or Jabin flowers), (2) geographical origin (Acanceh or Tahdziu locations), and (3) raw material (flower or honey) using an analysis of variance (ANOVA) to determine the effects and their interactions. These analyses were performed using the statistical software Statgraphics Centurion XVII.II-X64 (Stat-graphics Technologies Inc., Virgin, UT, USA). XL-STAT software (version 2021.2.2, Addison, Paris, France) was utilized in the CATA analysis to obtain the principal components analysis of the metabolites obtained in the samples. The analysis of the linear correlation between the concentrations of total polyphenols and the antioxidant capacity obtained by the DPPH was carried out with a linear correlation utilizing Microsoft Excel (version 2019).
3. Results
3.1. Collection of Plant Material
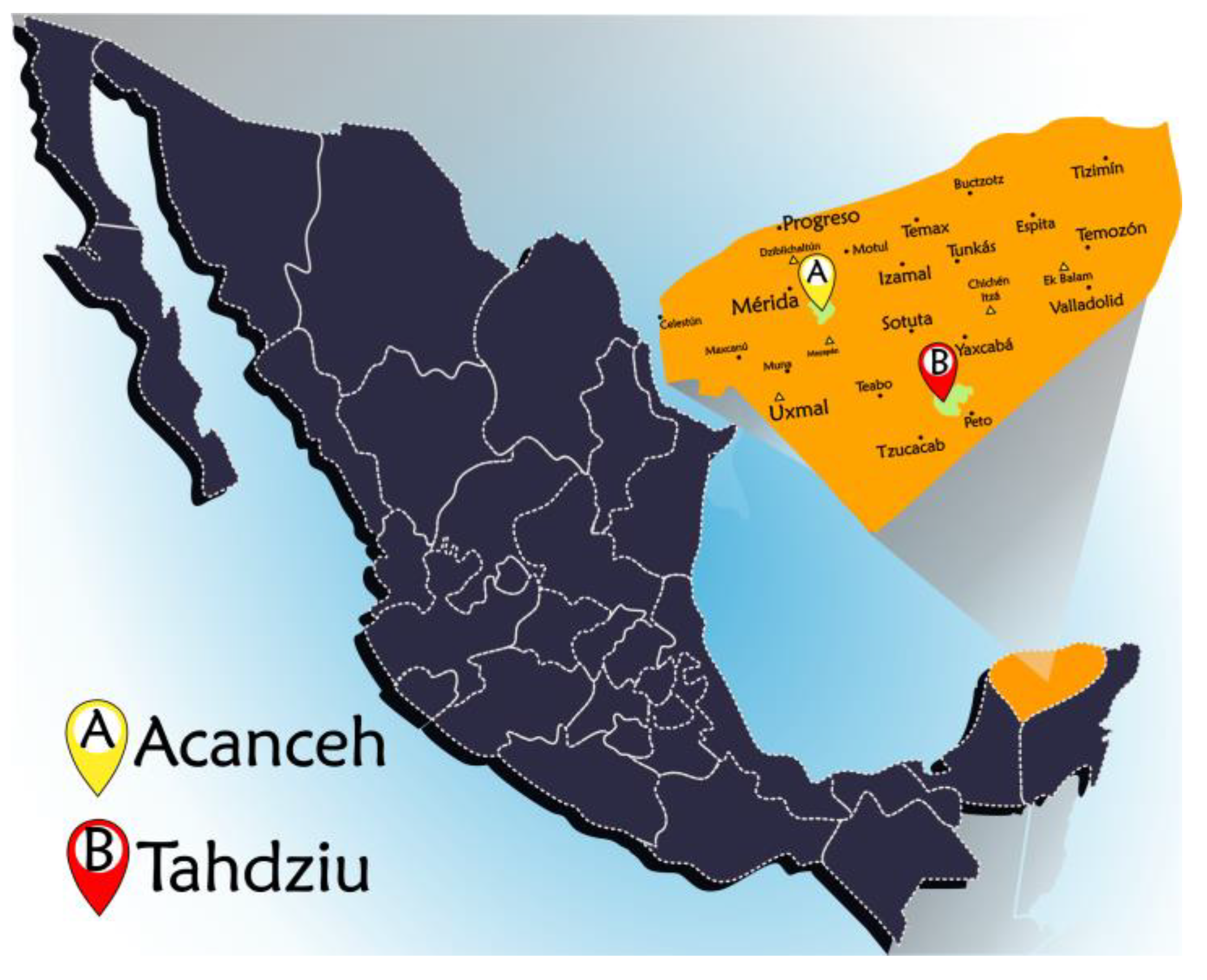
The sampled apiaries (Acanceh and Tahdziu locations) were in two different areas of the state of Yucatan, Mexico, as indicated in Figure 2. The apiary located in Tahdziu (20°19′22.4″ N 089°24′38.6″ W) had scattered vegetation; it was surrounded by hectares with agricultural activity, such as livestock farming, agriculture, and other apiaries. On the contrary, the apiary located in Acanceh (20°45′28.2″ N 089°23′18.5″ W) had dense and closed vegetation, surrounded by unexploited lowland jungle unaffected by commercial activities.
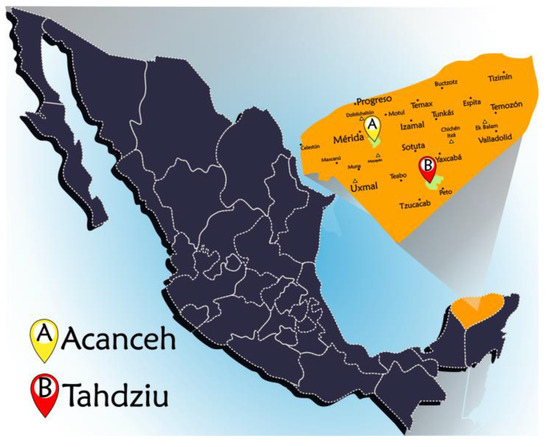
Figure 2.
Map of the state of Yucatan with the locations of the sampled apiaries.
The samples were labeled using codes for identification, which reflected their botanical origin (Jabin or Dzidzilche) and their geographical origin (Tahdziu or Acanceh). At the end of the plant material collection, 365 g of Dzidzilche material and 658 g of Jabin were obtained. The collected sample could contain additional structures other than flowers, principal stems, leaves, and secondary branching; however, these materials were discarded, as described in the following section.
3.2. Selection and Drying of Flowers
An initial weight of 365 g of Dzidzilche material was collected; after finalizing the process of classification and the selection of structures, 125 g remained that corresponded to flower structures. Similarly, Jabin decreased from the initial 658 g to 315 g of plant material corresponding to floral structures. After the drying and grinding process, 47 g and 39 g of floral flour were obtained from the Jabin and Dzidzilche species, respectively.
3.3. Moisture Determination in Flowers and Flower Flours
The Jabin species demonstrated a higher initial moisture percentage than the Dzidzilche species in both the Tahdziu (80.25 ± 0.19%) and Acanceh (72.26 ± 0.62%) apiaries. On the other hand, the Dzidzilche species showed the lowest initial moisture percentage in the Tahdziu locality (56.53 ± 1.09). Statistically significant differences (p < 0.0001) were found in the initial moisture between the species, resulting in three homogeneous groups: ACA-DZI and TAH-DZI, ACA-JA, and TAH-JA. Regarding the final moisture, a significant difference was also obtained (p = 0.0082), as shown in Table 2.

Table 2.
Moisture percentages in Jabin and Dzidzilche flowers from Tahdziu and Acanceh apiaries.
3.4. TPC and AC of Flower Flours and Honeys
The results of the total polyphenol content (TPC) and the antioxidant capacity (AC, with DPPH methodology) as response variables of the 23 factorial designs experiments are presented in Table 3. The data were obtained from two geographical locations (Tahdziu and Acanceh) and two different species (Jabin and Dzidzilche) using honey and flowers as raw materials.

Table 3.
Results of total polyphenol content and antioxidant capacity for the experimental design (23).
The results showed that the highest concentration of total polyphenols (1431.24 ± 15.38 mg GAE/100 g dry matter) was obtained in the flower with the botanical origin of Dzidzilche and the geographical origin of Acanceh. In general, flowers exhibited higher values of total polyphenol content than the BH.
On the other hand, the BH contained a low concentration of polyphenols, in which the lowest TPC (5.28 ± 0.47 mg GAE/100 g dry matter) was obtained in the Dzidzilche sample from Acanceh.
Meanwhile, flowers developed a higher antioxidant capacity than the BH. Flowers with the botanical origin of Dzidzilche and the geographical origin of Acanceh exhibited the highest antioxidant capacity (93.63% inhibition) (p < 0.05). By contrast, the lowest antioxidant capacity was observed in the BH with Jabin botanical origin and Tahdziu geographical origin (13.68% inhibition).
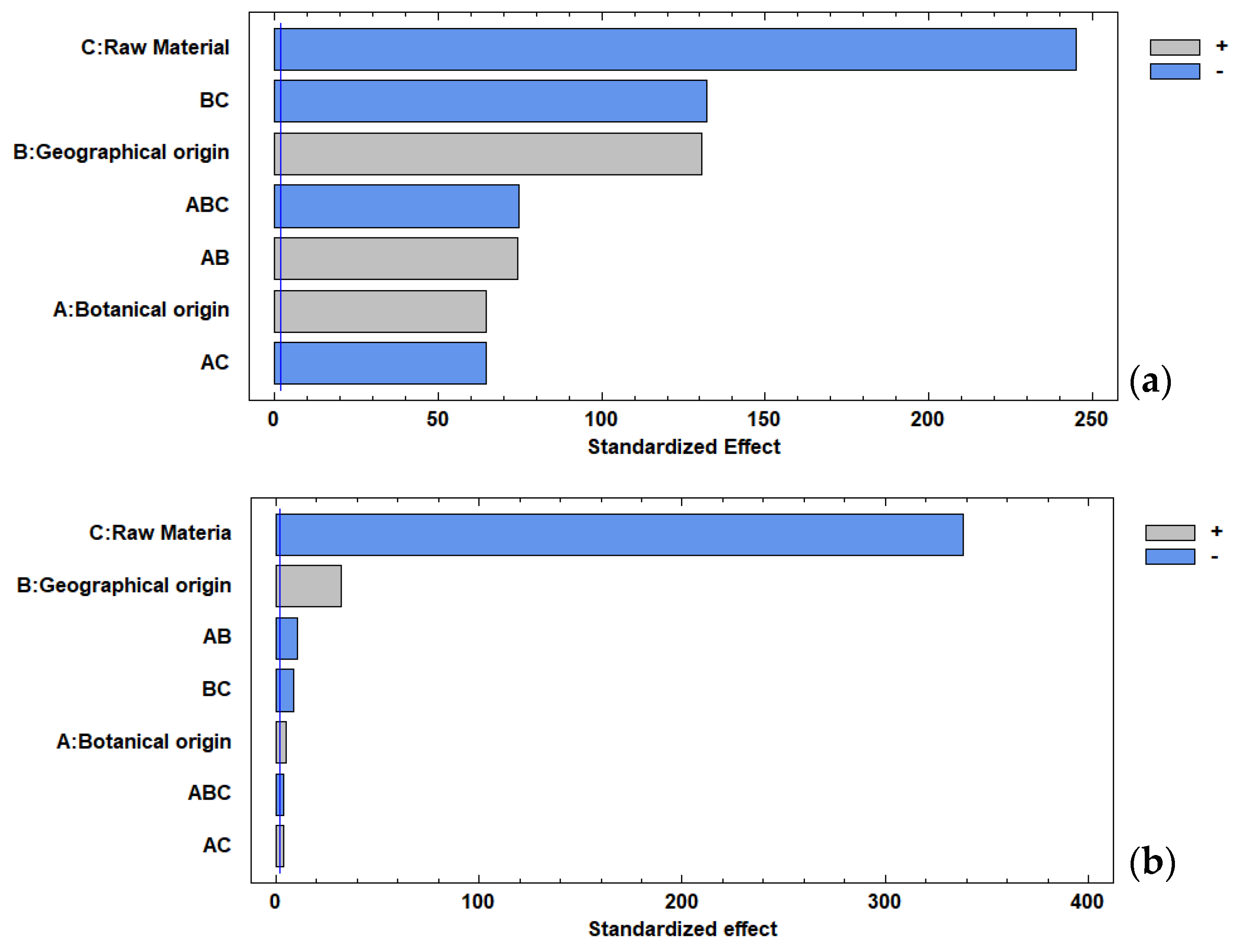
Figure 3 demonstrates the significant effect of all factors and interactions on the total polyphenol content. However, the raw material factor, as expected, presented a greater statistical effect due to the inherent differences in the materials. The Pareto chart revealed that the main factors, raw material (flowers) and geographical origin (Acanceh), presented significant effects (p < 0.05) on the antioxidant capacity. The effect of all factors on the TPC and AC was confirmed by the interaction chart (Figure S2) and the main factors chart (Figure S3).
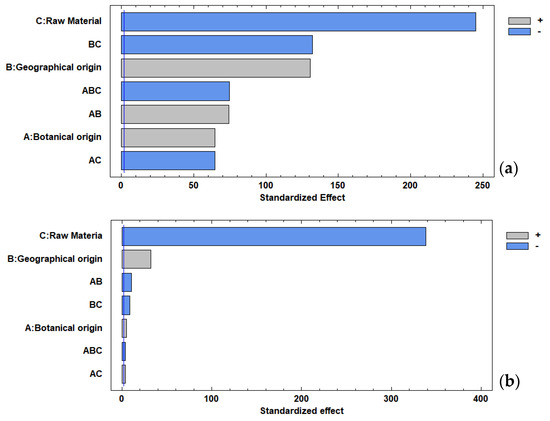
Figure 3.
Pareto chart: (a) total polyphenol content, (b) antioxidant capacity.
Figure S4 shows the effects of the individual factors (botanical origin and geographical origin) and their interaction on AC and TPC in the flowers and their honeys. In this sense, the geographical origin (Tahdziu) had a greater statistical effect than the botanical origin (Jabin); a similar trend was observed for the AC. In addition, the geographical origin (Acanceh) had a greater statistical effect than the botanical origin (Dzidzilche).
In the individual analysis, the flower (Figure S5) showed a significant effect of all factors and interactions on the TPC. However, the geographical origin (Acanceh) presented a greater statistical effect than the botanical origin (Dzidzilche). For AC, the geographical origin (Acanceh) and the interaction had a significant effect, while the botanical origin did not.
3.5. Identification of Metabolites in Flower Flours and Honey of Dzidzilche and Jabin
From the entire dataset, we obtained 312 peaks containing MS2. We focused solely on these peaks to obtain annotations at the molecular structure level through MS2 spectral matching against spectral libraries or through in silico predictions. After filtering out contaminants or low-quality annotations, a total of 55 metabolites in the BH (Table S1) and 69 metabolites in the flower (Table S2) samples were putatively identified, with an identification rate of approximately 24%. Notably, the annotations reported herein are putative, and due to the limitations of the technique, we could not differentiate between enantiomers. Therefore, the reported molecular structures are those automatically retrieved from the automated spectral match and in silico predictions.
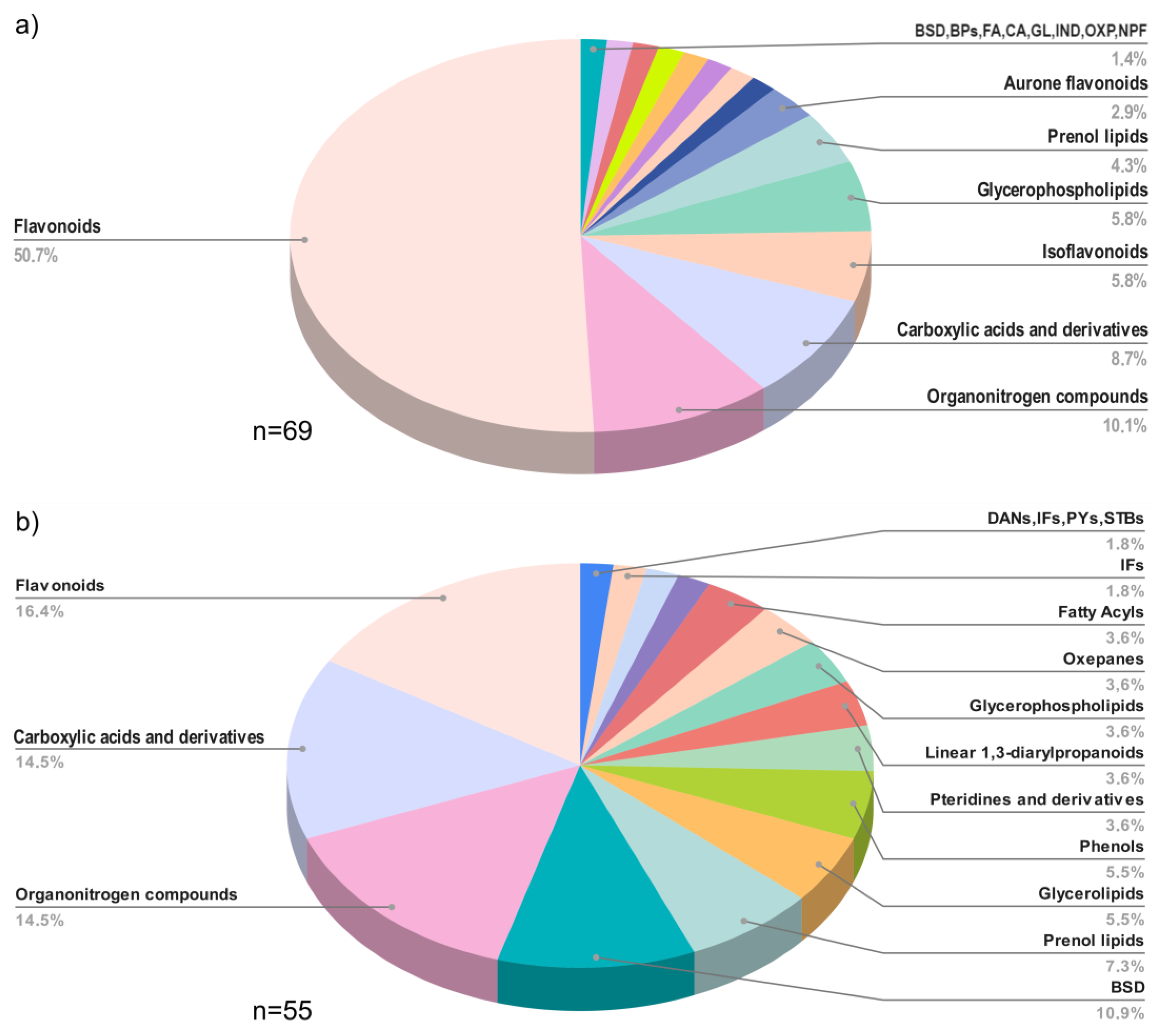
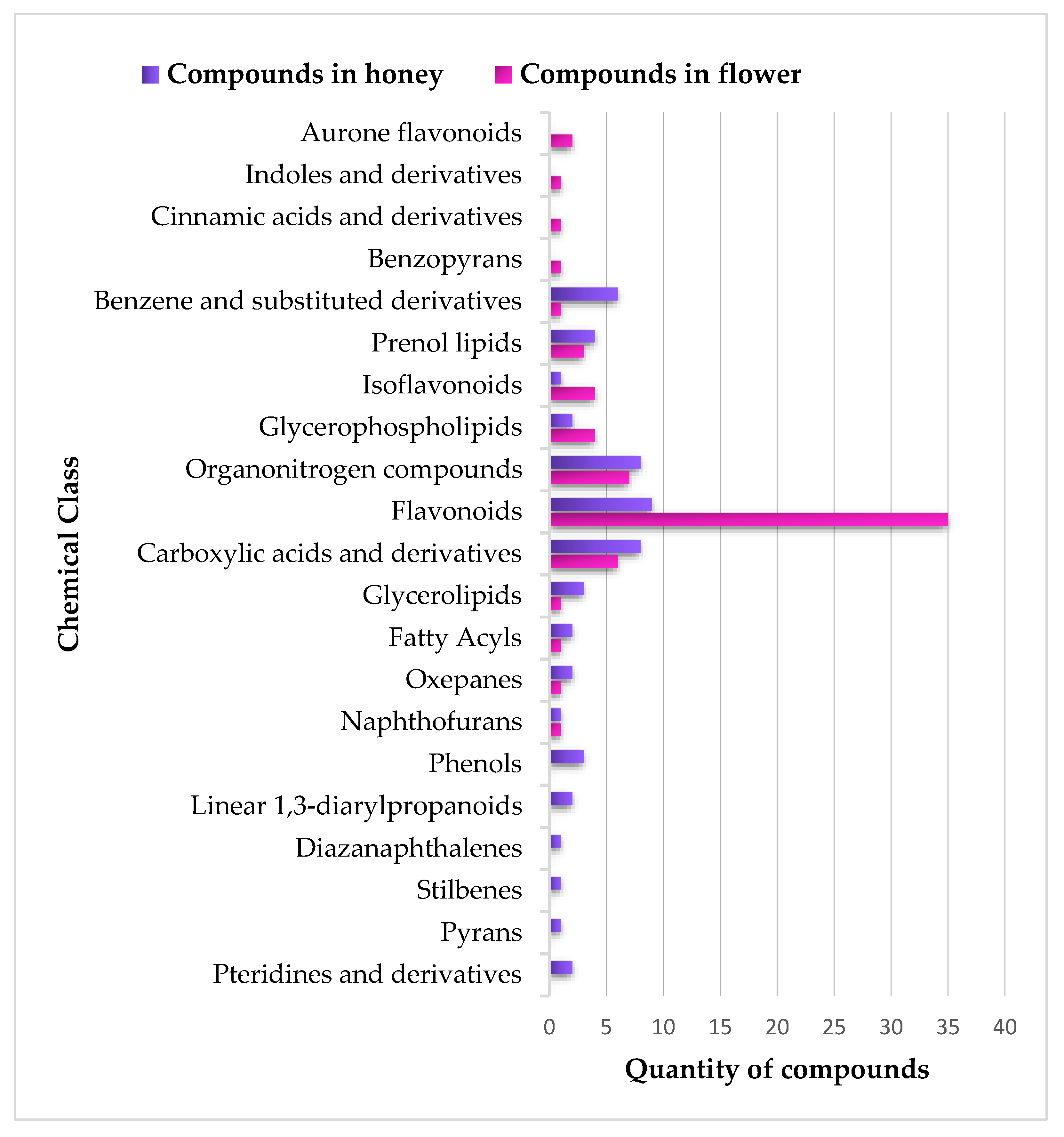
Raw materials (flowers and BH) were grouped to provide a comprehensive depiction of the chemical classes of the metabolites identified in both flowers and BH. A distinct chemical profile among flower and BH samples was observed. In flowers (Figure 4a), the 69 annotated compounds belonged to 15 chemical classes, and the flavonoid chemical class accounted for 50.7% of the total identified metabolites. By contrast, the 55 compounds annotated in BH (Figure 4b) spanned 16 chemical classes, with only one more chemical class than the flower repertoire. Despite this, the presence of flavonoids was lower in BH than in flowers, constituting 16.4% of BH metabolites. On the other hand, the percentage representation of carboxylic acids and their derivatives, organonitrogen compounds, and benzene and substituted derivative metabolites were more similar between flowers and BH.
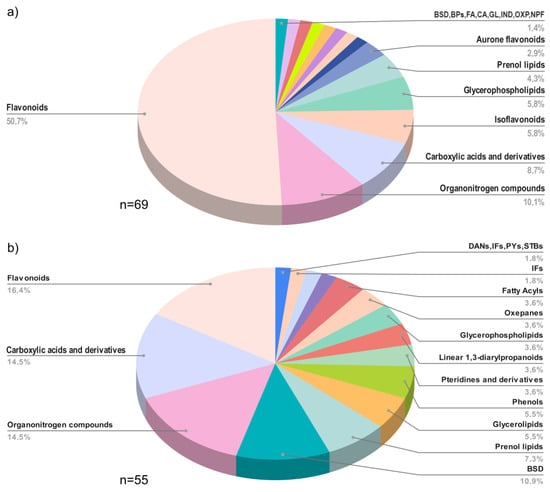
Figure 4.
Distribution of chemical classes in (a) flower samples, BSD = benzene and substituted derivatives; BPs = benzopyrans; FA = fatty Acyls; CA = cinnamic acids and derivatives; GL = glycerolipids; IND = indoles and derivatives; OXP = oxepanes; NPF = naphthofurans; and (b) honey samples, BSD = benzene and substituted derivatives; DANs = diazanaphthalenes; IFs = isoflavonoids; PYs = pyrans; STBs = stilbenes.
Four chemical classes (aurone flavonoids, indoles and their derivatives, cinnamic acids and their derivatives, and benzopyrans) were only observed in flower samples. Moreover, the BH samples exhibited six chemical classes (pteridines and their derivatives, pyrans, stilbenes, diazanaphthalenes, linear 1,3-diarylpropanoids, and phenols) that were not present in the flower samples, as shown in Figure 5.
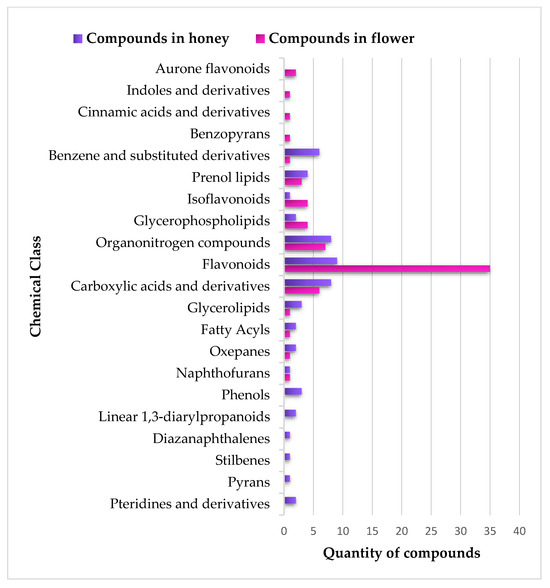
Figure 5.
Comparative global analysis of the distribution of metabolites from various chemical classes among flower and honey samples.
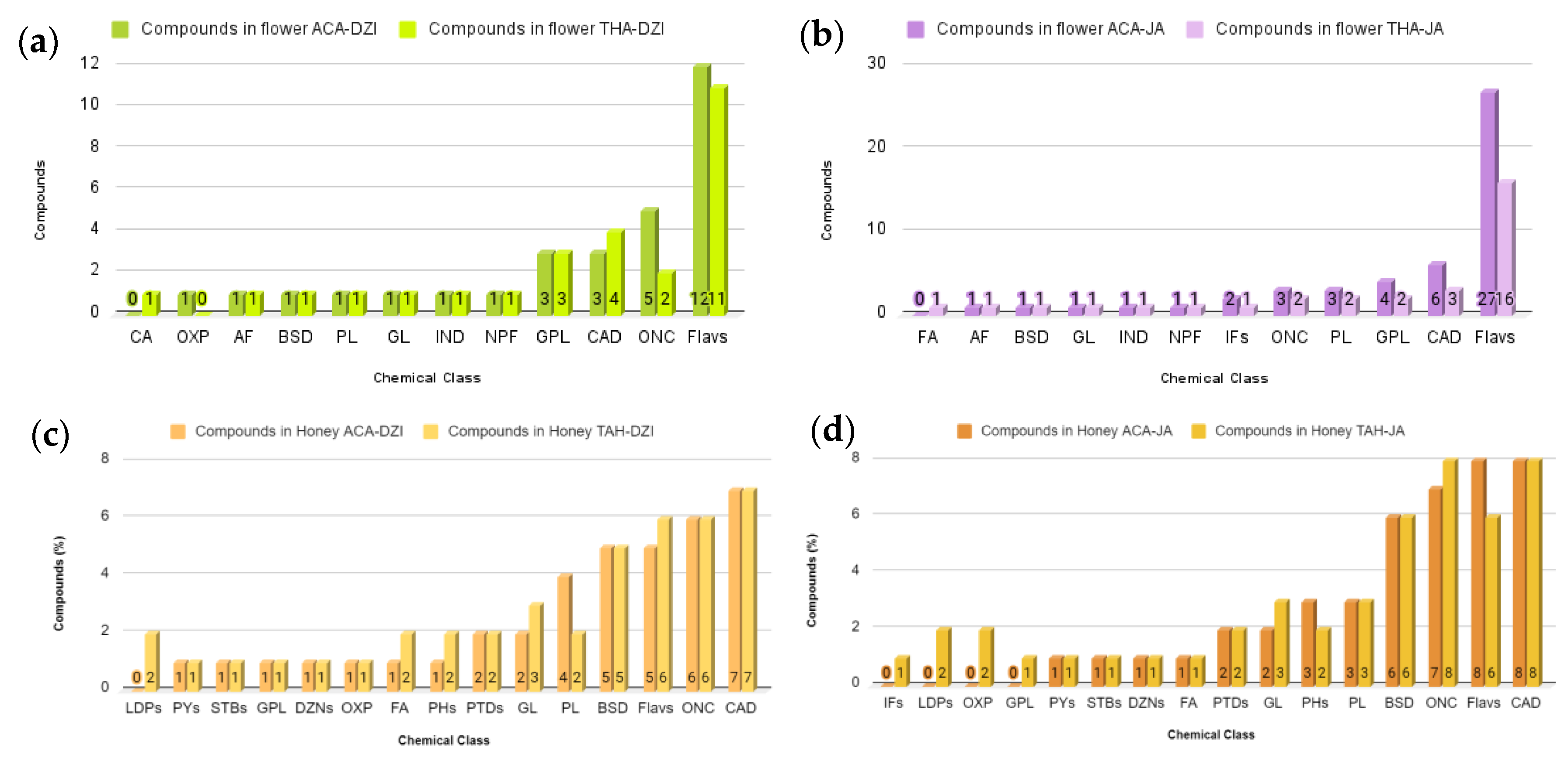
The comparative analysis of floral samples revealed a higher number of compounds (30 compounds) in Dzidzilche flowers from Acanceh compared with those from Tahdziu (27 compounds). Both Dzidzilche and Tahdziu samples displayed an equal number of chemical classes (n = 11). However, while cinnamic acids and their derivatives were present in Tahdziu, oxepanes were identified in the Acanceh samples (Figure 6a). Regarding botanical origin, Jabin flowers from Acanceh presented a higher number of compounds (50 compounds) than those from Tahdziu (32 compounds). These 50 metabolites were categorized into 12 different chemical classes, with the notable difference that fatty acyls were present in the Tahdziu samples and absent in the Acanceh samples (Figure 6b). For bee honey originating from Dzidzilche, a higher number of compounds were found in the Tahdziu location than in the Acanceh location (42 and 38 compounds, respectively). These compounds were classified into 15 chemical classes, wherein linear 1,3-diarylpropanoids were solely found in the Tahdziu location (Figure 6c). BH with Jabin botanical origin displayed a higher number of compounds in Tahdziu than Acanceh (48 and 43 compounds, respectively), categorized into 16 chemical classes. Among these isoflavonoids, linear 1,3-diarylpropanoids, oxepanes, and glycerophospholipids were present in the Tahdziu location (Figure 6d).
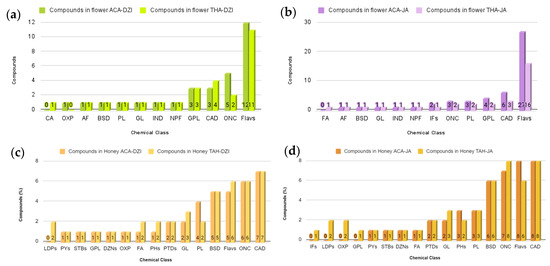
Figure 6.
Comparative analysis of the distribution of metabolites from various chemical classes in flower and honey samples according to geography. (a) Dzidzilche flowers in Acanceh and Tahdziu. (b) Jabin flowers in Acanceh and Tahdziu. (c) Honey from Dzidzilche in Acanceh and Tahdziu. (d) Honey from Jabin in Acanceh and Tahdziu. BSD = benzene and substituted derivatives; BPs = benzopyrans; FA= fatty acyls; CA = cinnamic acids and derivatives; GL = glycerolipids; IND= indoles and derivatives; OXP = oxepanes; NPF = naphthofurans; DANs = diazanaphthalenes; IFs = isoflavonoids; PYs = pyrans; STBs = stilbenes; AF = aurone flavonoids; GPL = glycerophospholipids; CAD = carboxylic acids and derivatives; PL= prenol lipids; ONC = organonitrogen compounds; Flavs = flavonoids; DZNs = diazanaphthalenes; PHs = phenols; PTDs = pteridines and derivatives; LDPs = linear 1,3-diarylpropanoids. ACA = Acanceh (geographical origin); TAH = Tahdziu (geographical origin); DZI = Dzidzilche (botanical origin); JA = Jabin (botanical origin).
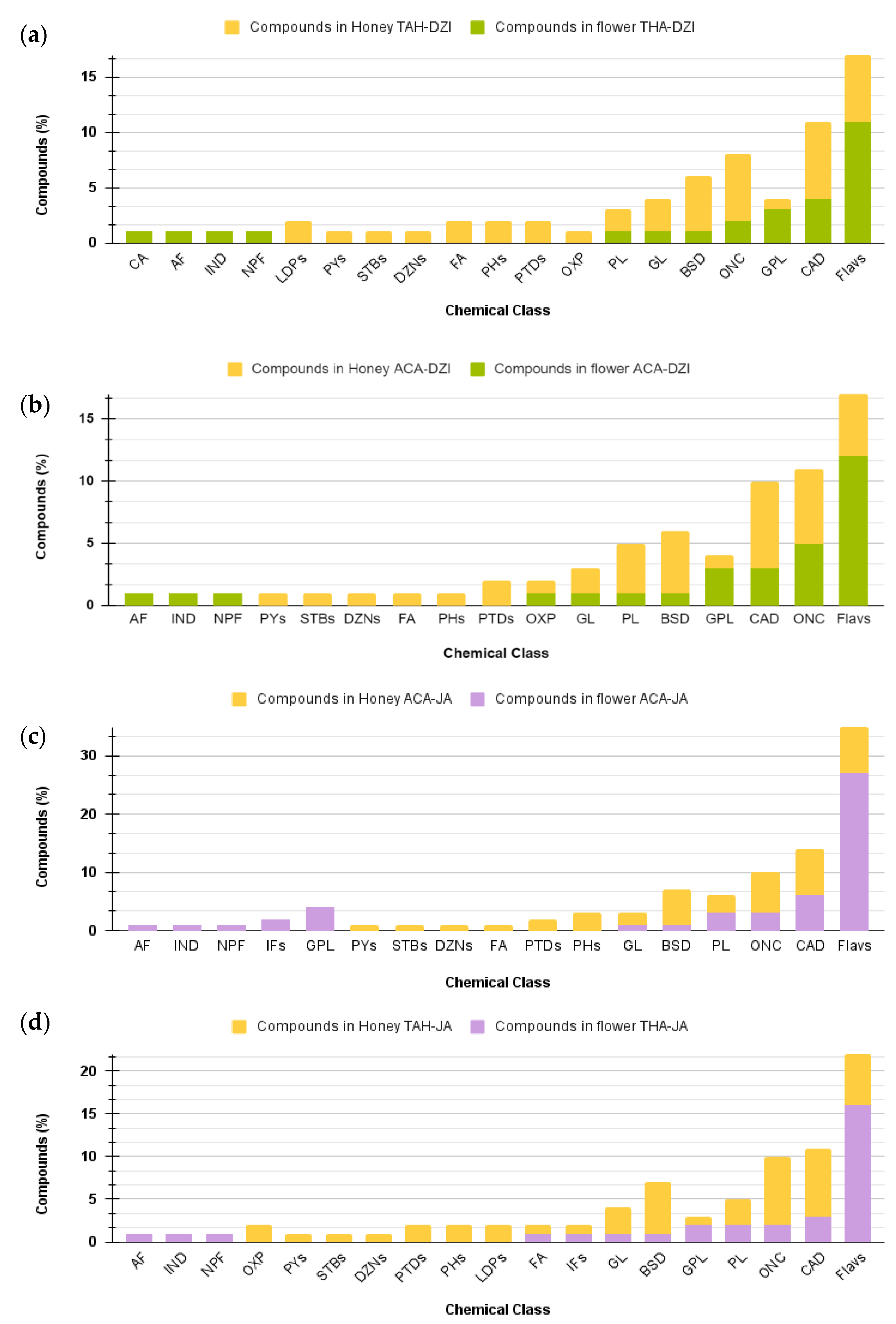
Although the BH and flower samples shared botanical and geographical origin, differences at the chemical class level were observed. For instance, flowers of Dzidzilche botanical origin from the Tahdziu location exhibited the presence of cinnamic acids and their derivatives, aurone flavonoids, indoles and their derivatives, and naphthofurans (Figure 7a), while BH (associated with the same botany and geography) differentially presented linear 1,3-diarylpropanoids, pyrans, stilbenes, diazanaphthalenes, fatty acyls, phenols, and oxepanes. Bee honey associated with Acanceh flowers from Dzidzilche lacked aurone flavonoids, indoles and their derivatives, and naphthofurans but presented pyrans, stilbenes, diazanaphthalenes, fatty acyls, phenols, and pteridines and their derivatives (Figure 7b). Jabin flowers from Acanceh uniquely presented aurone flavonoids, indoles and their derivatives, naphthofurans, isoflavonoids, and glycerophospholipids, while BH (associated with the same botany and geography) uniquely presented pyrans, stilbenes, diazanaphthalenes, fatty acyls, and phenols (Figure 7c).
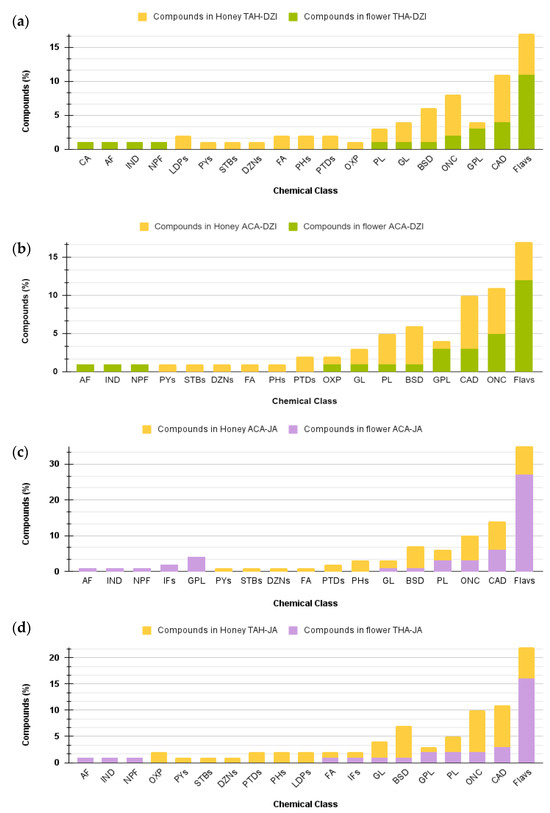
Figure 7.
Comparative analysis of the distribution of metabolites from various chemical classes among flower and honey samples from the same botany and geography. (a) Dzidzilche flowers in Acanceh and Tahdziu. (b) Jabin flowers in Acanceh and Tahdziu. (c) Honey from Dzidzilche in Acanceh and Tahdziu. (d) Honey from Jabin in Acanceh and Tahdziu. Abbreviations: BPs = benzopyrans; FA = fatty Acyls; CA = cinnamic acids and derivatives; GL = glycerolipids; IND = indoles and derivatives; OXP = oxepanes; NPF = naphthofurans; DANs = diazanaphthalenes; IFs = isoflavonoids; PYs = pyrans; STBs = stilbenes; AF = aurone flavonoids; GPL = glycerophospholipids; CAD = carboxylic acids and derivatives; PL = prenol lipids; ONC = organonitrogen compounds; Flavs = flavonoids; DZNs = diazanaphthalenes; PHs = phenols; PTDs = pteridines and derivatives; LDPs = linear 1,3-diarylpropanoids. ACA = Acanceh (geographical origin); TAH = Tahdziu (geographical origin); DZI = Dzidzilche (botanical origin); JA = Jabin (botanical origin).
Jabin flowers from Tahdziu uniquely presented aurone flavonoids, indoles and their derivatives, and naphthofurans, while BH (associated with the same botany and geography) uniquely presented oxepanes, pyrans, stilbenes, diazanaphthalenes, pteridines and their derivatives, phenols, and linear 1,3-diarylpropanoids. Overall, aurone flavonoids, indoles and their derivatives, naphthofurans, isoflavonoids, and glycerophospholipids were found in flowers, while BH (associated with the same botany and geography) uniquely presented pyrans, stilbenes, diazanaphthalenes, fatty acyls, and phenols (Figure 7c). Naphthofurans were consistently solely found in flowers when compared with their BH counterpart (same botany and geography).
Principal Component Analysis (PCA) and Lineal Correlation
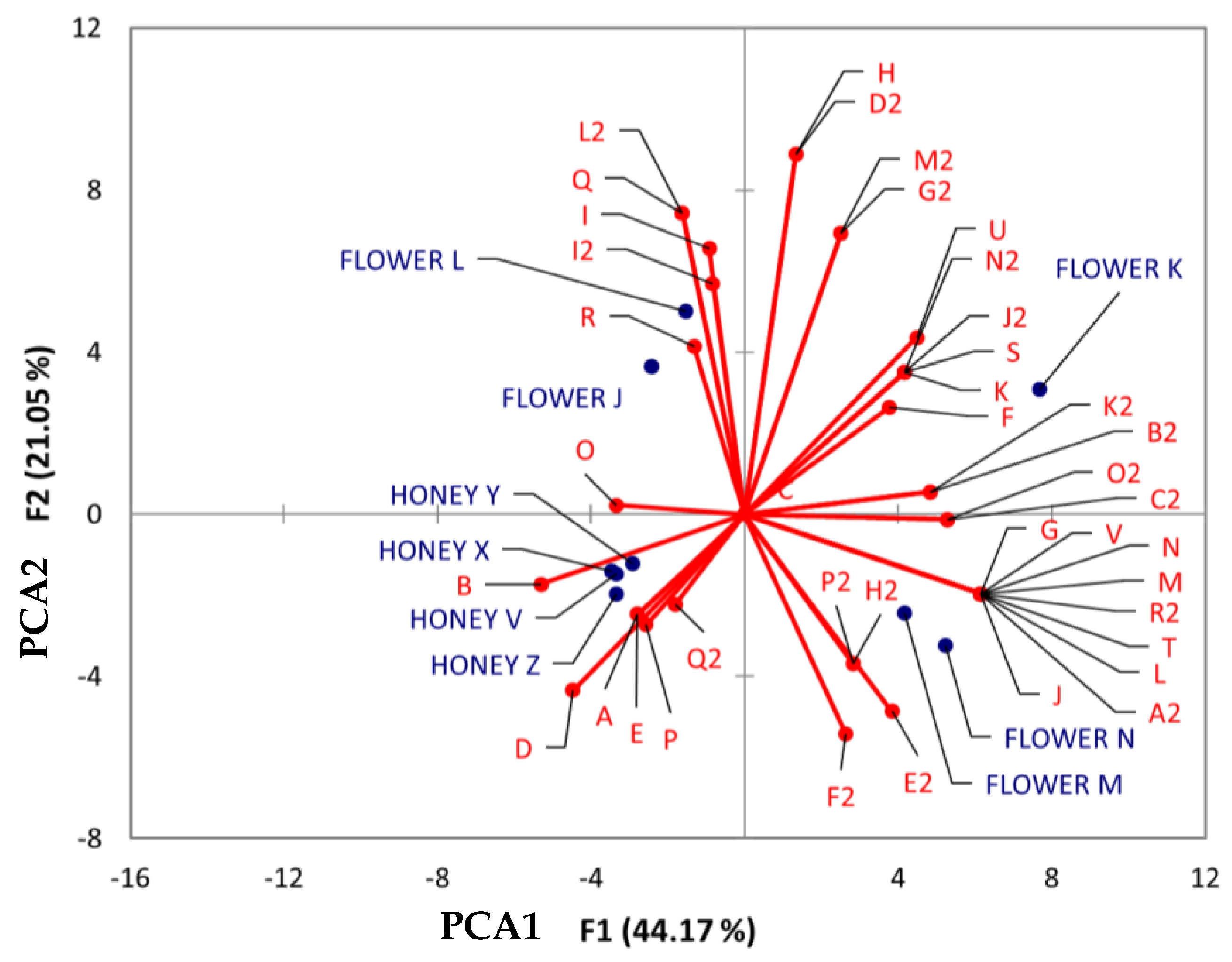
To examine similarities between flowers and BH considering their botany and geography, PCA was conducted. This analysis involved three distinct groups of chemical classes: (1) flavonoids, (2) benzenes and their derivatives, and (3) cinnamic acid and its derivatives. These groups were chosen due to their prominence as representative classes in honey and flowers.
In the PCA chart for flavonoids (Figure 8), the proximity between flower M (Tahdziu–Jabin) and N (Tahdziu–Jabin, oven-dried) suggests a shared similarity. Conversely, all honey samples exhibited close distances, implying common traits. The metabolite afzelin (O) demonstrated a close relationship between Dzidzilche flowers and honey from Acanceh and Tahdziu.
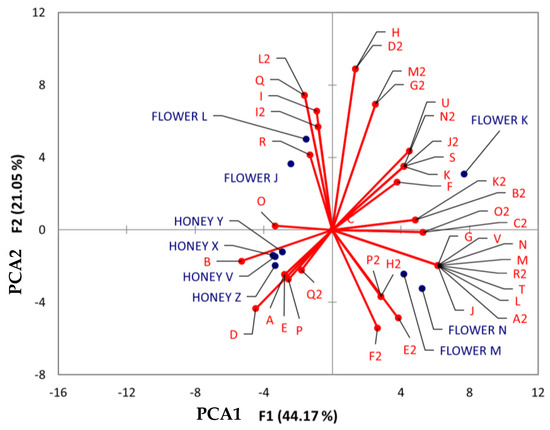
Figure 8.
Principal component analysis (PCA) biplot of Dzidzilche and Jabin flower and honey samples based on the identified flavonoid metabolites. Abbreviations: A = naringin; B = vitexin; C = quercitrin; D = quercetin-3-O-rhamnoside; E = kaempferol-3-rhamninoside; F = kaempferol; G = aromadendrin; H = cianidanol; I = quercetin; J = taxifolin; K = isorhamnetin; L = 3,6,3′,4′-tetramethoxyflavone; M = retusin; N = tangeretin; O = afzelin; P = 3,5-dihydroxy-2-(4-hydroxyphenyl)-7-[(3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl) oxy]-4H-1-benzopyran-4-one; Q = reynoutrin; R = (-)-epicatechin gallate; S = luteolin-4′-O-glucoside; T = sinensetin; U = 3-[5-(1,2-dihydroxyethyl)-3,4-dihydroxyoxolan-2-yl] oxy-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one; V = taxifolin-3-glucoside; A2 = isorhamnetin 3-galactoside; B2 = 2S,3S)-3,5,7-trihydroxy-2-[4-hydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-2,3-dihydrochromen-4-one; C2 = luteolin 7-(6″ ″-malonylglucoside); D2 = procyanidin B2; E2 = 6″-O-(3-hydroxy-3-methylglutaroyl)astragalin; F2 = 3″-O-L-rhamnopyranosylastragalin; G2 = rutin; H2 = hesperidin; I2 = 2′-O-galloylhyperin; J2 = 3-[3,4-dihydroxy-6-(hydroxymethyl)-5-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one; K2 = rhamnetin 3-sophoroside; L2 = procyanidin B5, 3′-O-gallate; M2 = 2-(3,4-dihydroxyphenyl)-6,8-bis [2-(3,4-dihydroxyphenyl)-3,7,8-trihydroxy-3,4-dihydro-2H-chromen-5-yl]-3,4-dihydro-2H-chromene-3,5,7-triol; N2 = (2S,3S)-3,5,7-trihydroxy-2-[4-hydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl) oxan-2-yl]oxyphenyl]-2,3-dihydrochromen-4-one; O2 = 11-hydroxytephrosin; P2 = amorphigenin; Q2 = puerarin; R2 = 6-Hydroxysumatrol; FLOWER J = Acanceh–Dzidzilche; FLOWER K = Acanceh–Jabin; FLOWER L = Tahdziu–Dzidzilche; FLOWER M = Tahdziu–Jabin; FLOWER N = Tahdziu–Jabin oven-dried; HONEY V = Acanceh–Dzidzilche; HONEY X = Acanceh–Jabin; HONEY Y = Tahdziu–Dzidzilche; HONEY Z = Tahdziu–Jabin.
Compounds such as vitexin (B), pueratin (Q2), naringin (A), quercetin (D), kaempferol (E), and 3,5-dihydroxy-2-(4-hydroxyphenyl)-7-[(3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl) oxy]-4H-1-benzopyran-4-one (P) showed a clear association with all honey samples, irrespective of their botanical or geographical origins. Regarding the flowers, those stemming from Jabin demonstrated an association with phenolic compounds, including aromadendrin (G), taxifolin-3-glucoside (V), retusin (M), tangeretin (N), 3,6,3′,4′-tetramethoxyflavone (L), sinensetin (T), taxifolin (J), isorhamnetin 3-galactoside (A2), 6″-O-(3-hydroxy-3-methylglutaroyl) astragalin (E2), 6-hydroxysumatrol (R2), 3″-O-L-rhamnopyranosylastragalin (F2), amorphigenin (P2), and hesperidin (H2). On the other hand, flowers of Dzidzilche origin were associated with compounds like (-)-epicatechin gallate (R), reynoutrin (Q), 2′-O-galloylhyperin (I2), and procyanidin B5, and 3′-O-gallate (L2).
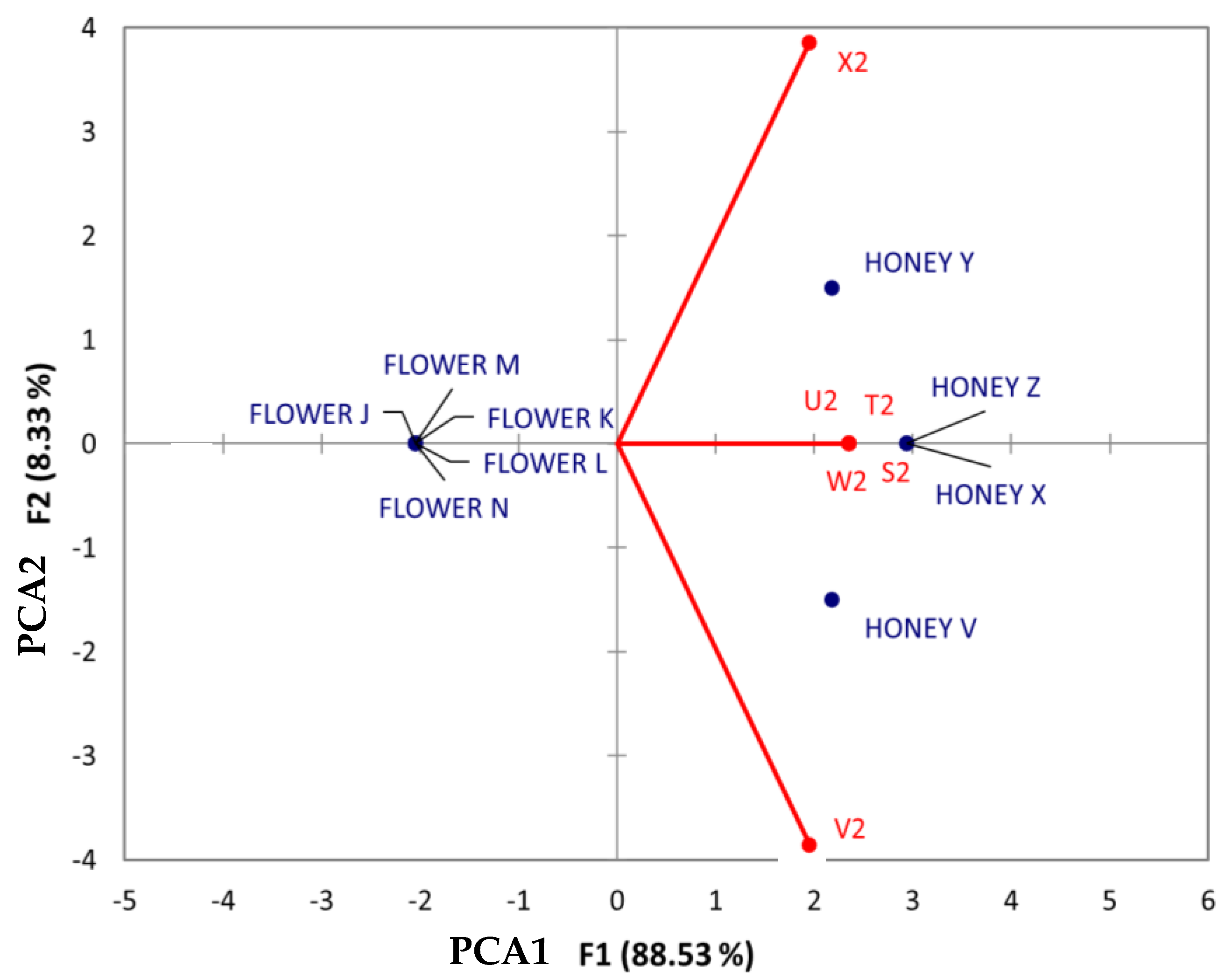
When analyzing benzenes and substituted derivatives (Figure 9), the analysis showed the following outcomes: compounds such as 3-methoxybenzoic acid (T2), benzylideneacetone (S2), tyramine (W2), and veratric acid (U2) were denoted as having a strong association with honey from Jabin, regardless of the geographical origin. Dzidzilche honey from Tahdziu showed an association with phenylalanine, whereas honey displayed an association with the compound 3-methylsalicylic acid (V2). Notably, no associations between flowers and compounds from this chemical class were found.
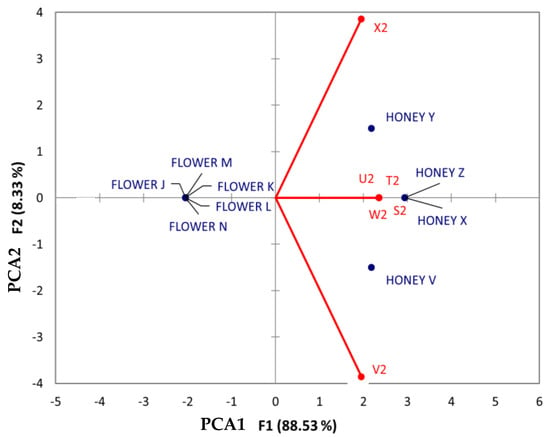
Figure 9.
Principal Component Analysis (PCA) biplot of Dzidzilche and Jabin flowers and honey samples based on the identified benzenes and substituted derivate metabolites. Abbreviations: S2 = benzylideneacetone; T2 = 3-methoxybenzoic acid; U2 = veratric acid; V2 = 3-methylsalicylic acid; W2 = tyramine; X2 = n-(phenylacetyl)phenylalanine; FLOWER J = Acanceh–Dzidzilche; FLOWER K = Acanceh–Jabin; FLOWER L = Tahdziu–Dzidzilche; FLOWER M = Tahdziu–Jabin; FLOWER N = Tahdziu–Jabin, oven-dried; HONEY V = Acanceh–Dzidzilche; HONEY X = Acanceh–Jabin; HONEY Y = Tahdziu–Dzidzilche; HONEY Z = Tahdziu–Jabin.
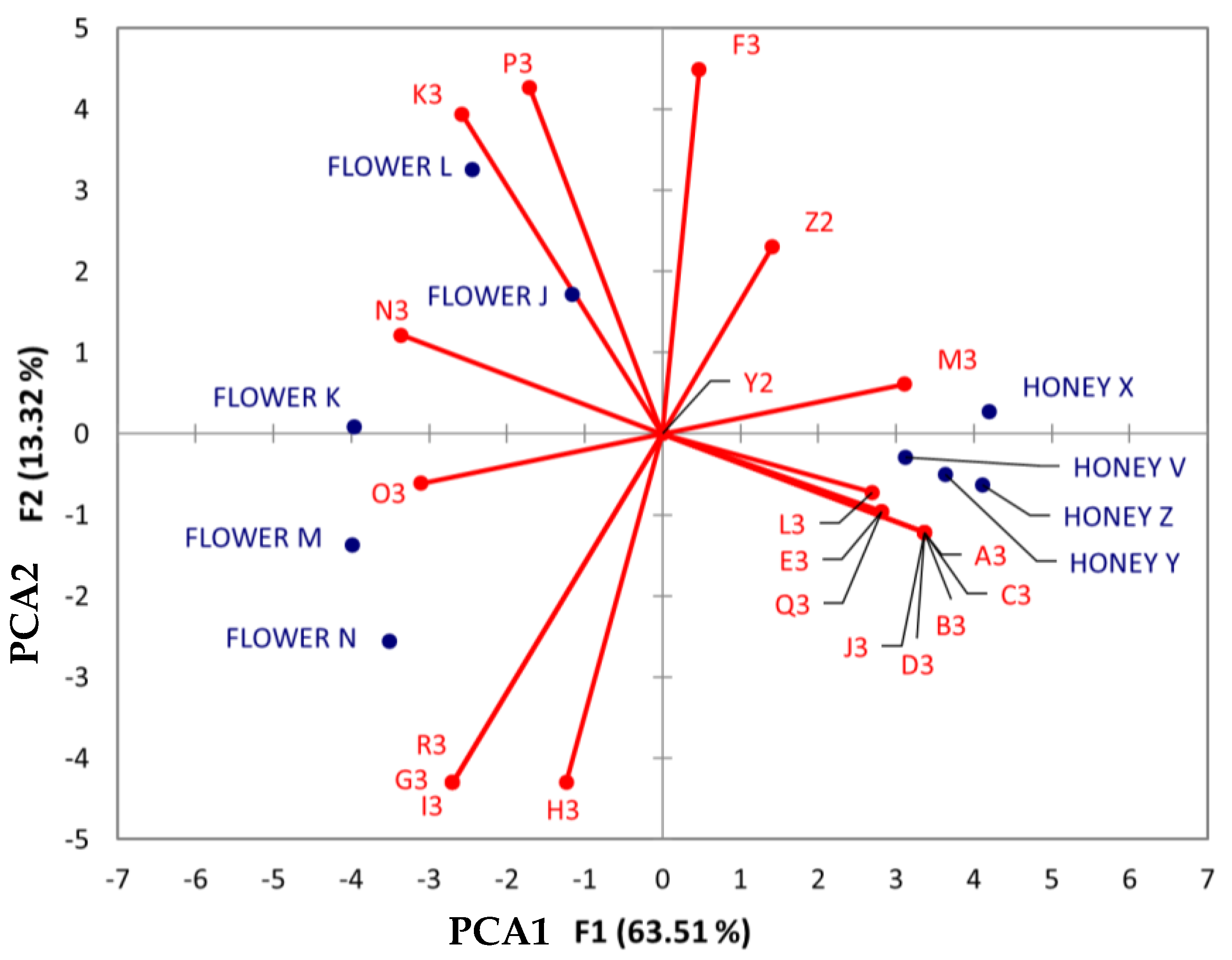
As shown in Figure 10, various compounds (listed from M3-R3) linked to the carboxylic acid and derivative chemical class were associated with different honey samples, regardless of their botanical and geographical origins. Notably, asparagine (O3) was associated with the Jabin botanical origin, while proline (N3), 4-hydroxybenzoic acid (K3), and n-methyl-tryptophan (P3) were associated with Dzidzilche.
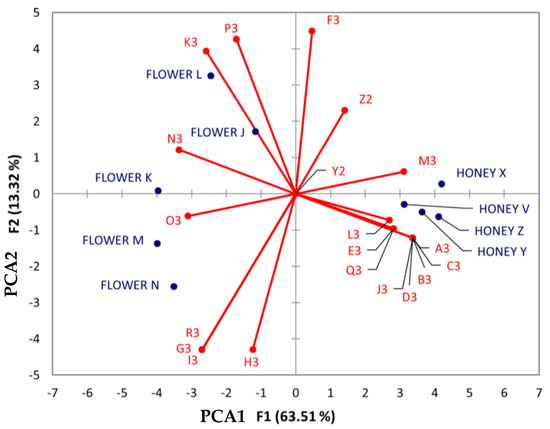
Figure 10.
Principal component analysis (PCA) biplot of Dzidzilche and Jabin flower and honey samples based on the identified carboxylic acids and derivatives and phenols metabolites. Abbreviations: Y2 = phenylalanine; Z2 = tyrosine A3 = n-(phenylacetyl)phenylalanine; B3 = n-Fructosyl-isoleucine; C3 = n-fructosyl-phenylalanine; D3 = [6]-gingerol; E3 = vanillin; F3 = moupinamide; G3 = (2Z)-4,6-dihydroxy-2-[(4-hydroxy-3,5-dimethoxyphenyl) methylidene]-1-benzofuran-3-one; H3 = methyl (1S,3S,4S,4aS,9aR)-7-[(5R,6R,8R,8aS,10aS)-1,5,8,8a-tetrahydroxy-10a-methoxycarbonyl-6-methyl-9-oxo-5,6,7,8-tetrahydroxanthen-2-yl]-1,4,8,9a-tetrahydroxy-3-methyl-9oxo1,2,3,tetrahydroxanthene-4a-carboxylate; I3 = 3,4-dihydroxy cinnamic acid; J3 = resveratrol; K3 = 4-hydroxybenzoic acid; L3 = n-jasmonoyl-leucine; M3 = n-(1-deoxy-1-fructosyl) phenylalanine; N3 = proline; O3 = asparagine (0.93722378); P3 = n-methyl-tryptophan; Q3 = n-Acetyl-phenylalanine; R3 = chicoric acid. FLOWER J = Acanceh–Dzidzilche; FLOWER K = Acanceh–Jabin; FLOWER L = Tahdziu–Dzidzilche; FLOWER M = Tahdziu–Jabin; FLOWER N = Tahdziu–Jabin, oven-dried; HONEY V = Acanceh–Dzidzilche; HONEY X = Acanceh–Jabin; HONEY Y = Tahdziu–Dzidzilche; HONEY Z = Tahdziu–Jabin.
Furthermore, a lineal correlation was found between total polyphenols and antioxidant capacity in Dzidzilche and Jabin flowers (p < 0.05).
4. Discussion
The highest concentration of polyphenols (1431.24 ± 15.38 mg GAE/100 g DM) was observed in Dzidzilche flowers originating from Acanceh, which also exhibited the highest antioxidant capacity (93.63 ± 0.22% inhibition). By contrast, Jabin honey (botanical origin) had the lowest TPC concentration (5.38 ± 0.14 mg GAE/100 g DM) in Acanceh and the lowest AC (13.68 ± 0.75% inhibition) in Tahdziu. While there were differences in TPC between flowers and honey, their antioxidant capacity displayed a similar trend. This variation in TPC is mainly attributed to the higher percentage of flavonoids present in the flowers (50.7%) compared with that in honey (14.7%), consequently influencing the antioxidant capacity. According to Ghasemzadeh, et al. [48], these bioactive compounds demonstrate a significant positive correlation with the antioxidant capacity of plant matrix extracts, including flower extracts [49].
The antioxidant properties of flavonoids are derived from their ability to donate a hydrogen proton to an oxidizing molecule, thereby promoting molecular stability and inhibiting oxidative processes [50]. On the other hand, the higher percentage of phenolic compounds in flowers than in honey arises because these are secondary metabolites exclusively synthesized by plants [51], regardless of their botanical and geographical origins. By contrast, the concentration of phenolic compounds in honey is influenced by external factors, such as the availability of biological material for bees, the honey production process, and the amalgamation of flower or plant secretions with the bee’s bodily fluids during the transport and storage of nectar [52].
Another crucial factor to consider when characterizing flowers and BH involves the edaphological conditions of the flower’s geographical origin. In this study, Acanceh, a municipality situated in the central area of Yucatan, was selected, which is characterized by Mollic Leptosol soil (as per the World Reference Base for Soil Resources) or Box lu’um (according to the Mayan classification as described by Bautista et al.) [53]. These soils manifest distinctive features, including a black color, an intermediate percentage of gravel and stones (20–60%), a substantial organic matter content (greater than 10%), good drainage capacity, and a high concentration of exchangeable cations like calcium, phosphorus, sulfur, and sodium. On the other hand, the red soils (WRB) or Hay lu’um (Mayan classification) found in Tahdziu present a lower content of exchangeable cations than Mollic Leptosol soils, red color, clayey texture, reduced moisture retention, lower organic matter content (<15%), and occasional rocky outcrops [53,54].
The soil characteristics influence the polyphenol content of products such as plants, fruits, and their by-products. Numerous studies have evaluated the impact of black, brown, and red soils on the TPC and AC of habanero chili products from the Yucatan Peninsula. In one study, it was observed that plants cultivated in black soils presented a notably higher TPC (217.13 ± 28.04 mg GAE/100 g dry pepper) and AC (86.51 ± 0.82% inhibition) compared with those grown in other soil types, including red soil (135.17 ± 14.24 mg GAE/100 g dry pepper, with inhibition below 86%) and brown soil, which displayed intermediate behavior [54]. This study suggests that these differences were primarily attributed to variations in electrical conductivity, organic matter content, and increased nitrogen presence [55,56]. Overall, such factors are thought to impact nutrient availability and uptake in plants, ultimately modulating the polyphenol content and thereby the AC of the products.
Botanical origin emerged as a pivotal factor in assessing the TPC, AC, and metabolite chemical classes of honey. The Dzidzilche tree (Gymnopodium floribundum) has adapted to the climatic and soil conditions of the Yucatan Peninsula’s tropical forest in Mexico and is notably abundant in the region [19]. This tree plays a crucial role in providing biological materials, such as flowers, nectar, and pollen to honeybees (Apis mellifera) [5]. It has also been identified as a producer of various secondary metabolites, mainly phenolic compounds, including flavonoids and tannins [19,57,58]. In this context, Ortíz-Ocampo et al. [19] conducted a study collecting biological materials (stems, leaves, and flowers) from Dzidzilche trees in the “Cuxtal” natural reserve from December 2017 to December 2018. They aimed to analyze changes in the concentration of phenolic compounds across the seasons (spring, summer, autumn, and winter). Their findings indicated significantly higher concentrations of phenolic compounds (p < 0.05) during the months of January, February, and March, coinciding with the same sampling period as the present study (March). During this period, Dzidzilche flowers presented a greater concentration of TPC than Jabin (another botanical origin). However, it is worth noting that this difference may also be attributed to the fact that Dzidzilche trees belong to the Polygonaceae family, which has demonstrated higher concentrations of phenolic compounds (1310 ± 100 mg GAE/100 g DM) than the Fabaceae family (713.3 ± 37.1 mg GAE/100 g DM), the family to which Jabin (Piscidia piscipula) belongs. This trend aligns with the current study’s findings, in which Dzidzilche exhibited a significantly higher concentration of phenolic compounds than Jabin [57,59,60]. Furthermore, the concentrations of phenolic compounds exhibited a correlation with the AC in both Dzidzilche and Jabin flowers. For instance, Hallman [59] reported a significant linear correlation between the phenolic compound content in extracts of the Fabaceae family and their AC. Similarly, Feduraev et al. [57] reported a significant linear correlation between phenolic-rich extracts from plants of the Polygonaceae family and their AC. This same trend was observed in the present study when linearly correlating the phenolic compounds of Dzidzilche (Polygonaceae family) and Jabin (Fabaceae family) flower extracts with their respective antioxidant capacities, reaffirming the relevance of polyphenols in the antioxidant bioactivity of these plants.
Notably, the TCP and AC are not only affected by botanical origin but also by the presence of specific secondary metabolites. For example, within the Fabaceae family, plants are characterized by their repertoire of a set of compounds belonging to the chemical class of isoflavonoids. This type of compound is a subclass of the flavonoid family featuring a benzene ring B at the carbon 3 position of the pyran ring [61] and is synthesized de novo through the Shikimic acid pathway. At a certain stage, the activation of chalcone isomerase and isoflavone synthase is promoted, working as a defense mechanism in response to stressors, predominantly of microbiological origin [62]. Additionally, these compounds are regarded as pathogen resistance biomarkers [63]. On the other hand, plants belonging to the Polygonaceae family are renowned for their substantial content of flavonoids, particularly quercetin and epicatechin [51]. These metabolites are found in numerous genera within this family and are linked to pleiotropic effects, including a robust antioxidant capacity [64] along with antidiabetic, neuroprotective, cardioprotective, and anticancer effects [65,66].
In this study, the presence of 12 chemical classes of the samples analyzed was observed. However, it is worth noting that Jabin flowers contained two secondary metabolites classified as isoflavones, specifically 11-hydroxytephrosin and 6-hydroxysumatrol, which were absent in the Dzidzilche samples. Conversely, a notable association was observed between the flavanol catechin (or one of its stereoisomers) and the botanical origin of Dzidzilche. It is important to highlight that these phenolic compounds were also identified in honey. Although these metabolites are solely synthesized by plants, they can make their way into honey through the collection of nectar, propolis, and/or resins by honeybees, which are subsequently processed in the beehive [66].
Some authors, including Gašić et al. [67], Cheung et al. [68], and Kasiotis et al. [69], have proposed phenolic compounds as potential biomarkers for identifying the botanical and even geographical origin of honey, mainly monofloral honey, to ensure their authenticity in global markets. According to Lawag et al. [70], characteristic phenolic compounds found in honey sourced from plants of the Polygonaceae family across various geographical regions (China, Finland, Italy, Lithuania, Poland, Turkey, USA, etc.) encompass apigenin, luteolin, chrysin, vitexin, quercetin, kaempferol, rutin, phenylacetic acid, salicylic acid, p-coumaric acid, and chlorogenic acid, among others. On the other hand, representative phenolic compounds of the Fabaceae family identified from different geographical regions (China, Argentina, Italy, Turkey, Germany, Spain, Austria, USA, Poland, etc.) include vanillin, apigenin, luteolin, quercetin, quercitrin, rutin, myricetin, vanillic acid, p-coumaric acid, gallic acid, and protocatechuic acid. These reported results aligned with the findings of the present study. For example, vitexin (apigenin glycoside) was identified (Figure S6) in honey originating from Dzidzilche (Polygonaceae) but not in honey from Jabin (Fabaceae), while a characteristic metabolite of the Fabaceae family, vanillin, was only identified in honey with the botanical origin of Jabin (Figure S7).
Moreover, we detected numerous phenolic compounds of interest in both honeys (Dzidzilche and Jabin), including quercetin, kaempferol, naringenin, and quercitrin, with the latter being notably characteristic of the Fabaceae family. Although these honeys are considered monofloral, as they predominantly originate from a single plant species, it is important to acknowledge that metabolites from other plant species may be also present [71]. Additionally, during the honey production process, phenolic compounds can undergo hydrolysis or form glycosylated derivatives due to the activities of enzymes and microorganisms within the honeybee’s digestive system [69,72]. Given the variability of phenolic compounds influenced by abiotic and biotic factors, there has been an exploration of alternative markers, such as nitrogenous compounds (e.g., essential amino acids), in honey [69,73,74,75]. However, no discernible differences in amino acids have been identified regarding botanical origin. For instance, Hermosín et al. [74] examined 31 Spanish honeys from various botanical (rosemary, eucalyptus, lavender, thyme, and orange blossom) and geographic (Alcarria, Cabañeros, Cáceres, Valencia, and Cantabria) origins and found that it was not feasible to definitively distinguish the botanical origin solely on the basis of the presence and concentration of amino acids. In this study, an association was found through PCA between tyrosine and honey sourced from Jabin collected from both geographic origins (Acanceh and Tahdziu). Moreover, samples from Dzidzilche and Tahdziu presented an association with phenylalanine. However, no such association with any amino acid was observed in honey originating from Dzidzilche or Acanceh. As highlighted by Kowalski et al. [76], the detection of amino acids from different botanical origins within the same geographic area may be possible due to environmental conditions and the proximity of flowering periods.
5. Conclusions
With the assistance of mass spectrometry and comprehensive chemoinformatics, a detailed analysis was conducted on the metabolome found in flowers and their associated honeys from Yucatan, Mexico. The aim was to uncover how the chemistry of these samples is influenced by their botanical and geographical origins. The flowers of the Gymnopodium floribundum and Piscidia piscipula (L.) genera presented a notable abundance of phenolic compounds, mainly flavonoids, and a remarkable AC, above 90% inhibition (DPPH). These characteristics were distinctly mirrored in the resulting honey; flavonoids and carboxylic acids and their derivatives were the main chemical classes detected, and they showed an AC range between 18 and 21% inhibition (DPPH). The intricate interplay of geographical and botanical origin factors emerged as a crucial determinant in shaping the presence of secondary metabolites (e.g., flavonoids and carboxylic acids and their derivatives), along with fostering a robust AC in both the flowers and the honey. Secondary metabolites previously suggested as biomarkers were identified by liquid chromatography–tandem mass spectrometry (LC–MS2) in honey from Gymnopodium floribundum (vitexin) and Piscidia piscipula (L.) (vanillin). This substantiates and reinforces the ability to determine the botanical origin of Yucatan, México, honey through the presence of phenolic compounds, offering a valuable complement to melissopalynology. Such an approach not only serves as a potent tool for quality control but also substantiates the bioactive properties of honey sourced from this Mexican region, thereby enhancing its market value and streamlining export opportunities to global destinations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr11103028/s1, Figure S1. (a) Total polyphenol content (TPC) calibration curve for flower sample analysis. (b) Total polyphenol content (TPC) calibration curve for honey sample analysis; Figure S2. Interaction chart; total polyphenol content (TPC) (a,b) antioxidant capacity (AC). Capital letters represent a main factor, where A: botanical origin; B: geographical origin; and C: raw material; Figure S3. Main effect plot; total polyphenol content (TPC) (a), antioxidant capacity (AC) (b). Capital letters represent a main factor, where BO: botanical origin; GO: geographical origin; and RM: raw material; Figure S4. Pareto chart in raw material honey: (a) total polyphenol content, (b) antioxidant capacity; Figure S5. Pareto chart in raw material flower: (a) total polyphenol content, (b) antioxidant capacity; Figure S6. Representative chromatogram used for the analysis of vitexin. (A) Extracted ion chromatogram for Gymnopodium floribundum flower from Acanceh; (B) Extracted ion chromatogram for Gymnopodium floribundum honey from Acanceh; (C) Extracted ion chromatogram for Gymnopodium floribundum flower from Tahdziu; (D) Extracted ion chromatogram for Gymnopodium floribundum honey from Tahdziu; Figure S7. Representative chromatogram used for the analysis of vanillin. (A) Extracted ion chromatogram for Piscidia piscipula flower from Acanceh; (B) Extracted ion chromatogram for Piscidia piscipula honey from Acanceh; (C) Extracted ion chromatogram for Piscidia piscipula flower from Tahdziu; (D) Extracted ion chromatogram for Piscidia piscipula honey from Tahdziu; Table S1. List of metabolites putatively annotated using spectral libraries and in silico predictions in honey; Table S2. List of metabolites putatively annotated using spectral libraries and in-silico predictions in flour flower.
Author Contributions
Conceptualization, I.M.R.-B. and A.E.M.-O.; methodology, I.M.R.-B., A.M.-U. and R.C.-C.; software, A.M.-U., R.C.-C., A.E.M.-O. and K.A.A.-B.; validation, I.M.R.-B., M.O.R.-S. and A.M.-U.; formal analysis, I.M.R.-B.; investigation, I.M.R.-B., A.E.M.-O., A.M.-U., R.C.-C., A.U.-V. and K.A.A.-B.; resources, I.M.R.-B.; data curation, I.M.R.-B., M.O.R.-S. and A.M.-U.; writing—original draft preparation A.E.M.-O. and K.A.A.-B.; writing—review and editing, I.M.R.-B., M.O.R.-S., A.M.-U., R.C.-C. and K.A.A.-B.; visualization, I.M.R.-B.; supervision, I.M.R.-B.; project administration, I.M.R.-B.; funding acquisition, I.M.R.-B. All authors have read and agreed to the published version of the manuscript.
Funding
The National Council of Humanities, Sciences, and Technologies of Mexico (CONAHCYT) which financed scholarship 1192863 for Andrea Elizabeth Mendoza-Osorno and scholarship 661099 for Kevin Alejandro Avilés-Betanzos. A.M.-U. thanks, CONAHCYT for grant F0001-2020-02-314964.
Data Availability Statement
The parameters and data for GNPS-derived analysis are available at the following links: (a) classical molecular networking/spectral matching, https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=2d10247511654d2eaaeef0ba768bde5f (accessed on 25 August 2023) (b) MolDiscovery, https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=ed9f60679c504868b7cfc7f119e867bd (accessed on 25 August 2023); (c) Dereplicator+, https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=ee920092b09a4b0cb5db9ee734727e89 (accessed on 25 August 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carnevali, F.C.G.; Tapia–Muñoz, J.L.; Duno de Stefano, R.; Ramírez Morillo, I. (Eds.) Flora Ilustrada de la Penín-sula de Yucatán: Listado Florístico; Centro de Investigación Científica de Yucatán, A.C: Mérida, Mexico, 2010; p. 328. [Google Scholar]
- Gobierno de México. Yucatán Se Encuentra Entre Los Principales Productores de Miel Del País. Available online: https://www.gob.mx/agricultura/yucatan/articulos/yucatan-se-encuentra-entre-los-principales-productores-de-miel-del-pais?idiom=es (accessed on 14 August 2023).
- Rogel, F.J.; Carlos, E.G.; Juan Manuel, R.; Álvarez, G. La Apicultura En La Península de Yucatán. Actividad de Subsistencia En un Entorno Globalizado. Rev. Mex. Caribe 2003, 8, 16. [Google Scholar]
- Cairns, C.E.; Villanueva-Gutiérrez, R.; Koptur, S.; Bray, D.B. Bee Populations, Forest Disturbance, and Africanization in Mexico. Biotropica J. Biol. Conserv. 2005, 37, 686–692. [Google Scholar] [CrossRef]
- Santiago, C.B.; Sosa, J.C.; Díaz, A.R.; Trejo, R.N.; May, D.C. Estudio de la flora presente en apiarios de tres municipios en el estado de Yucatán, México. Polibotánica 2022, 3, 1–15. [Google Scholar] [CrossRef]
- Arteaga-Hernández, V.; González-Ávila, M.; Cauich-Rodríguez, J.V. Compuestos fenólicos y actividad antioxidante de plantas de la península de Yucatán. Rev. U.D.C.A. Actual. Divulg. Científica 2014, 17, 29–38. [Google Scholar]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Actividad anti-oxidante y contenido fenólico total de extractos de hojas de doce especies de plantas seleccionadas de la Península de Yucatán, México. Rev. Mex. Cienc. Forest. 2011, 2, 7–18. [Google Scholar]
- Stefano, R.D.; Morillo, I.M.; Tapia-Muñoz, J.L.; Hernández-Aguilar, S.; Can, L.L.; Cetzal-Ix, W.; Méndez-Jiménez, N.; Zamora-Crescencio, P.; Gutiérrez-Báez, C.; Carnevali-Fernaández-Concha , G. Aspectos generales de la flora vascular de la Península de Yucatán, México. Bot. Sci. 2018, 96, 512–532. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Pérez-Sarabia, J.E.; Duno de Stefano, R.; Carnevali Fernández-Concha, G.; Ramírez Morillo, I.; Méndez-Jiménez, N.; Zamo-ra-Crescencio, P.; Gutierrez-Báez, C.; Cetzal-Ix, W. El conocimiento florístico de la Península de Yucatán, México, actualización y colecciones botánicas. In Proceedings of the XX Congreso Mexicano de Botánica, Ciudad de, Mexico, Mexico, 4–9 September 2016. [Google Scholar]
- Rosado-Vallado, M.; Brito-Loeza, W.; Mena-Rejón, G.; Quintero-Marmol, E.; Flores-Guido, J. Antimicrobial activity of Fabaceae species used in Yucatan traditional medicine. Fitoterapia 2000, 71, 570–573. [Google Scholar] [CrossRef]
- Canché-Collí, C.; Jiménez, L.N.L.; Rodríguez, R.; Canto, A. El jabín y los secretos de su néctar. Ecofronteras 2022, 26, 2–5. [Google Scholar]
- Zamora Crescencio, P.; Flores Guido, J.S.; Ruenes Morales, R. Flora útil y su manejo en el cono sur del estado de Yucatán, México. Polibotánica 2009, 28, 227–250. [Google Scholar]
- Villanueva-Gutiérrez, R.; Moguel-Ordóñez, Y.B.; Echazarreta-González, C.M.; Arana-López, G. Monofloral honeys in the Yucatán Peninsula, Mexico. Grana 2009, 48, 214–223. [Google Scholar] [CrossRef]
- Escobar-Rivera, P.; González-Mujica, F.; Rodríguez-Amado, J.; lejandro Cruz Sánchez, T. Composición química, actividades antioxidantes y antiinflamatorias de la corteza de Piscidia piscipula L. Aliment. Chem. Toxicol. 2019, 123, 476–483. [Google Scholar] [CrossRef]
- Hernández-Moreno, L.V.; Salazar, J.R.; Pabón, L.C.; Hernández-Rodríguez, P. Antioxidant activity and quantification of phenols and flavonoids of Colombian plants used in urinary tract infections. Rev. U.D.C.A Actual Divulg. Cient. 2022, 25, 1–7. Available online: http://www.scielo.org.co/pdf/rudca/v25n1/0123-4226-rudca-25-01-e1690.pdf (accessed on 23 August 2023).
- Mohtashami, L.; Amiri, M.S.; Ayati, Z.; Ramezani, M.; Jamialahmadi, T.; Emami, S.A.; Sahebkar, A. Ethnobotanical uses, phytochemistry and pharmacology of different Rheum Species (Polygonaceae): A. Review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Advances in Experimental Medicine and Biology Series; Springer: Cham, Switzerland, 2021; Volume 1308, pp. 1–22. [Google Scholar] [CrossRef]
- Moguel-Ordóñez, Y.; Echazarreta-Gonzalez, C.; Mora-Escobedo, R. Fisicoquímica de la miel de abeja Apis mellifera producida en el estado de Yucatán durante diferentes etapas del proceso de producción y tipos de floración. Técnica Pecu. México 2005, 43, 323–334. [Google Scholar]
- Ortíz-Ocampo, G.I.; Sandoval-Castro, C.A.; González-Pech, P.G.; Mancilla-Montelongo, G.; Ventura-Cordero, J.; Castañeda-Ramírez, G.S.; Tun-Garrido, J.; Torres-Acosta, J.F.d.J. Month of Harvest and Leaf Age Impact the Bromatological Composition and Polyphenol Content of Gymnopodium floribundum Rolfe Leaves. Agriculture 2022, 12, 1110. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.; Sosa-Moguel, O.; Ortiz-Vázquez, E.; Sauri-Duch, E.; Pino, A. Volatile constituents of tzizilché flower (Gymnopodium floribundum Rolfe) from Yucatán Peninsula, Mexico. J. Essent. Oil Res. 2012, 24, 359–361. [Google Scholar] [CrossRef]
- Ortíz-Ocampo, G.I.; Sandoval-Castro, C.A.; González-Pech, P.G.; Mancilla-Montelongo, G.; Ventura-Cordero, J.; Castañeda-Ramírez, G.S.; Pérez, J.I.C.; Leal, C.C.; Torres-Acosta, J.F.d.J. El Mes de Cosecha y la Edad de la Hoja Impact. Rev. Mex. Cienc. Pecu. 2020, 12, 1289–1303. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- García-Lara, S.; Dzib, G.; Cetzal-Ix, W. Flora Melífera de la Península de Yucatán, México: Estrategia Para Incrementar la Pro-ducción de Miel en los Periodos de. CICY. Available online: https://www.cicy.mx/Documentos/CICY/Desde_Herbario/2019/2019-09-05-Cetzal-Noguera-Martinez-Flora-melifera-de-PY.pdf (accessed on 25 August 2023).
- Wang, J.; Qing, X.L. Chapter 3—Chemical Composition, Characterization, and Differentiation of Honey Botanical and Geo-Graphical Origins; Steve Taylor, L., Ed.; Advances in Food and Nutrition Research; Academic Press: New York, NY, USA, 2011; Volume 62, pp. 89–137. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Guo, P.; Deng, Q.; Lu, Q. Anti-alcoholic effects of honeys from different floral origins and their correlation with honey chemical compositions. Food Chem. 2019, 286, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Kortesniemi, M.; Rosenvald, S.; Laaksonen, O.; Vanag, A.; Ollikka, T.; Vene, K.; Yang, B. Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem. 2018, 246, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, A.; Spanedda, L.; Tuberoso, C.; Cabras, P. Solid-phase extraction and high-performance liquid chromatographic determination of organic acids in honey. J. Chromatogr. A 1994, 669, 59–64. [Google Scholar] [CrossRef]
- Toma´s-Barbera´n, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Toma´s-Barbera´n, F.A.; Garcı´a-Viguera, C.; Vit-Olivier, P.; Ferreres, F.; Toma´s-Lorente, F. Flavonoids in honey of different geographical origin. Z. Lebensm. Unters. Forsch. 1993, 196, 38–44. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Avilés-Betanzos, K.A.; Ramírez-Rivera, E.J.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants 2020, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Chel-Guerrero, L.D.; Oney-Montalvo, J.E.; Rodríguez-Buenfil, I.M. Phytochemical Characterization of By-Products of Habanero Pepper Grown in Two Different Types of Soils from Yucatán, Mexico. Plants 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Optimization of Spray-Drying Conditions of Microencapsulated Habanero Pepper (Capsicum chinense Jacq.) Extracts and Physicochemical Characterization of the Microcapsules. Polibotánica 2023, 11, 1238. [Google Scholar] [CrossRef]
- Contreras-Angulo, L.A.; Moreno-Ulloa, A.; Carballo-Castañeda, R.A.; León-Felix, J.; Romero-Quintana, J.G.; Aguilar-Medina, M.; Ramos-Payán, R.; Heredia, J.B. Metabolomic Analysis of Phytochemical Compounds from Agricultural Residues of Eggplant (Solanum melongena L.). Molecules 2022, 27, 7013. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant Activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to convert raw mass spectrometry data. Curr. Protoc. Bioinform. 2014, 46, 13.24.1–13.24.9. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Res-olution Mass Spectrometry: Communicating Confidence. Env. Sci Technol 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Guler, M.; Tagirdzhanov, A.; Lee, Y.-Y.; Gurevich, A.; Mohimani, H. MolDiscovery: Learning mass spectrometry fragmentation of small molecules. Nat. Commun. 2021, 12, 3718. [Google Scholar] [CrossRef] [PubMed]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.-F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Feunang, Y.D.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Chemin. 2016, 8, 61. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Ludwig, M.; Nothias, L.-F.; Dührkop, K.; Koester, I.; Fleischauer, M.; Hoffmann, M.A.; Petras, D.; Vargas, F.; Morsy, M.; Aluwihare, L.; et al. Database-independent molecular formula annotation using Gibbs sampling through ZODIAC. Nat. Mach. Intell. 2020, 2, 629–641. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.B.; Ghasemzadeh, N.C.D. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Bautista, F.; Zinck, J.A. Construction of an Yucatec Maya soil classification and comparison with the WRB framework. J. Ethnobiol. Ethnomed. 2010, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Zúñiga, F.; Jiménez-Osornio, J.; Navarro-Alberto, J.; Manu, A.; Lozano, R. Microrelieve y color del suelo como pro-piedades de diagnóstico en leptosoles cársticos. Tierra Latinoame-Ricana 2003, 21, 1–11. Available online: http://www.redalyc.org/pdf/573/57321101.pdf (accessed on 25 August 2023).
- Oney-Montalvo, J.; Uc-Varguez, A.; Ramírez-Rivera, E.; Ramírez-Sucre, M.; Rodríguez-buenfili, I.M. influence of soil composition on the profile and content of polyphenols in habanero peppers (capsicum Chinese Jacq). Agronomy 2020, 10, 1234. [Google Scholar] [CrossRef]
- Montalvo, J.E.O.; Madrigal, A.C.d.S.; Sucre, M.O.R.; Rodríguez-Buenfil, I.M. Effect of the Soil and Ripening Stage in Capsicum chinense var. Jaguar on the Content of Carotenoids and Vitamins. Horticulturae 2021, 7, 442. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef]
- Hussein, S.; El-Magly, U.; Tantawy, M.; Kawashty, S.; Saleh, N. Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt. Arab. J. Chem. 2017, 10, 76–81. [Google Scholar] [CrossRef]
- Hallmann, E. Quantitative and qualitative identification of bioactive compounds in edible flowers of black and bristly locust and their antioxidant activity. Biomolecules 2020, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Drouet, S.; Lorenzo, J.M.; Hano, C. Characterization of Bioactive Phenolics and Antioxidant Capacity of Edible Bean Extracts of 50 Fabaceae Populations Grown in Thailand. Foods 2021, 10, 3118. [Google Scholar] [CrossRef] [PubMed]
- Desta, K.T.; El-Aty, A.M.A. Millettia isoflavonoids: A comprehensive review of structural diversity, extraction, isolation, and pharmacological properties. Phytochem. Rev. 2023, 22, 275–308. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Abdel-Kader, M.S. Isoflavonoids. In Flavonoids—From Biosynthesis to Human Health; InTech: Mexico City, Mexico, 2017. [Google Scholar] [CrossRef]
- Araya-Cloutier, C.; den Besten, H.M.W.; Aisyah, S.; Gruppen, H.; Vincken, J.-P. The position of prenylation of isoflavonoids and stilbenoids from legumes (Fabaceae) modulates the antimicrobial activity against Gram positive pathogens. Food Chem. 2017, 226, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Robert, P.; Holgado, F.; Velasco, J.; Márquez-Ruiz, G. Antioxidant Activity and Kinetics Studies of Quercetin, Epicatechin and Naringenin in Bulk Methyl Linoleate. J. Am. Oil Chem. Soc. 2017, 94, 1189–1196. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. In Oxidative Medicine and Cellular Longevity; Hindawi Publishing Corporation: London, UK, 2015. [Google Scholar] [CrossRef]
- Shabir, I.; Pandey, V.K.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, L. Polyphenols as possible markers of botanical origin of honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Baira, E.; Iosifidou, S.; Bergele, K.; Manea-Karga, E.; Theologidis, I.; Barmpouni, T.; Tsipi, D.; Machera, K. Characterization of Ikaria Heather Honey by Untargeted Ultrahigh-Performance Liquid Chromatography-High Resolution Mass Spectrometry Metabolomics and Melissopalynological Analysis. Front. Chem. 2022, 10, 924881. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Lim, L.-Y.; Joshi, R.; Hammer, K.A.; Locher, C. A Comprehensive Survey of Phenolic Constituents Reported in Monofloral Honeys around the Globe. Foods 2022, 11, 1152. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Finotello, C.; Uddin, J.; Mammi, S.; Piana, L. Objective Definition of Monofloral and Polyfloral Honeys Based on NMR Metabolomic Profiling. J. Agric. Food Chem. 2016, 64, 3645–3652. [Google Scholar] [CrossRef]
- Deadman, B.J. The Flavonoid Profile of New Zealand Manuka Honey. Master Thesis, The University of Waikato, Hamilton, New Zealand, 2009; pp. 1–270. Available online: https://hdl.handle.net/10289/5443 (accessed on 1 March 2023).
- Kuś, P.; Jerković, I.; Jakovljević, M.; Jokić, S. Extraction of bioactive phenolics from black poplar (Populus nigra L.) buds by supercritical CO2 and its optimization by response surface methodology. J. Pharm. Biomed. Anal. 2018, 152, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Hermosín, I.; Chicón, R.M.; Cabezudo, M.D. Free amino acid composition and botanical origin of honey. Food Chem. 2003, 83, 263–268. [Google Scholar] [CrossRef]
- Dimins, F.; Cinkmanis, I.; Radenkovs, V.; Augspole, I.; Valdovska, A. Analysis of 18 Free Amino Acids in Honeybee and Bumblebee Honey from Eastern and Northern Europe and Central Asia Using HPLC-ESI-TQ-MS/MS Approach Bypassing Derivatization Step. Foods 2022, 11, 2744. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Kopuncová, M.; Ciesarová, Z.; Kukurová, K. Free amino acids profile of Polish and Slovak honeys based on LC–MS/MS method without the prior derivatisation. J. Food Sci. Technol. 2017, 54, 3716–3723. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).