New Insights into the Chemical Compatibility of Nitrochitosan with Potential Energetic Molecules
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Sample Preparation
2.2. Compatibility Assessment
3. Results and Discussion
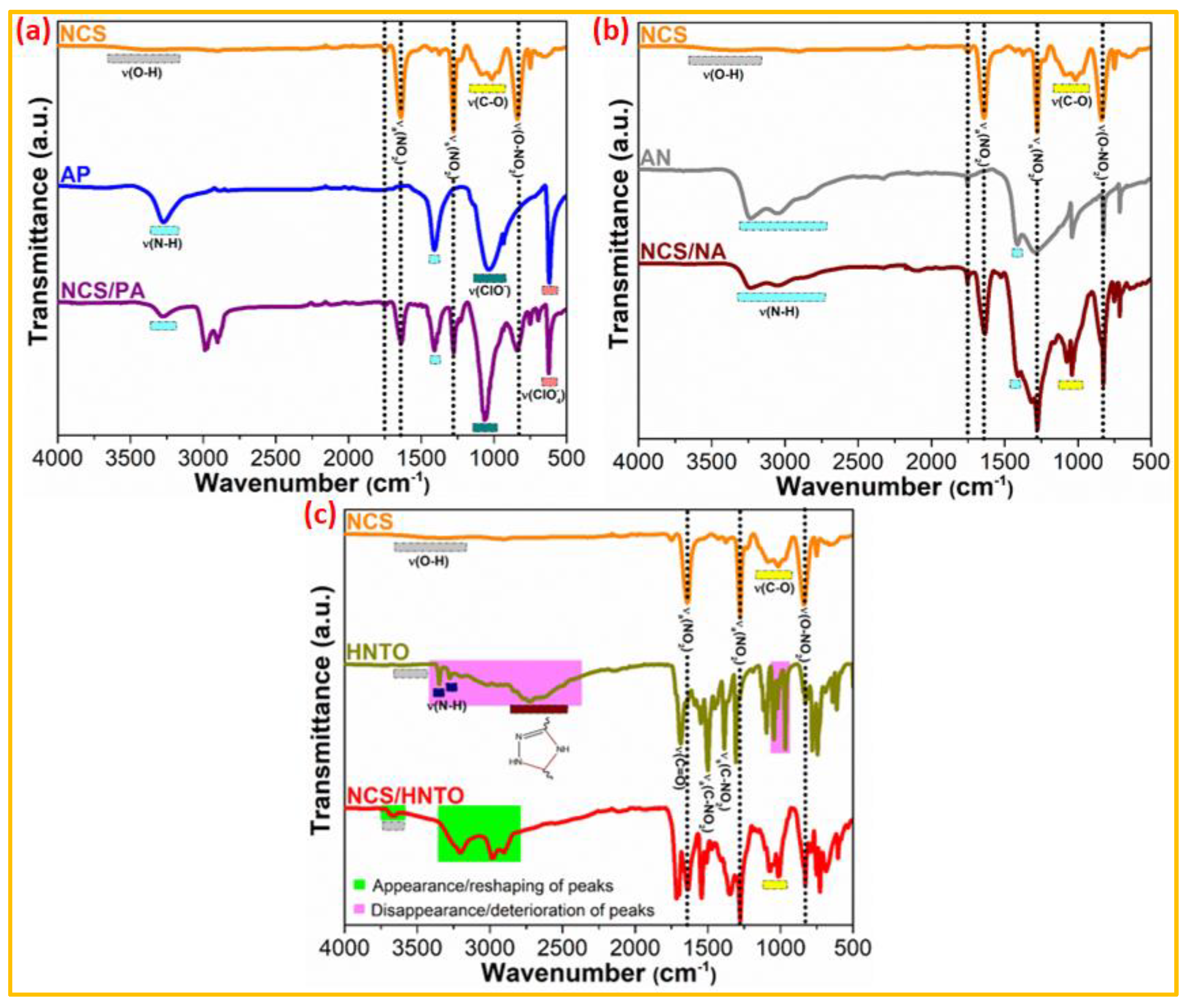
3.1. Non-Thermal Characterization
3.2. Thermal Analyses
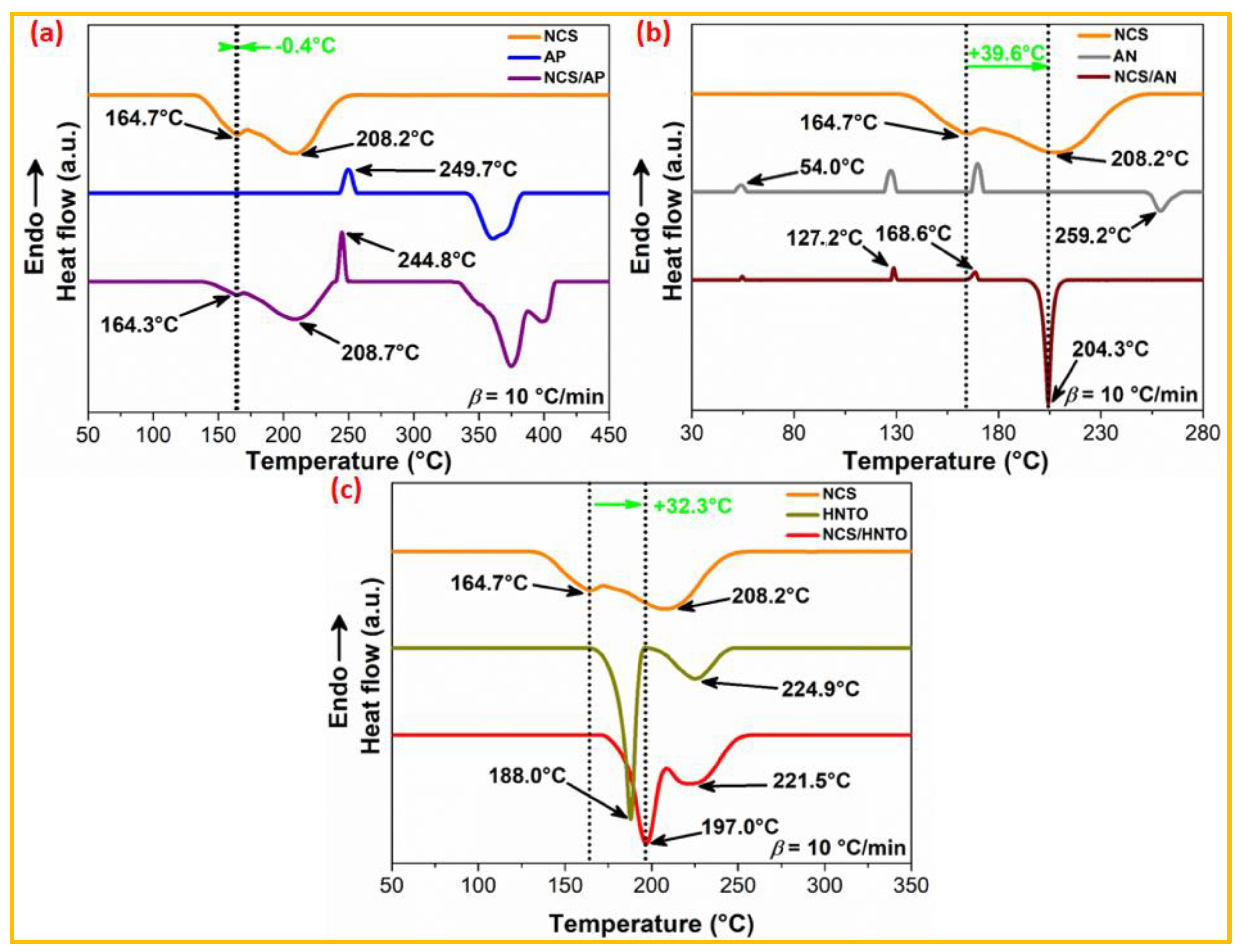
3.2.1. DSC-Based Compatibility
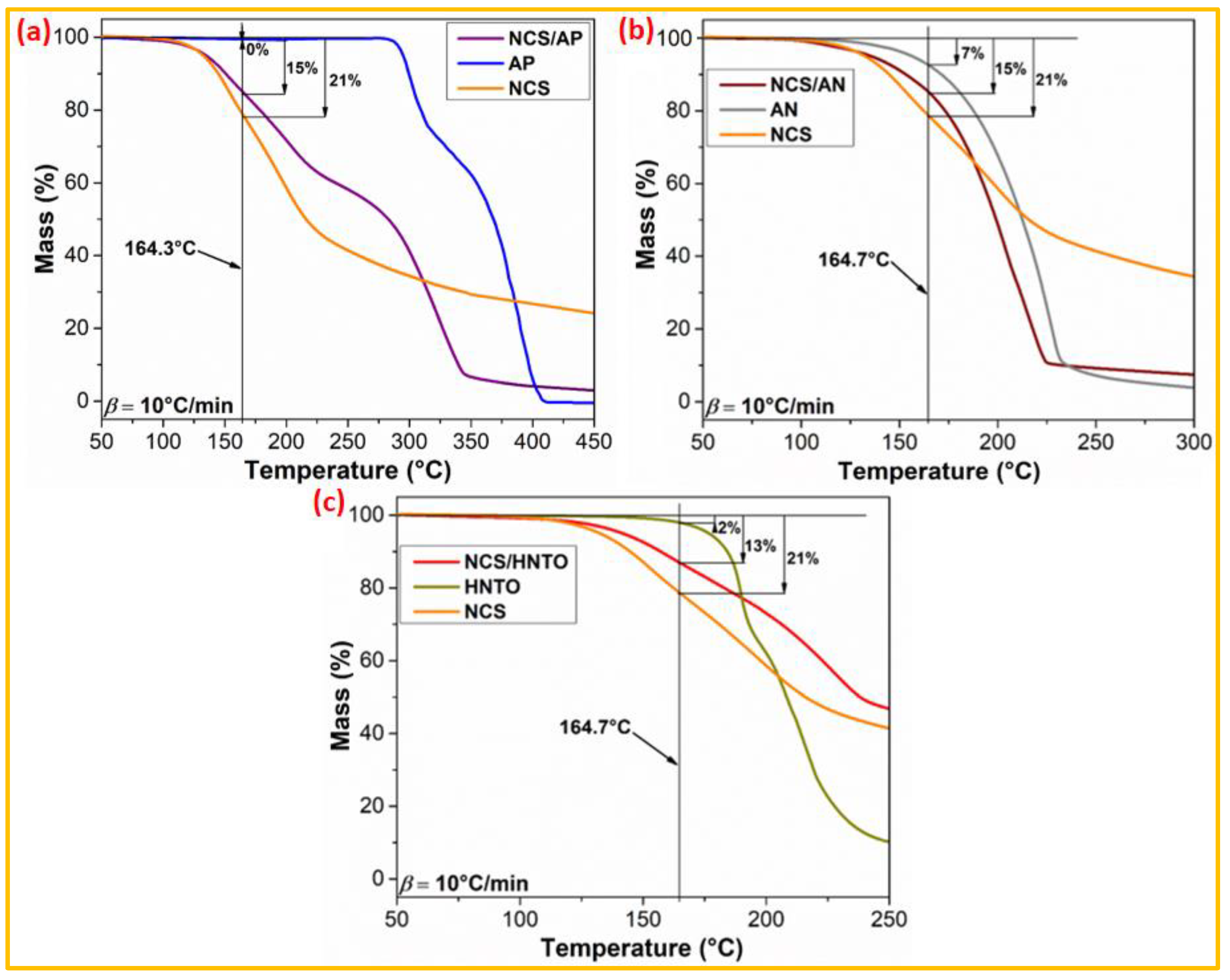
3.2.2. TGA-Based Compatibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anniyappan, M.; Talawar, M.B.; Sinha, R.K.; Murthy, K.P.S. Review on advanced energetic materials for insensitive munition formulations. Combust. Explos. Shock. Waves 2020, 56, 495–519. [Google Scholar] [CrossRef]
- Liu, R.; Wen, Y.; Pang, W. Advanced Energetic Materials: Testing and Modeling. Crystals 2023, 13, 1100. [Google Scholar] [CrossRef]
- Pang, W.; DeLuca, L.T. Nano and Micro-Scale Energetic Materials: Propellants and Explosives; John Wiley & Sons: Weinheim, Germany, 2023. [Google Scholar]
- Sabatini, J.J.; Johnson, E.C. A short review of nitric esters and their role in energetic materials. ACS Omega 2021, 6, 11813–11821. [Google Scholar] [CrossRef]
- Mattar, H.; Baz, Z.; Saleh, A.; Shalaby, A.S.; Azzazy, A.E.; Salah, H.; Ismail, I. Nitrocellulose: Structure, synthesis, characterization, and applications. Water Energy Food Environ. J. 2020, 3, 1–15. [Google Scholar]
- Wang, Y.; Luo, T.; Song, X.; Li, F. Electrospinning Preparation of NC/GAP/Submicron-HNS Energetic Composite Fiber and its Properties. ACS Omega 2019, 4, 14261–14271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, Z.; Yuan, J.; Jia, S.; Wang, F.; Li, Y.; Guo, B.; Yang, Z.; Zuo, Y. Structure and Properties of Chain Branched Nitrocellulose. Cent. Eur. J. Energetic Mater. 2021, 18, 448–476. [Google Scholar] [CrossRef]
- Jain, S.; Chakraborty, S.; Qiao, L. Burn rate enhancement of ammonium perchlorate–nitrocellulose composite solid propellant using copper oxide–graphene foam micro-structures. Combust. Flame 2019, 206, 282–291. [Google Scholar] [CrossRef]
- Boukeciat, H.; Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Hamada, R.A.; Bouhantala, A.; Bousstila, C.; Hanafi, S.; Dourari, M.; Klapötke, T.M. Towards Investigating the Effect of Ammonium Nitrate on the Characteristics and Thermal Decomposition Behavior of Energetic Double Base NC/DEGDN Composite. Materials 2022, 15, 8138. [Google Scholar] [CrossRef]
- Yi, J.H.; Zhao, F.Q.; Ren, Y.H.; Xu, S.Y.; Ma, H.X.; Hu, R.Z. Thermal decomposition mechanism and quantum chemical investigation of hydrazine 3-nitro-1,2,4-triazol-5-one (HNTO). J. Therm. Anal. Calorim. 2010, 100, 623–627. [Google Scholar] [CrossRef]
- Chen, L.; Nan, F.; Li, Q.; Zhang, J.; Jin, G.; Wang, M.; Cao, X.; Liu, J.; He, W. Sol–gel synthesis of insensitive nitrated bacterial cellulose/cyclotrimethylenetrinitramine nano-energetic composites and its thermal decomposition property. Cellulose 2022, 29, 7331–7351. [Google Scholar] [CrossRef]
- Pu, C.; Yi, M.; Luan, Y.; Xiao, Z. A balanced strategy for energy level, mechanical properties and sensitivity of the gun propellants based on step ladder-structured nitrocellulose. Cellulose 2023, 30, 2367–2384. [Google Scholar] [CrossRef]
- Pu, C.K.; Luan, Y.; Yi, M.J.; Xiao, Z.G. Investigation on thermal characteristics and desensitization mechanism of improved step ladder-structured nitrocellulose. Def. Technol. 2023, 20, 93–102. [Google Scholar] [CrossRef]
- Gismatulina, Y.A. Promising Energetic Polymers from Nanostructured Bacterial Cellulose. Polymers 2023, 15, 2213. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, L.; Huang, J.; Meng, D.; Nan, F.; He, W. Electrostatic spraying construction strategy and performance of NGEC-based core-shell nanoenergetic material. FirePhysChem 2023, 3, 48–53. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Krumm, B.; Mezroua, A.; Derradji, M.; Bessa, W. Design and characterization of new advanced energetic biopolymers based on surface functionalized cellulosic materials. Cellulose 2021, 28, 6107–6123. [Google Scholar] [CrossRef]
- Gozin, M.; Fershtat, L.L. Recent Advances in Chemistry of Nitrogen-Rich Energetic Polymers and Plasticizers. Nitrogen-Rich Energetic Mater. 2023, 189–238. [Google Scholar]
- Tarchoun, A.F.; Trache, D.; Hamouche, M.A.; Abdelaziz, A.; Boukeciat, H.; Chentir, I.; Touidjine, S.; Klapötke, T.M. Elucidating the characteristics of a promising nitrate ester polysaccharide derived from shrimp shells and its blends with cellulose nitrate. Cellulose 2023, 30, 4941–4955. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Hamouche, M.A.; Bessa, W.; Abdelaziz, A.; Boukeciat, H.; Bekhouche, S.; Belmehdi, D. Insights into characteristics and thermokinetic behavior of potential energy-rich polysaccharide based on chitosan. Cellulose 2022, 29, 8085–8101. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Xu, K. High-Substitute Nitrochitosan Used as Energetic Materials: Preparation and Detonation Properties. Carbohydr. Polym. 2020, 237, 116176. [Google Scholar] [CrossRef]
- Wan, C.; Guo, Z.; Zhang, W.; Chen, S.; Qin, Z.; Xu, K. A new ternary high-energy composite based on nano titanium powder with low sensitivity and stable combustion. Combust. Flame 2023, 247, 112480. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Li, F. Thermal behavior and decomposition mechanism of ammonium perchlorate and ammonium nitrate in the presence of nanometer triaminoguanidine nitrate. ACS Omega 2019, 4, 214–225. [Google Scholar] [CrossRef]
- Lv, M.; Qian, H.; Dong, Z.; Ye, Z. Compatibility study of erythritol tetranitrate with some energetic materials. J. Therm. Anal. Calorim. 2023, 148, 711–719. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Pan, J.; Wang, W.; Pan, R.; Zhu, W. Synthesis, Characterization and Compatibility Studies of Poly (DFAMO/NIMMO) with Propellant and PBX Ingredients. Cent. Eur. J. Energetic Mater. 2018, 15, 85–99. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Phan, D.N.; Nguyen, D.C.; Do, V.T.; Bach, L.G. The chemical compatibility and adhesion of energetic materials with several polymers and binders: An experimental study. Polymers 2018, 10, 1396. [Google Scholar] [CrossRef]
- Malik, S.; Singh, A.; Kumar, R.; Soni, P.K.; Kaur, A. Compatibility and thermokinetics studies of 4-amino triazolium picrate with various polymers. J. Therm. Anal. Calorim. 2023, 148, 6371–6387. [Google Scholar] [CrossRef]
- B08-ATT 0011-4147; Chemical Compatibility of Ammunition Components with Explosives (Non-Nuclear Applications), 2nd ed. NATO Military Agency for Standardization: Brussels, Belgium, 2001; pp. 12–16.
- Gołofit, T.; Zakościelny, B.; Maksimowski, P.; Chmielarek, M.; Cieślak, K.; Sałaciński, T. Review of Methods for Testing the Compatibility of High Energy Mixed Components. Cent. Eur. J. Energetic Mater. 2021, 18, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Tarchoun, A.F.; Chelouche, S.; Khimeche, K. New insights on the compatibility of nitrocellulose with aniline-based compounds. Propellants Explos. Pyrotech. 2019, 44, 970–979. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, N.; Zhang, T.; Gong, H.; Ma, H.; An, T.; Zhao, F.; Hu, R. Compatibility and thermal decomposition mechanism of nitrocellulose/Cr2O3 nanoparticles studied using DSC and TG-FTIR. RSC Adv 2019, 9, 3927–3937. [Google Scholar] [CrossRef]
- Chalghoum, F.; Trache, D.; Benziane, M.; Chelouche, S. Effect of complex metal hydride on the thermal decomposition behavior of AP/HTPB-based aluminized solid rocket propellant. J. Therm. Anal. Calorim. 2022, 147, 11507–11534. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Cao, X.; Hu, L.; Deng, P.; Xie, Z. Ammonium perchlorate-based molecular perovskite energetic materials: Preparation, characterization, and thermal catalysis performance with MoS2. J. Energetic Mater. 2020, 38, 162–169. [Google Scholar] [CrossRef]
- Suppajariyawat, P.; Elie, M.; Baron, M.; Gonzalez-Rodriguez, J. Classification of ANFO samples based on their fuel composition by GC–MS and FTIR combined with chemometrics. Forensic Sci. Int. 2019, 301, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.H.; Zhao, F.Q.; Gao, H.X.; Xu, S.Y.; Wang, M.C. Preparation, characterization, non-isothermal reaction kinetics, thermodynamic properties, and safety performances of high nitrogen compound: Hydrazine 3-nitro-1,2,4-triazol-5-one complex. J. Hazard. Mater. 2008, 153, 261–268. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, Z.; Yi, J.; Chen, S.; Xu, K. Laser ignition and combustion properties of composite with high-substituted nitrochitosan and nano-Ti powder. Combust. Flame 2022, 240, 112056. [Google Scholar] [CrossRef]
- Gong, L.; Guo, Y.; Meng, L.; Li, J.; Yang, R. Kinetics model reconstruction for multistep overlapping thermal decomposition of ammonium perchlorate with and without the copper oxide compound catalyst. Combust. Sci. Technol. 2021, 193, 2856–2871. [Google Scholar] [CrossRef]
- Nourine, M.; Boulkadid, M.K.; Touidjine, S.; Akbi, H.; Belkhiri, S. Preparation of surface modified ammonium perchlorate with green iron oxide nitrocellulose nanocomposite for catalytic thermal decomposition of ammonium perchlorate. Mater. Chem. Phys. 2023, 303, 127784. [Google Scholar] [CrossRef]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.; Gao, H.; Fu, W.; Li, Y.; Tang, L.; Zhou, Z. Promising hydrazinium 3-Nitro-1,2,4-triazol-5-one and its analogs. J. Mater. Sci. 2016, 51, 10849–10862. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarchoun, A.F.; Trache, D.; Hamouche, M.A.; Abdelaziz, A.; Chelouche, S.; Boukeciat, H.; Klapötke, T.M. New Insights into the Chemical Compatibility of Nitrochitosan with Potential Energetic Molecules. Processes 2023, 11, 3060. https://doi.org/10.3390/pr11113060
Tarchoun AF, Trache D, Hamouche MA, Abdelaziz A, Chelouche S, Boukeciat H, Klapötke TM. New Insights into the Chemical Compatibility of Nitrochitosan with Potential Energetic Molecules. Processes. 2023; 11(11):3060. https://doi.org/10.3390/pr11113060
Chicago/Turabian StyleTarchoun, Ahmed Fouzi, Djalal Trache, Mohamed Abderrahim Hamouche, Amir Abdelaziz, Salim Chelouche, Hani Boukeciat, and Thomas M. Klapötke. 2023. "New Insights into the Chemical Compatibility of Nitrochitosan with Potential Energetic Molecules" Processes 11, no. 11: 3060. https://doi.org/10.3390/pr11113060





