Removal of Pyridine from Aqueous Solutions Using Lignite, Coking Coal, and Anthracite: Adsorption Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Analysis of Test Samples
2.3. Batch Adsorption Studies
2.4. The UV Analysis Method
2.5. Adsorption Models
2.5.1. Pseudo-First-Order Kinetic Equation
2.5.2. Pseudo-Second-Order Kinetic Equation
2.5.3. The Intraparticle Diffusion Model
2.5.4. Bangham Model
3. Results
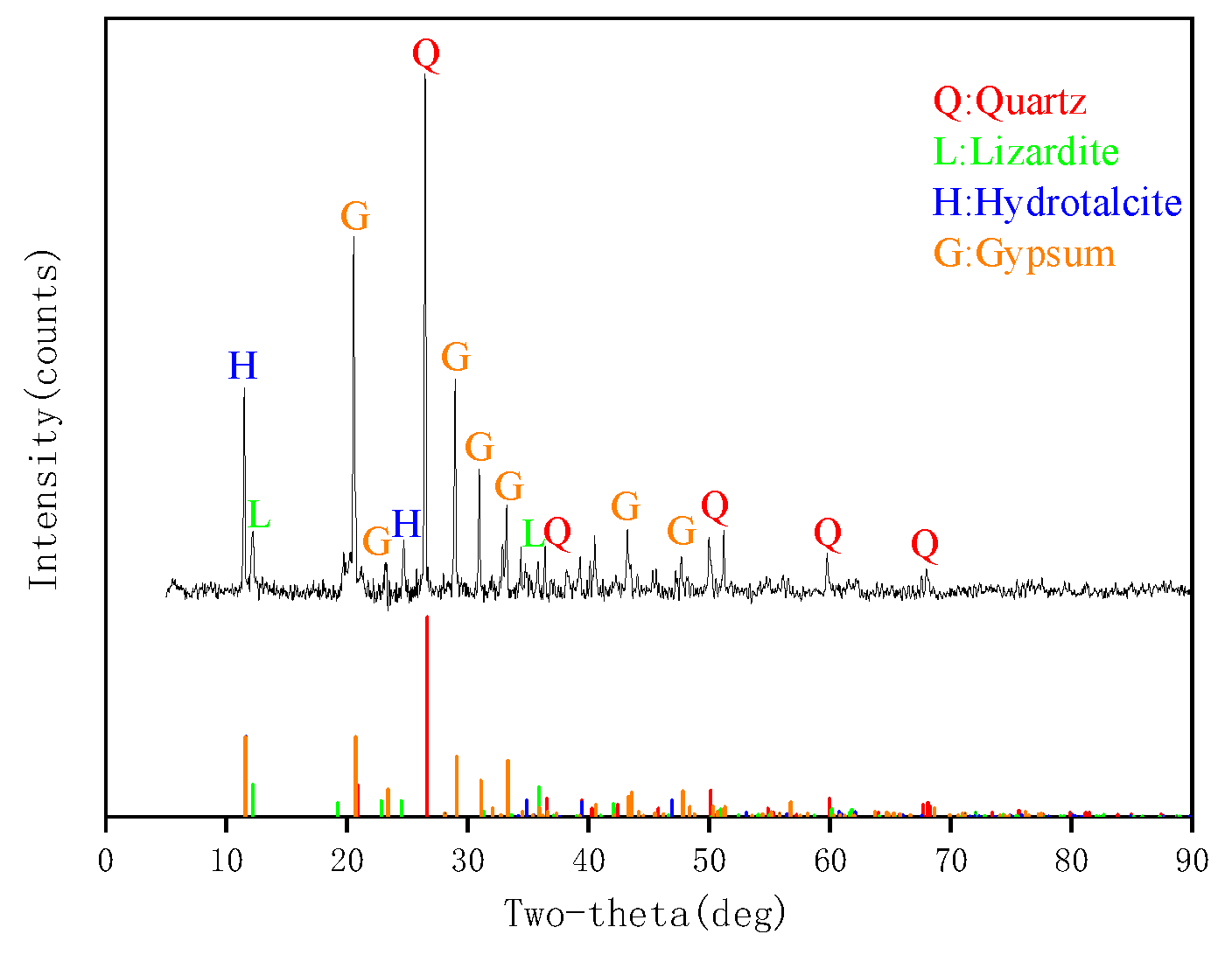
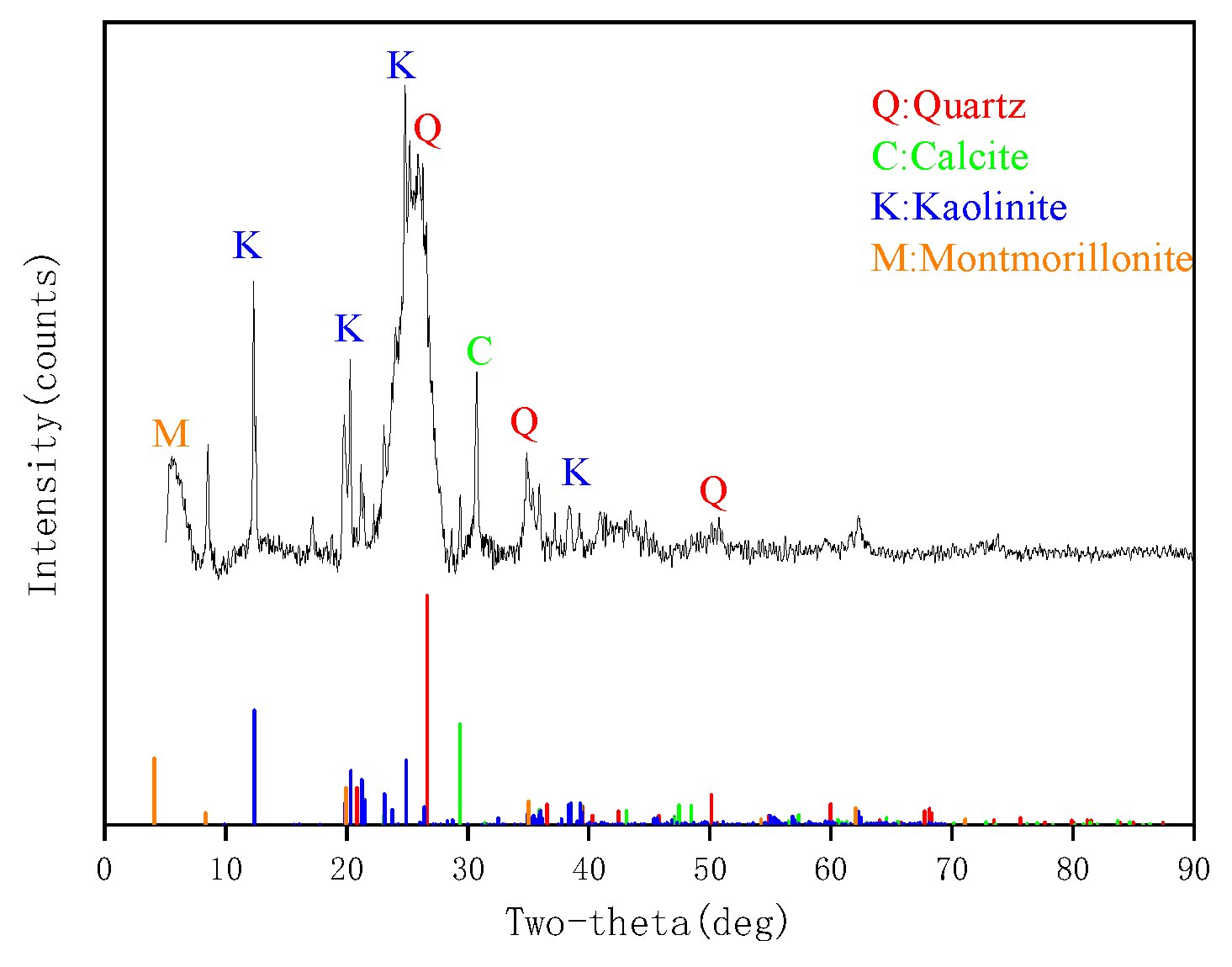
3.1. Composition of Lignite, Coking Coal, and Anthracite
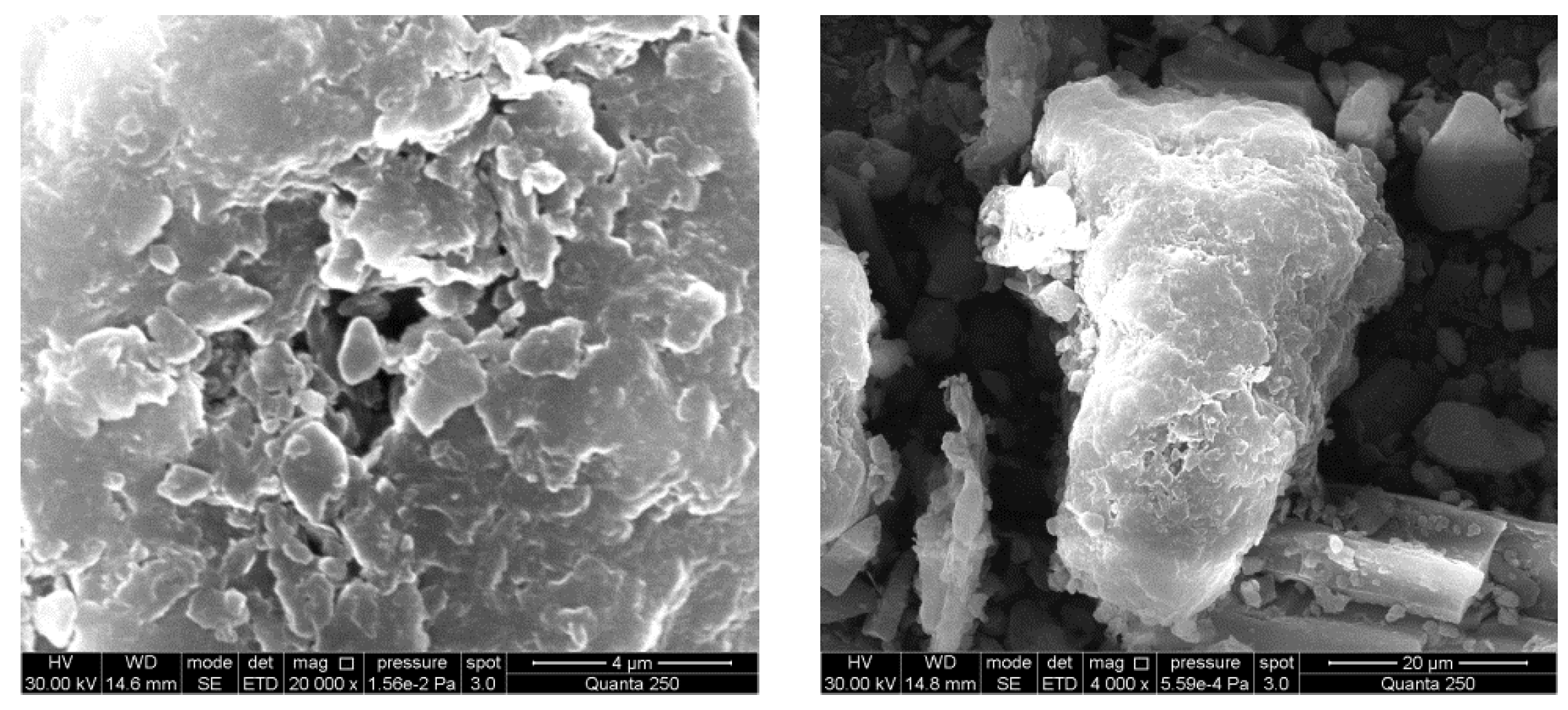
3.2. SEM Analysis of Adsorbents
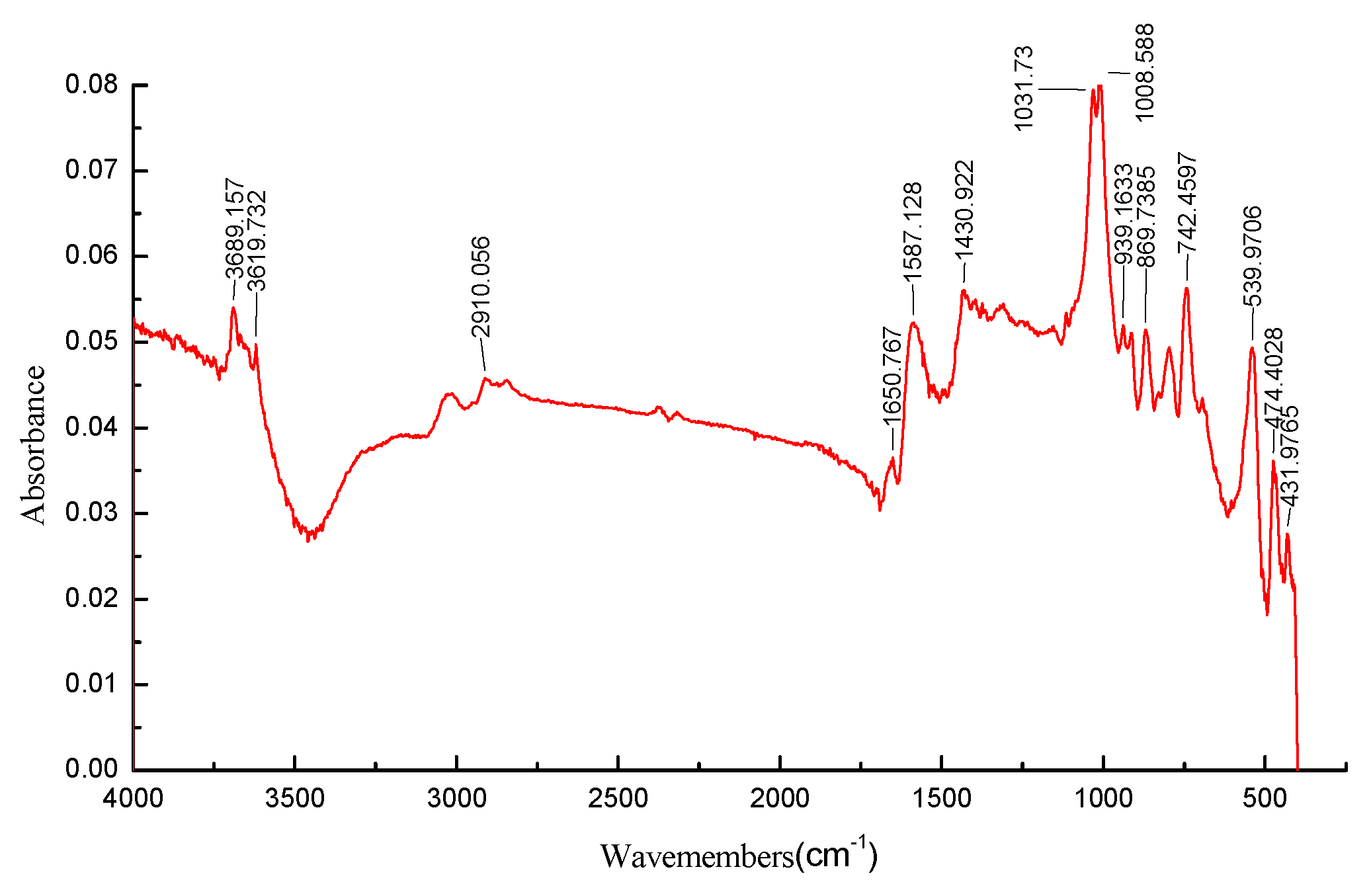
3.3. FTIR Analysis
3.4. The Specific Surface Area
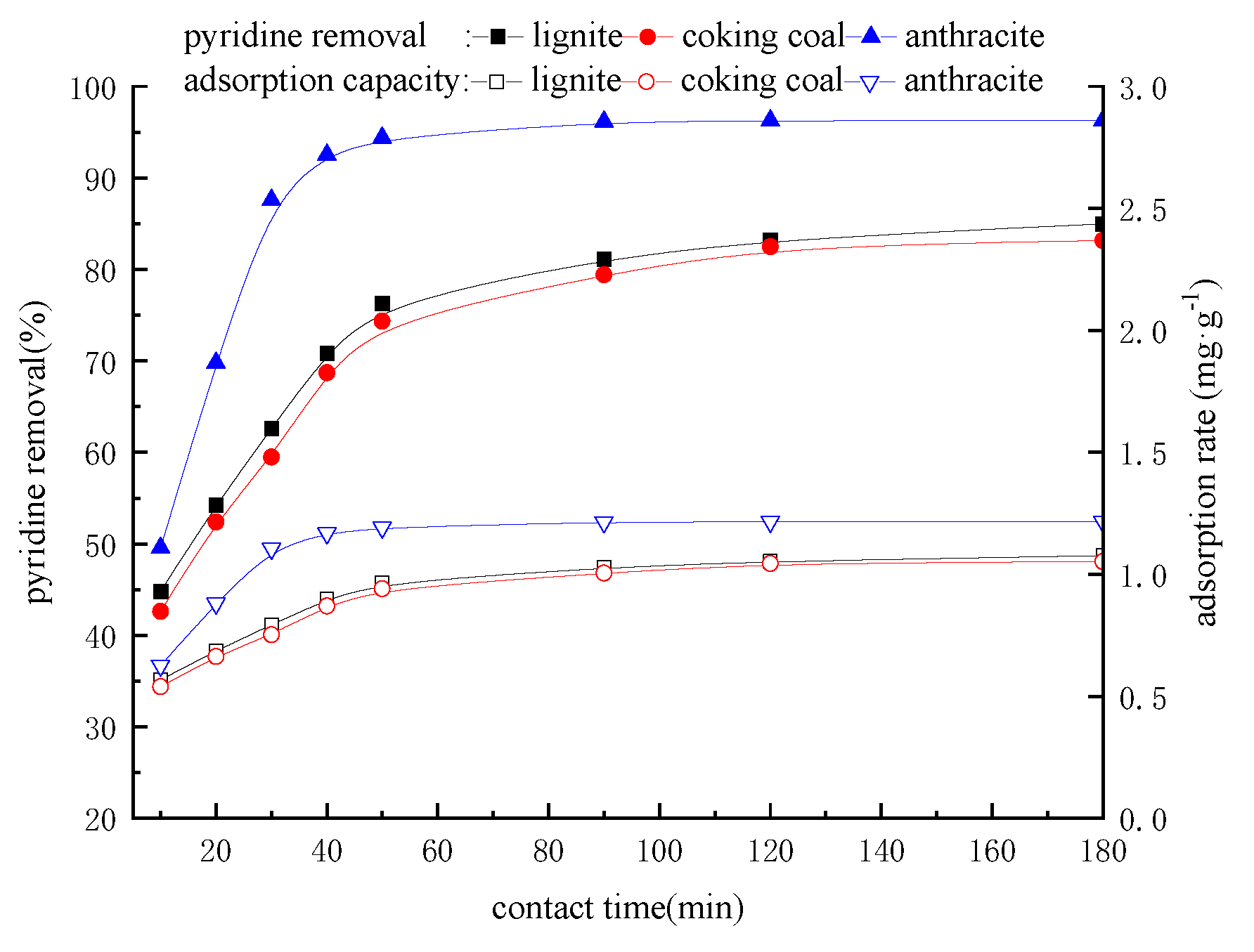
3.5. Effect of Contact Time
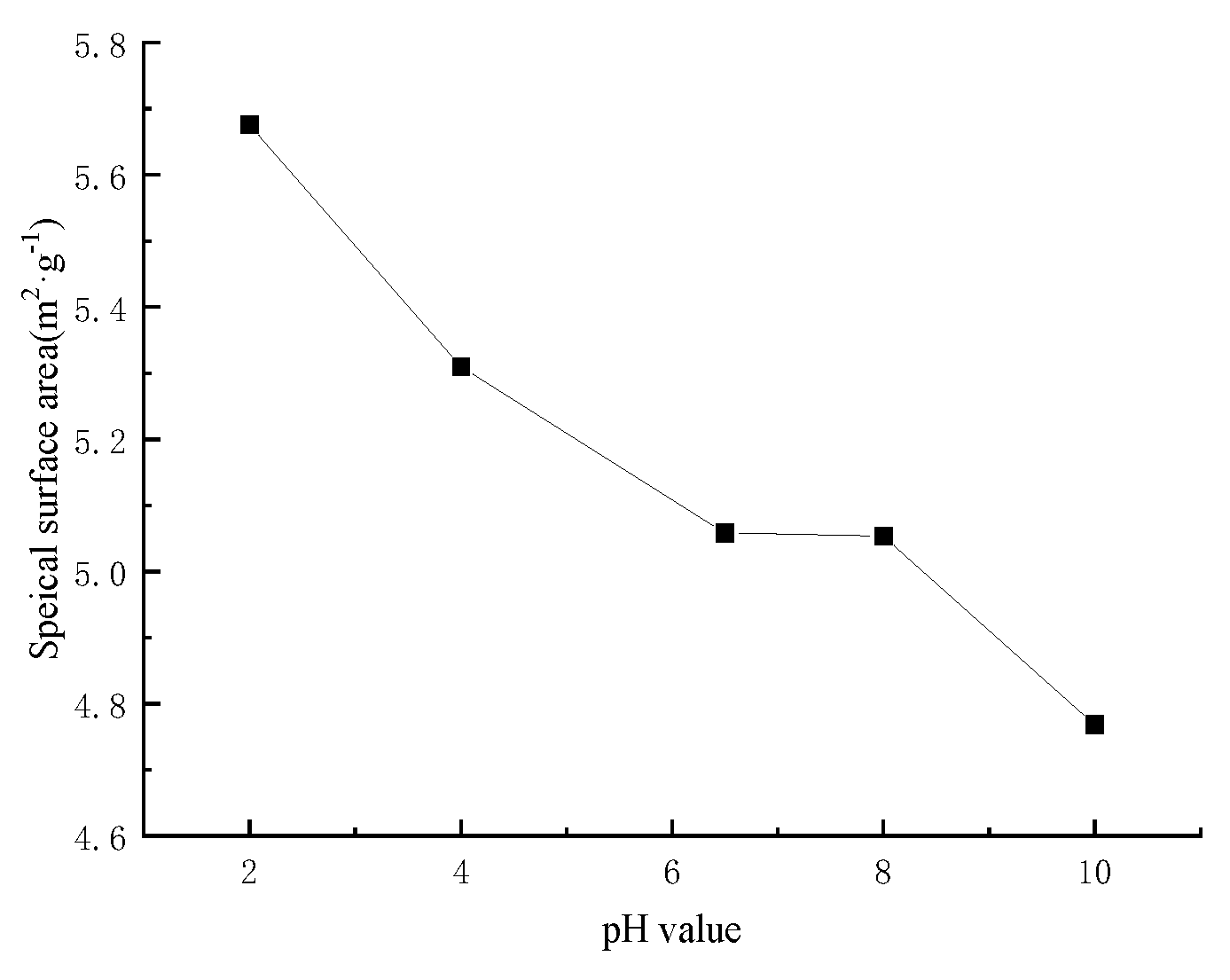
3.6. Effect of pH on Specific Surface Area
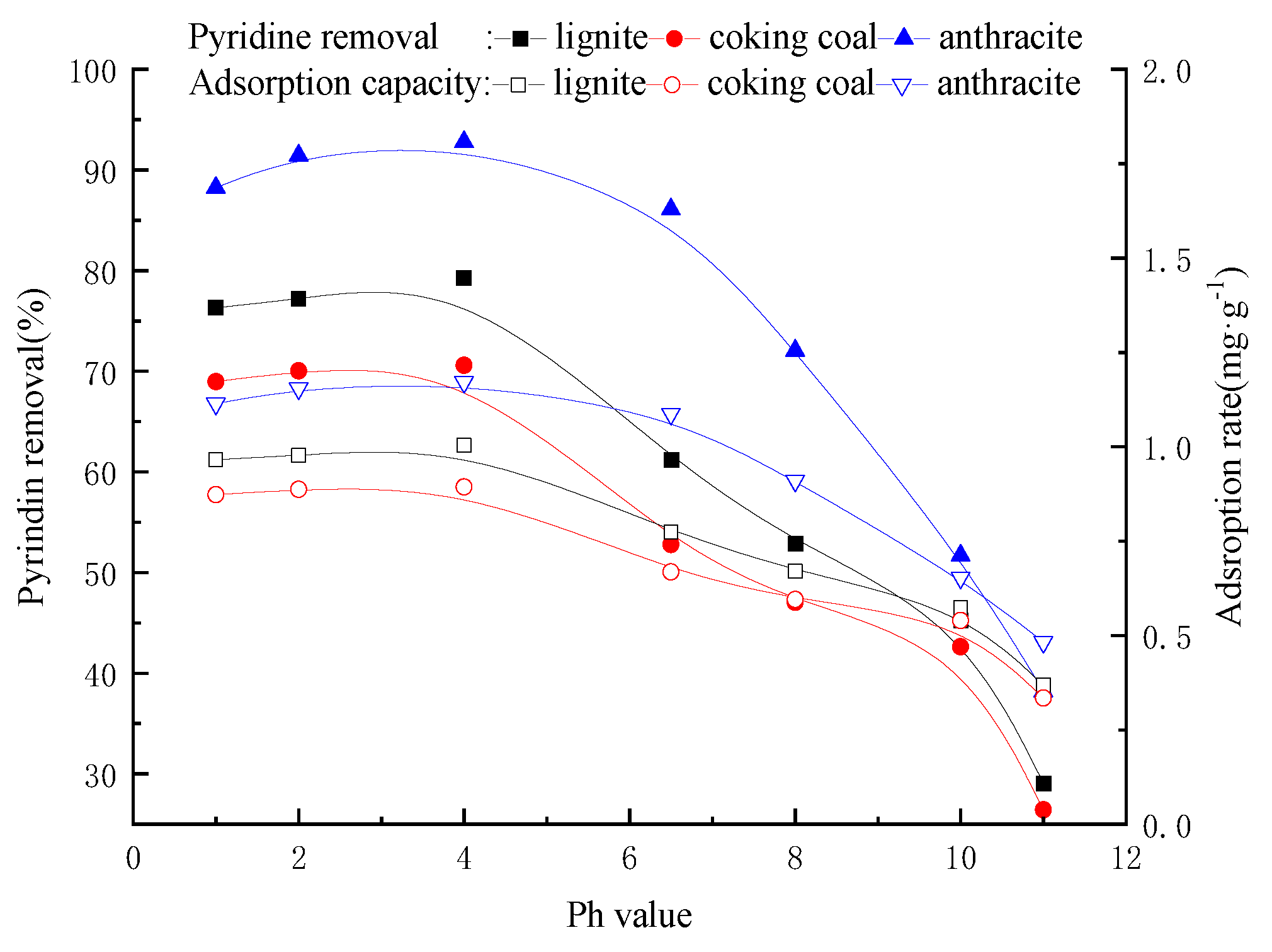
3.7. Effect of pH on Coal Adsorption
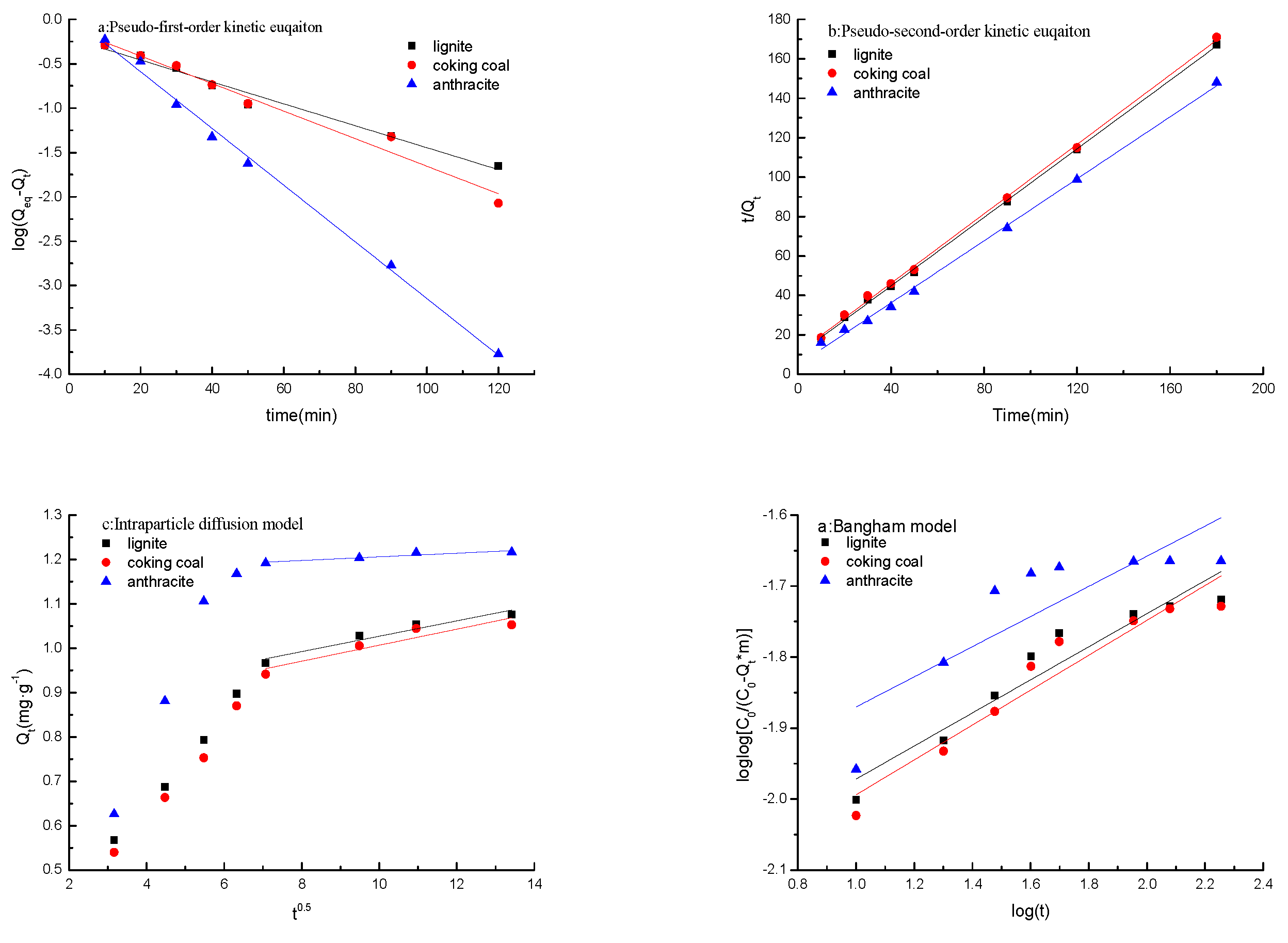
3.8. Kinetics Models
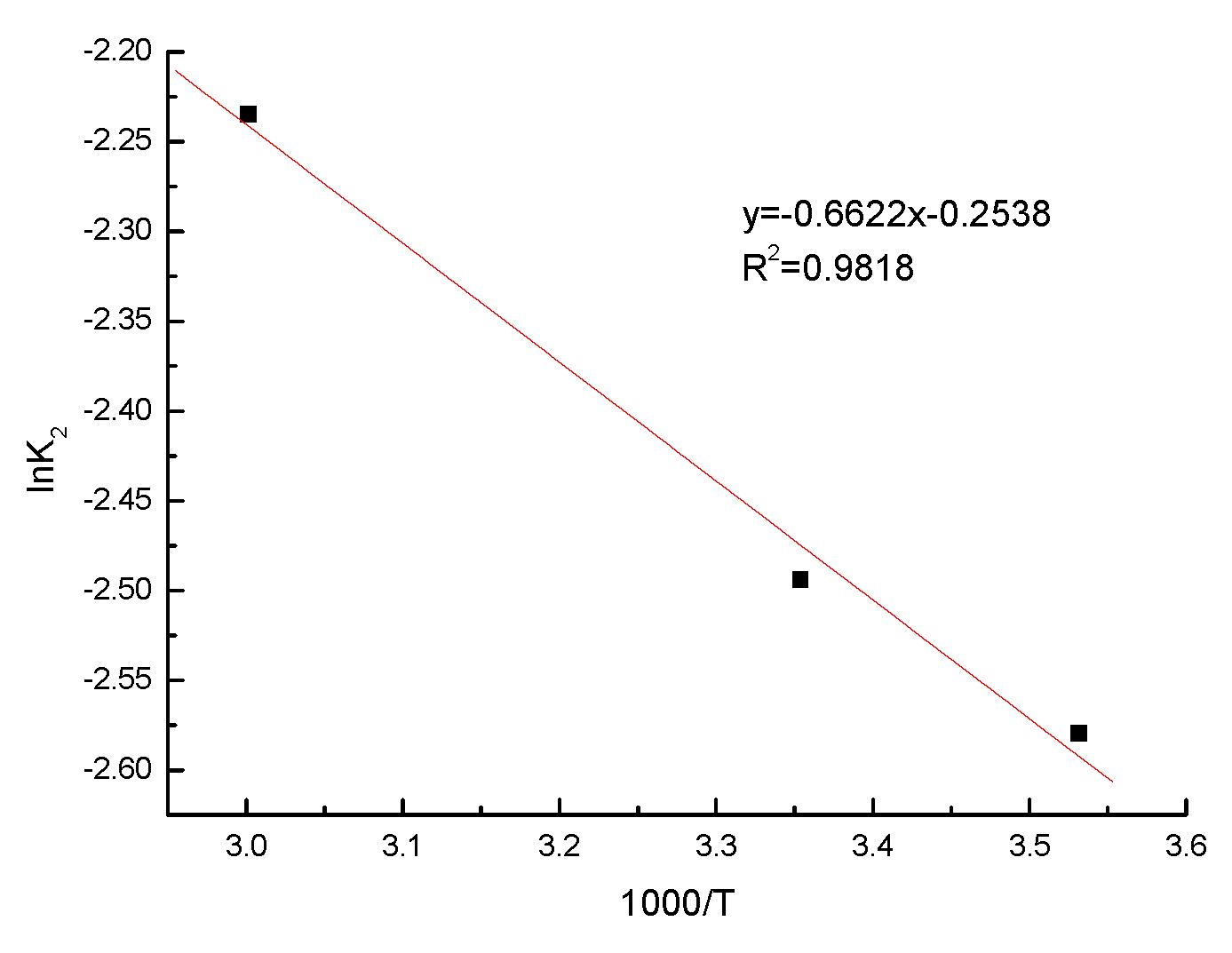
3.9. Adsorption Activation Energy Calculation
4. Conclusions
- The experimental data were analyzed using various kinetic models. Among them, the pseudo-second-order rate kinetics model provided the most accurate representation of the adsorption processes.
- The surface of all three coal types predominantly featured acidic oxygen-containing functional groups. When treated with hydrochloric acid solutions, the specific surface area of the coking coal increased. Of the three coal varieties, anthracite showed the highest pyridine adsorption rate.
- Insights from the Bangham model support that the sorption processes encompass both intraparticle and boundary-layer diffusions. The activation energy for pyridine adsorption onto coking coal was determined to be 5.51 kJ·mol−1, indicating that the process primarily involves physical adsorption.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, X.Y.; Wang, X.C.; Liu, Y.J.; Gao, J.; Wang, Y.K. Variations in toxicity of semi-coking wastewater treatment processes and their toxicity prediction. Ecotoxcol. Environ. Safe 2017, 138, 163–169. [Google Scholar]
- Meng, X.; Ning, P.; Xu, G.; Cao, H. Removal of coke powder from coking wastewater by extraction technology. Sep. Purif. Technol. 2017, 175, 506–511. [Google Scholar]
- Tamang, M.; Paul, K.K. Advances in treatment of coking wastewater—A state of the art review. Water Sci. Technol. 2022, 85, 449–473. [Google Scholar] [PubMed]
- Ma, D.H.; Liu, C.; Zhu, X.B.; Liu, R.; Chen, L.J. Acute toxicity and chemical evaluation of coking wastewater under biological and advanced physicochemical treatment processes. Environ. Sci. Pollut. R. 2016, 23, 18343–18352. [Google Scholar]
- Kwarciak-Kozlowska, A.; Worwag, M. The Impact of an Ultrasonic Field on the Efficiency of Coke Wastewater Treatment in a Sequencing Batch Reactor. Energies 2021, 14, 17. [Google Scholar]
- Liu, Y.; Wu, Z.-y.; Peng, P.; Xie, H.-B.; Li, X.-Y.; Xu, J.; Li, W.-H. A pilot-scale three-dimensional electrochemical reactor combined with anaerobic-anoxic-oxic system for advanced treatment of coking wastewater. J. Environ. Manag. 2020, 258. [Google Scholar] [CrossRef]
- Li, B.; Sun, Y.-L.; Li, Y.-Y. Pretreatment of coking wastewater using anaerobic sequencing batch reactor (ASBR). J. Zhejiang Univ. Sci. B 2005, 6, 1115–1123. [Google Scholar]
- Qin, Z.; Wei, C.; Wei, T.; Li, Z.M.; Pang, Z.J.; Luo, P.; Feng, C.H.; Qiu, G.L.; Wei, C.H.; Wu, H.Z.; et al. Evolution of biochemical processes in coking wastewater treatment: A combined evaluation of material and energy efficiencies and secondary pollution. Sci. Total Environ. 2022, 807, 13. [Google Scholar]
- Dong, Y.R.; Zhang, J.T. Testing the genotoxicity of coking wastewater using Vicia faba and Hordeum vulgare bioassays. Ecotoxicol. Environ. Safe 2010, 73, 944–948. [Google Scholar]
- Zhao, J.L.; Jiang, Y.X.; Yan, B.; Wei, C.H.; Zhang, L.J.; Ying, G.G. Multispecies Acute Toxicity Evaluation of Wastewaters from Different Treatment Stages in a Coking Wastewater-Treatment Plant. Environ. Toxicol. Chem. 2014, 33, 1967–1975. [Google Scholar]
- Zhang, T. Research on coking wastewater treatment technology in the metallurgical industry. Sichuan Nonferrous Met. 2022, 2, 55–57+65. [Google Scholar]
- Fu, X.; Zhou, S. Application of coking wastewater treatment in metallurgical industry. Metall. Equip. 2017, 23, 67–69. [Google Scholar]
- Song, X.L.; Wang, C.; Liu, M.Q.; Zhang, M. Advanced treatment of biologically treated coking wastewater by persulfate oxidation with magnetic activated carbon composite as a catalyst. Water Sci. Technol. 2018, 77, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.X.; Zhang, W.; Li, B.J.; Duan, J.; Lv, Y.; Liu, W.D.; Ying, W.C. Combined Fenton oxidation and biological activated carbon process for recycling of coking plant effluent. J. Hazard. Mater. 2011, 189, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, S.; Wang, Y.; Gui, X.; Xu, H. Pretreatment of Coking Wastewater by an Adsorption Process Using Fine Coking Coal. Physicochem. Probl. Miner. Process. 2016, 52, 422–436. [Google Scholar]
- Lian, X.G.; An, X.C.; Jing, S.J. Research on the Joint Application of Bio-process and Absorbent Addition in Treatment of Waste Water from Coke Chemistry Plant. Coal Process. Compr. Util. 2000, 4, 27–29. [Google Scholar]
- Burmistrz, P.; Rozwadowski, A.; Burmistrz, M.; Karcz, A. Coke dust enhances coke plant wastewater treatment. Chemosphere 2014, 117, 278–284. [Google Scholar] [CrossRef]
- Gao, Q.Y.; Wang, L.; Li, Z.P.; Xie, Y.Q.; He, Q.Q.; Wang, Y.T. Adsorptive Removal of Pyridine in Simulation Wastewater Using Coke Powder. Processes 2019, 7, 459. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Biswas, P.; Bhunia, P.; Kadukar, S.; Banerjee, S.K.; Ghosh, R.; Sarkar, S. Application of coke breeze for removal of color from coke plant wastewater. J. Environ. Manag. 2022, 302, 12. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Z.L.; Li, J.F.; Guo, Y.M.; Cheng, F.Q. Removal of recalcitrant organic pollutants from bio-treated coking wastewater using coal-based carbonaceous materials. Desalination Water Treat. 2017, 88, 75–84. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Chaudhari, P.K. Removal of pollutants of coking wastewater by adsorption. Desalination Water Treat. 2017, 75, 45–57. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, R.; Das, N. Adsorptive removal of phenol from aqueous solutions and coking wastewater by coke produced from hard and soft coking coals. Desalination Water Treat. 2017, 86, 139–149. [Google Scholar] [CrossRef]
- Xu, H.X.; Wang, Y.T.; Huang, G.; Fan, G.X.; Gao, L.H.; Li, X.B. Removal of Quinoline from Aqueous Solutions by Lignite, Coking Coal and Anthracite. Adsorption Kinetics. Physicochem. Probl. Miner. Process. 2016, 52, 397–408. [Google Scholar]
- Rodriguez-Narciso, S.; Lozano-Alvarez, J.A.; Salinas-Gutierrez, R.; Castaneda-Leyva, N. A Stochastic Model for Adsorption Kinetics. Adsorpt. Sci. Technol. 2021, 2021, 21. [Google Scholar] [CrossRef]
- Islam, M.A.; Chowdhury, M.A.; Mozumder, M.S.I.; Uddin, M.T. Langmuir Adsorption Kinetics in Liquid Media: Interface Reaction Model. ACS Omega 2021, 6, 14481–14492. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 12. [Google Scholar] [CrossRef]
- Regazzoni, A.E. Adsorption kinetics at solid/aqueous solution interface: On the boundaries of the pseudo-second-order rate equation. Colloids Surface A 2020, 585, 124093. [Google Scholar] [CrossRef]
- Sun, X.F.; Ma, L.Q.; Ye, G.C.; Wu, L.; Li, J.H.; Xu, H.X.; Huang, G. Phenol adsorption kinetics and isotherms on coal: Effect of particle size. Energy Sources Part A 2021, 43, 461–474. [Google Scholar] [CrossRef]
- Xu, H.J.; Zhang, X.P.; Zhang, Y.D. Modification of biochar by Fe2O3 for the removal of pyridine and quinoline. Environ. Technol. 2018, 39, 1470–1480. [Google Scholar] [CrossRef]
- Wang, J.L.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 18. [Google Scholar] [CrossRef]
- Zhang, J.F. Physical insights into kinetic models of adsorption. Sep. Purif. Technol. 2019, 229, 11. [Google Scholar] [CrossRef]
- Reczek, L.; Michel, M.M.; Kusmierek, K.; Swiatkowski, A.; Siwiec, T. Sorption of 4-chlorophenol and lead(II) on granular activated carbon: Equilibrium, kinetics and thermodynamics. Desalination Water Treat. 2017, 62, 369–376. [Google Scholar] [CrossRef]
- Alaei, R.; Javanshir, S.; Behnamfard, A. Treatment of gold ore cyanidation wastewater by adsorption onto a Hydrotalcite-type anionic clay as a novel adsorbent. J. Environ. Health Sci. 2020, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of copper from aqueous solution by chemically activated pine cone: A kinetic study. Chem. Eng. J. 2011, 175, 260–270. [Google Scholar] [CrossRef]
- Korkut, F.; Saloglu, D. Synthesis, characterization, and tetracycline adsorption behavior of activated carbon dopped alginate beads: Isotherms, kinetics, thermodynamic, and adsorption mechanism. Desalination Water Treat. 2020, 206, 315–330. [Google Scholar] [CrossRef]
- Secula, M.S.; Cretescu, I.; Diaconu, M. Adsorption of Acid Dye Eriochrome Black t from Aqueous Solutions onto Activated Carbon. Kinetic and Equilibrium Studies. J. Environ. Prot. Ecol. 2014, 15, 1583–1593. [Google Scholar]
- Varank, G.; Demir, A.; Yetilmezsoy, K.; Top, S.; Sekman, E.; Bilgili, M.S. Removal of 4-nitrophenol from aqueous solution by natural low-cost adsorbents. Indian J. Chem. Technol. 2012, 19, 7–25. [Google Scholar]
- Hao, P.Y.; Meng, Y.J.; Zeng, F.G.; Yan, T.T.; Xu, G.B. Quantitative Study of Chemical Structures of Different Rank Coals Based on Infrared Spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 787–792. [Google Scholar]
- Hu, Y.Y.; Zhang, Q.T.; Zhou, G.; Wang, H.Y.; Bai, Y.L.; Liu, Y.J. Influence Mechanism of Surfactants on Wettability of Coal with Different Metamorphic Degrees Based on Infrared Spectrum Experiments. ACS Omega 2021, 6, 22248–22258. [Google Scholar] [CrossRef]
- Ghorai, S.; Ghosh, B.; Chandaliya, V.K.; Singh, R.; Dash, P.S.; Mal, D. Difference in structural chemistry of non-coking and coking coal using acid treatment demineralization technique. Int. J. Coal Prep. Util. 2019, 42, 788–808. [Google Scholar] [CrossRef]
- Jaiswal, Y.; Pal, S.L.; Jaiswal, H.; Jain, A.; Kush, L.; Rai, D.; Tatar, D. An investigation of changes in structural parameters and organic functional groups of inertinite rich lignite during acid treatment processes. Energy Sources Part A 2021. [Google Scholar] [CrossRef]
- Mou, P.W.; Pan, J.N.; Niu, Q.H.; Wang, Z.Z.; Li, Y.B.; Song, D.Y. Coal Pores: Methods, Types, and Characteristics. Energy Fuel 2021, 35, 7467–7484. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, Z.H.; Yang, Y.L.; Zhou, Y.B.; Kong, B.; Li, J.H.; Si, L.L. Effect of acid treatment on the characteristics and structures of high-sulfur bituminous coal. Fuel 2016, 184, 418–429. [Google Scholar] [CrossRef]
- Bai, W.L.; Qian, M.M.; Li, Q.X.; Atkinson, S.; Tang, B.; Zhu, Y.C.; Wang, J.F. Rice husk-based adsorbents for removing ammonia: Kinetics, thermodynamics and adsorption mechanism. J. Environ. Chem. Eng. 2021, 9, 11. [Google Scholar] [CrossRef]
- Zheng, L.P.; Yan, G.Y.; Gu, Q.; Xie, L.S.; Long, J.L. Mesoporous V-doped TiO2 Nanoparticles as New Adsorbents for the Removal of Methylene Blue from Color Textile Wastewater. Chin. J. Struct. Chem. 2015, 34, 56–68. [Google Scholar]
- Wei, S.X.; Chen, W.; Li, Z.L.; Liu, Z.Z.; Xu, A. Synthesis of cationic biomass lignosulfonate hydrogel for the efficient adsorption of Cr(VI) in wastewater with low pH. Environ. Technol. 2022, 14, 2134–2147. [Google Scholar] [CrossRef]
- Al-Shehri, H.S.; Alanazi, H.S.; Shaykhayn, A.M.; Alharbi, L.S.; Alnafaei, W.S.; Alorabi, A.Q.; Alkorbi, A.S.; Alharthi, F.A. Adsorption of Methylene Blue by Biosorption on Alkali-Treated Solanum incanum: Isotherms, Equilibrium and Mechanism. Sustainability 2022, 14, 2644. [Google Scholar] [CrossRef]





| Method | Specific Surface Area S/ m2·g−1 | ||
|---|---|---|---|
| Lignite | Coking Coal | Anthracite | |
| BET method | 6.0876 | 5.7864 | 6.1479 |
| Adsorbents | Pseudo-first-order kinetics model | |||
| Qeq(exp)/mg·g−1 | Qeq(calc)/mg·g−1 | K1/min−1 | R2 | |
| lignite | 1.08 | 0.62 | 0.0285 | 0.9790 |
| coking coal | 1.05 | 0.79 | 0.0358 | 0.9739 |
| anthracite | 1.22 | 1.12 | 0.0736 | 0.9956 |
| Adsorbents | Pseudo-second-order kinetics model | |||
| Qeq(calc)/mg·g−1 | K2/g·mg−1·min−1 | h/mg·g−1·min−1 | R2 | |
| lignite | 1.15 | 0.0861 | 0.11 | 0.9993 |
| coking coal | 1.14 | 0.0826 | 0.11 | 0.9988 |
| anthracite | 1.27 | 0.1403 | 0.23 | 0.9975 |
| Adsorbents | Intraparticle diffusion model | |||
| K3/mg·g−1·min−0.5 | intercept | R2 | ||
| lignite | 0.0173 | 0.8536 | 0.9116 | |
| coking coal | 0.0180 | 0.8263 | 0.8294 | |
| anthracite | 0.0041 | 1.1650 | 0.8264 | |
| Adsorbents | Bangham model | |||
| K4 | γ | R2 | ||
| lignite | 0.0072 | 0.2329 | 0.9072 | |
| coking coal | 0.0066 | 0.2456 | 0.9118 | |
| anthracite | 0.0095 | 0.2123 | 0.6682 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Li, S.; Wang, J.; Deng, J.; Huang, G.; Sang, Q.; Cui, J. Removal of Pyridine from Aqueous Solutions Using Lignite, Coking Coal, and Anthracite: Adsorption Kinetics. Processes 2023, 11, 3118. https://doi.org/10.3390/pr11113118
Xu H, Li S, Wang J, Deng J, Huang G, Sang Q, Cui J. Removal of Pyridine from Aqueous Solutions Using Lignite, Coking Coal, and Anthracite: Adsorption Kinetics. Processes. 2023; 11(11):3118. https://doi.org/10.3390/pr11113118
Chicago/Turabian StyleXu, Hongxiang, Shan Li, Jingzheng Wang, Jiushuai Deng, Gen Huang, Qun Sang, and Jiahua Cui. 2023. "Removal of Pyridine from Aqueous Solutions Using Lignite, Coking Coal, and Anthracite: Adsorption Kinetics" Processes 11, no. 11: 3118. https://doi.org/10.3390/pr11113118






