Production of High-Porosity Biochar from Rice Husk by the Microwave Pyrolysis Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermochemical Properties of RH
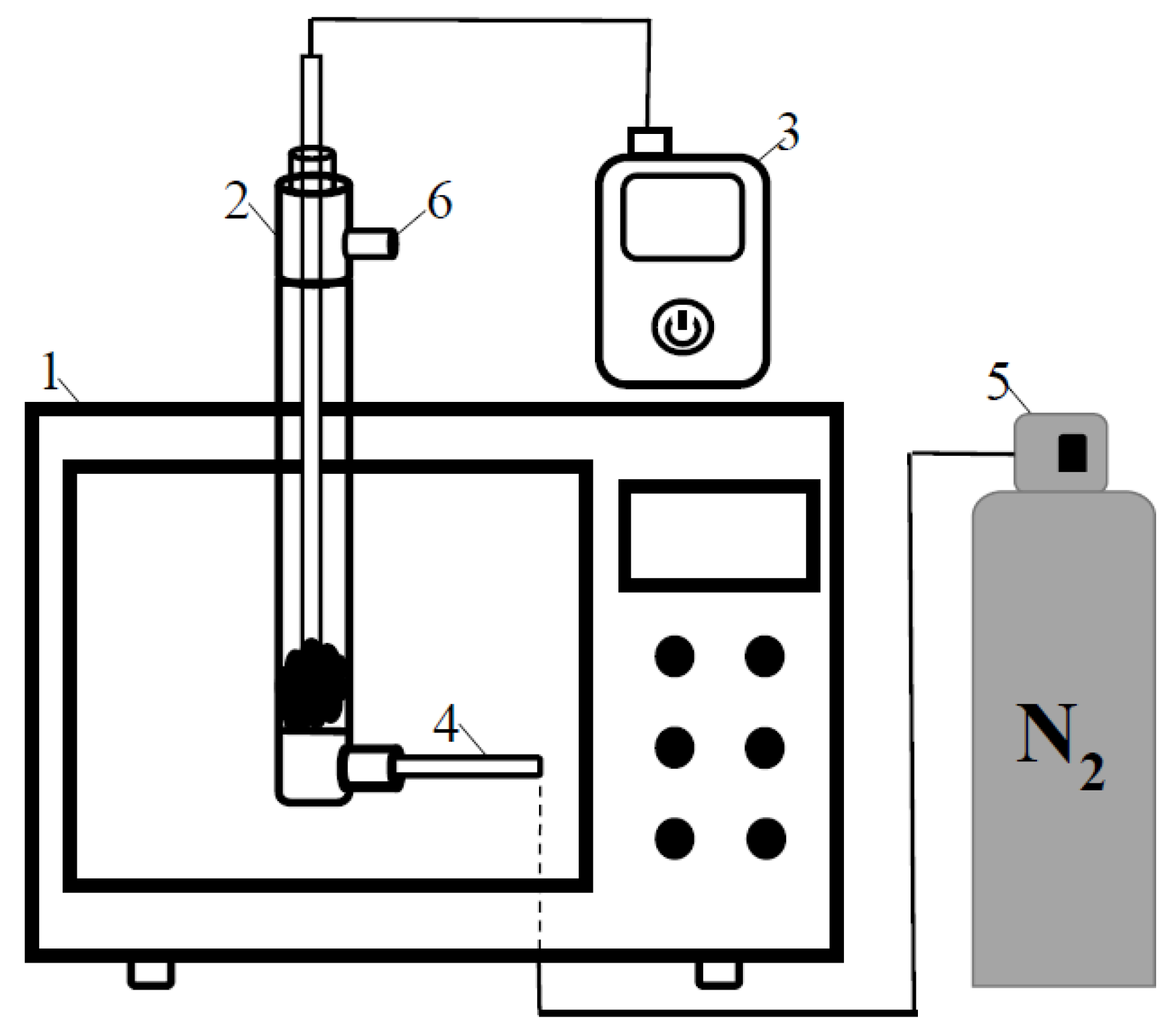
2.3. Microwave Pyrolysis Experiments
2.4. Determinations of Calorific Values and Textural Characteristics of RH-Based Biochar Products
3. Results and Discussion
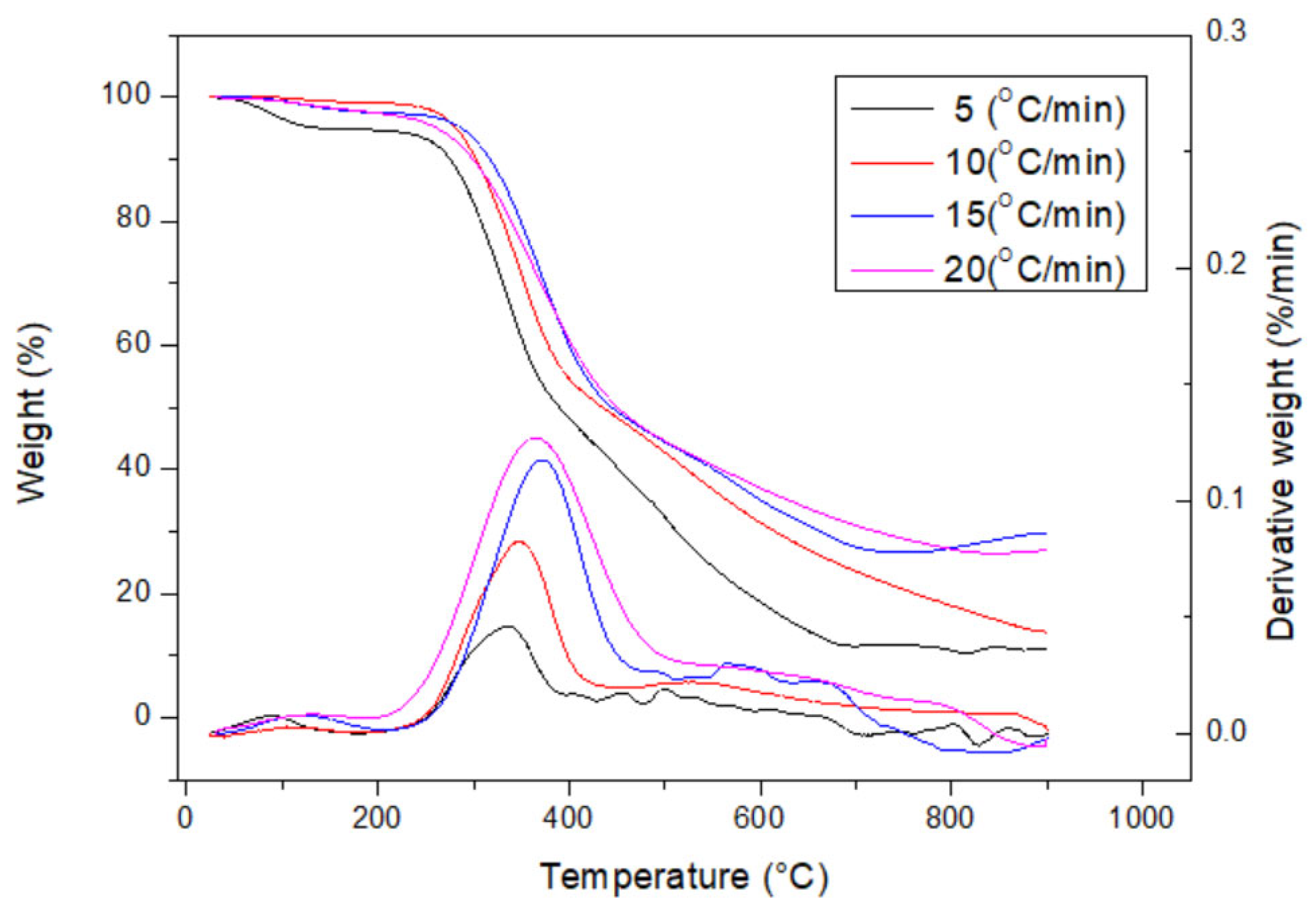
3.1. Thermochemical Characteristics of RH
3.2. Mass Yield and Calorific Value of RH-Based Biochar Products
3.3. Pore Properties of RH-Based Biochar Products
- The pore properties of the RH-based biochar products significantly increased as the microwave output power increased from 300 to 1000 W, with a holding time of 5 min, giving more pore formation and the increments of the surface area and pore volume. The maximal pore properties (i.e., BET surface area of 172.04 m2/g and total pore volume of 0.1229 cm3/g) were obtained at a microwave output power of 1000 W at a holding time of 5 min. Obviously, the pore formation was more developed as the pyrolysis reaction increased at a higher microwave output power, leading to larger pore properties;
- As shown in Table 1, the residence time also played a determining role in the pore properties of the RH-based biochar products in the microwave pyrolysis process. For example, the values of the BET surface area decreased with an extending residence time from 5 min to 15 min at a microwave output power of 1000 W, showing a BET surface area of 172.04 m2/g (BC-RH-1000W-5M) to 154.04 m2/g (BC-RH-1000W-10M) and 63.43 m2/g (BC-RH-1000W-15M). This result may be attributable to the collapse or destruction of the formed pores by severe microwave pyrolysis at longer reaction times. Therefore, the optimal microwave pyrolysis conditions for producing high porosity should be performed at a microwave power of 1000 W and a holding time of 5 min. The maximal BET surface area (i.e., 172.04 m2/g) and total pore volume (i.e., 0.1229 cm3/g) listed in Table 1 were slightly lower than those shown in similar studies [22,36,37];
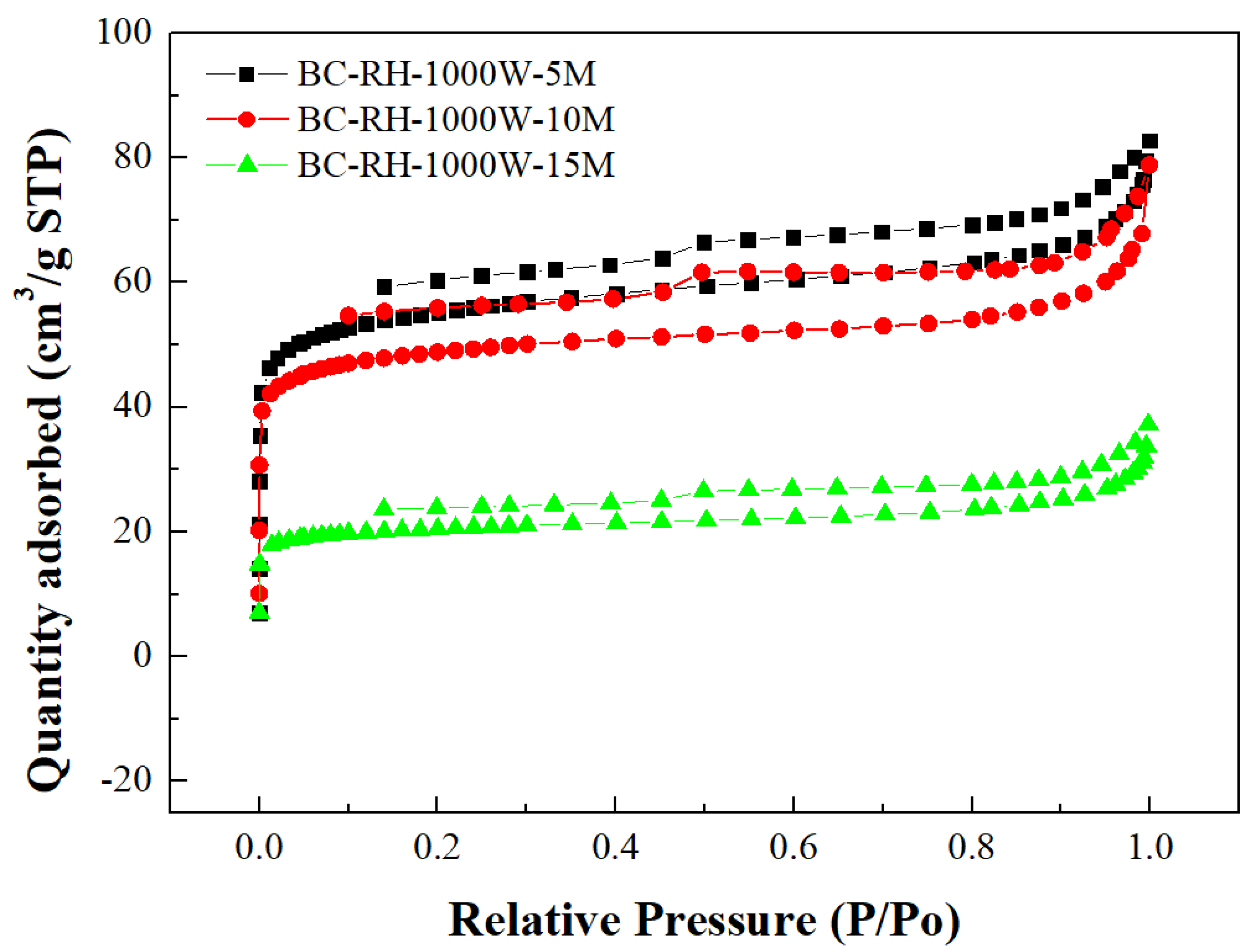
- As shown in Figure 3, the resulting biochar products are characteristic of microporous and mesoporous features, thus displaying Type I and Type VI isotherms [42,43]. It can be seen that the slight hysteresis loops (Type VI isotherms) start from approximately 0.15 of relative pressure in the N2 desorption isotherms. According to the classification by the International Union of Pure and Applied Chemistry (IUPAC) [43], the hysteresis loops should be associated with Type H4 loops, indicating narrow slit pores. In this work, the mesopore size distributions obtained by the BJH method using the N2 desorption isotherm data are depicted in Figure 4. It shows the peak at about 3.8 nm, displaying the mesopores (pore width in the range between 2 nm and 50 nm) in the resulting biochar products;
- Figure 5 further depicts the micropore size distribution of the optimal biochar product (i.e., BC-RH-1000W-5M), using the HK equation for a more accurate description of its micropores [43]. Obviously, the resulting biochar is a microporous material, which showed significant micropores at about 0.6 nm.
| Biochar Product a | SBET b (m2/g) | Smicro c (m2/g) | Vt d (cm3/g) | Vmicro c (cm3/g) |
|---|---|---|---|---|
| BC-RH-300W-5M d | 1.36 | 0.92 | 0.0030 | 0.000 |
| BC-RH-440W-5M | 8.64 | 6.67 | 0.0141 | 0.003 |
| BC-RH-600W-10M | 63.97 | 51.87 | 0.0476 | 0.027 |
| BC-RH-800W-5M | 75.34 | 49.47 | 0.0594 | 0.025 |
| BC-RH-800W-10M | 58.65 | 35.77 | 0.018 | 0.000 |
| BC-RH-1000W-5M | 172.04 | 120.48 | 0.1229 | 0.063 |
| BC-RH-1000W-10M | 154.04 | 116.36 | 0.1138 | 0.059 |
| BC-RH-1000W-15M | 63.43 | 47.87 | 0.0523 | 0.025 |


3.4. Textural and Chemical Characteristics of RH-Based Biochar Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice Knowledge Bank (International Rice Research Institute). Available online: http://www.knowledgebank.irri.org/ (accessed on 30 September 2023).
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Yan, J.; Oyedeji, O.; Leal, J.H.; Donohoe, B.S.; Semelsberger, T.A.; Li, C.; Hoover, A.N.; Webb, E.; Bose, E.A.; Zeng, Y.; et al. Characterizing variability in lignocellulosic biomass: A review. ACS Sustain. Chem. Eng. 2020, 8, 8059–8085. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Kieling, A.G.; Moraes, C.A.M.; Rocha, T.L.A.C.; Brehm, F.A.; Modolo, R.C.E. Characterization of rice husk ash produced using different biomass combustion techniques for energy. Fuel 2016, 165, 351–359. [Google Scholar] [CrossRef]
- Blissett, R.; Sommerville, R.; Rowson, N.; Jones, J.; Laughlin, B. Valorisation of rice husks using a TORBED® combustion process. Fuel Process. Technol. 2017, 159, 247–255. [Google Scholar] [CrossRef]
- Baetge, S.; Kaltschmitt, M. Rice straw and rice husks as energy sources-comparison of direct combustion and biogas production. Biomass Conv. Bioref. 2018, 8, 719–737. [Google Scholar] [CrossRef]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Yoon, S.J.; Son, Y.I.; Kim, Y.K.; Lee, J.G. Gasification and power generation characteristics of rice husk and rice husk pellet using a downdraft fixed-bed gasifier. Renew. Energy 2012, 42, 163–167. [Google Scholar] [CrossRef]
- Vaskalis, I.; Skoulou, V.; Stavropoulos, G.; Zabaniotou, A. Towards circular economy solutions for the management of rice processing residues to bioenergy via gasification. Sustainability 2019, 11, 6433. [Google Scholar] [CrossRef]
- Nyakuma, B.B.; Wong, S.; Mong, G.R.; Utume, L.N.; Oladokun, O.; Wong, K.Y.; Ivase, T.J.P.; Abdullah, T.A.T. Bibliometric analysis of the research landscape on rice husks gasification (1995–2019). Environ. Sci. Pollut. Res. 2021, 28, 49467–49490. [Google Scholar] [CrossRef]
- Dafiqurrohman, H.; Safitri, K.A.; Setyawan, M.I.B.; Surjosatyo, A.; Aziz, M. Gasification of rice wastes toward green and sustainable energy production: A review. J. Clean. Prod. 2022, 366, 132926. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Taiwan. Green National Income Account—Agricultural Solid Waste. Available online: https://agrstat.moa.gov.tw/sdweb/public/common/Download.aspx (accessed on 30 September 2023).
- Saeed, A.A.H.; Yub Harun, N.; Bilad, M.R.; Afzal, M.T.; Parvez, A.M.; Roslan, F.A.S.; Abdul Rahim, S.; Vinayagam, V.D.; Afolabi, H.K. Moisture content impact on properties of briquette produced from rice husk waste. Sustainability 2021, 13, 3069. [Google Scholar] [CrossRef]
- Kipngetich, P.; Kiplimo, R.; Tanui, J.K.; Chisale, P. Effects of carbonization on the combustion of rice husks briquettes in a fixed bed. Clean. Eng. Technol. 2023, 13, 100608. [Google Scholar] [CrossRef]
- Claoston, N.; Samsuri, A.; Ahmad Husni, M.; Mohd Amran, M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Phuong, H.T.; Uddin, M.A.; Kato, Y. Characterization of biochar from pyrolysis of rice husk and rice straw. J. Biobased Mater. Bioenergy 2015, 9, 439–446. [Google Scholar] [CrossRef]
- Menya, E.; Olupot, P.W.; Storz, H.; Lubwama, M.; Kiros, Y.; John, M.J. Optimization of pyrolysis conditions for char production from rice husks and its characterization as a precursor for production of activated carbon. Biomass Conv. Bioref. 2020, 10, 57–72. [Google Scholar] [CrossRef]
- Singh, S.V.; Chaturvedi, S.; Dhyani, V.C.; Kasivelu, G. Pyrolysis temperature influences the characteristics of rice straw and husk biochar and sorption/desorption behaviour of their biourea composite. Bioresour. Technol. 2020, 314, 123674. [Google Scholar]
- Vieira, F.R.; Luna, C.M.R.; Arce, G.L.A.F.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Yadav, K.; Jagadevan, S. Effect of pyrolysis of rice husk–derived biochar on the fuel characteristics and adsorption of fluoride from aqueous solution. Bioenerg. Res. 2021, 14, 964–977. [Google Scholar] [CrossRef]
- Sahoo, D.; Remya, N. Influence of operating parameters on the microwave pyrolysis of rice husk: Biochar yield, energy yield, and property of biochar. Biomass Conv. Bioref. 2022, 12, 3447–3456. [Google Scholar] [CrossRef]
- Anando, A.I.; Ehsan, M.M.; Karim, M.R.; Bhuiyan, A.A.; Ahiduzzaman, M.; Karim, A. Thermochemical pretreatments to improve the fuel properties of rice husk: A review. Renew. Energy 2023, 215, 118917. [Google Scholar] [CrossRef]
- Bushra, B.; Remya, N. Biochar from pyrolysis of rice husk biomass—Characteristics, modification and environmental application. Biomass Conv. Bioref. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Herrera, K.; Morales, L.F.; Tarazona, N.A.; Aguado, R.; Saldarriaga, J.F. Use of biochar from rice husk pyrolysis: Part a: Recovery as an adsorbent in the removal of emerging compounds. ACS Omega 2022, 7, 7625–7637. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Lee, J.; Kim, M.; Kwon, D.; Jung, S. Pyrolysis of rice husk using CO2 for enhanced energy production and soil amendment. Energy Environ. 2023, 34, 873–885. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Li, H.; Xu, D.; Li, X.; Xiang, L.; Tu, S. Review on rice husk biochar as an adsorbent for soil and water remediation. Plants 2023, 12, 1524. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Awang Biak, D.R.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Fodah, A.E.M.; Ghosal, M.K.; Behera, D. Microwave-assisted pyrolysis of agricultural residues: Current scenario, challenges, and future direction. Int. J. Environ. Sci. Technol. 2022, 19, 2195–2220. [Google Scholar] [CrossRef]
- Hadiya, V.; Popat, K.; Vyas, S.; Varjani, S.; Vithanage, M.; Gupta, V.K.; Delgado, A.N.; Zhou, Y.; Show, P.L.; Bilal, M.; et al. Biochar production with amelioration of microwave-assisted pyrolysis: Current scenario, drawbacks and perspectives. Bioresour. Technol. 2022, 355, 127303. [Google Scholar] [CrossRef]
- Narde, S.R.; Remya, N. Biochar production from agricultural biomass through microwave-assisted pyrolysis: Predictive modelling and experimental validation of biochar yield. Environ. Dev. Sustain. 2022, 24, 11089–11102. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Liu, T.; Fu, W.; Li, B. A review of biochar prepared by microwave-assisted pyrolysis of organic wastes. Sustain. Energy Technol. Assess. 2022, 50, 101873. [Google Scholar] [CrossRef]
- Potnuri, R.; Surya, D.V.; Rao, C.S.; Yadav, A.; Sridevi, V.; Remya, N. A review on analysis of biochar produced from microwave-assisted pyrolysis of agricultural waste biomass. J. Anal. Appl. Pyrolysis 2023, 173, 106094. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2016, 28, 317–330. [Google Scholar] [CrossRef]
- Bartoli, M.; Frediani, M.; Briens, C.; Berruti, F.; Rosi, L. An Overview of temperature issues in microwave-assisted pyrolysis. Processes 2019, 7, 658. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y.; Liu, X.; Zhu, S. Effects of water washing and torrefaction pretreatments on rice husk pyrolysis by microwave heating. Bioresour. Technol. 2015, 193, 442–448. [Google Scholar] [CrossRef]
- Shukla, N.; Sahoo, D.; Remya, N. Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J. Clean. Prod. 2019, 235, 1073–1079. [Google Scholar] [CrossRef]
- Halim, S.A.; Mohd, N.A.; Razali, A. A comparative assessment of biofuel products from rice husk and oil palm empty fruit bunch obtained from conventional and microwave pyrolysis. J. Taiwan Inst. Chem. Eng. 2022, 134, 104305. [Google Scholar] [CrossRef]
- Tsai, W.T.; Jiang, T.J.; Tang, M.S.; Chang, C.H.; Kuo, T.H. Enhancement of thermochemical properties on rice husk under a wide range of torrefaction conditions. Biomass Conv. Bioref. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Tsai, C.H.; Shen, Y.H.; Tsai, W.T. Effect of alkaline pretreatment on the fuel properties of torrefied biomass from rice husk. Energies 2023, 16, 679. [Google Scholar] [CrossRef]
- Tsai, C.H.; Shen, Y.H.; Tsai, W.T. Thermochemical characterization of rice-derived residues for fuel use and its potential for slagging tendency. Fire 2023, 6, 230. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Zhu, Y.; Niu, Y.; Tan, H.; Wang, X. Short review on the origin and countermeasure of biomass slagging in grate furnace. Front. Environ. Res. 2014, 2, 7. [Google Scholar] [CrossRef]
- Zhu, C.; Tu, H.; Bai, Y.; Ma, D.; Zhao, Y. Evaluation of slagging and fouling characteristics during Zhundong coal co-firing with a Si/Al dominated low rank coal. Fuel 2019, 254, 115730. [Google Scholar] [CrossRef]
- Lachman, J.; Balas, M.; Lisy, M.; Lisa, H.; Milcak, P.; Elbl, P. An overview of slagging and fouling indicators and their applicability to biomass fuels. Fuel Process. Technol. 2021, 217, 106804. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Li, H.; Liu, Z.; Ma, W.; Liang, X. Thermal stability of oxygen-containing functional groups on activated carbon surfaces in a thermal oxidative environment. J. Chem. Eng. Jpn. 2004, 47, 21–27. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Gharehkhani, S.; Afifi, A.B.M. Adsorption capability of activated carbon synthesized from coconut shell. Carbon Lett. 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Johnston, C.T. Biochar analysis by Fourier-transform infra-red spectroscopy. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 199–228. [Google Scholar]
- Qiu, C.; Jiang, L.; Gao, Y.; Sheng, L. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]



| Property | Value |
|---|---|
| Proximate analysis a,b | |
| Moisture (wt%) | 7.14 ± 0.78 |
| Ash (wt%) | 13.93 ± 0.09 |
| Volatile matter (wt%) | 70.60 ± 1.51 |
| Fixed carbon c (wt%) | 8.34 |
| Calorific value (MJ/kg) a,d | 16.94 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, L.-A.; Tsai, W.-T.; Yang, R.-Y.; Tsai, J.-H. Production of High-Porosity Biochar from Rice Husk by the Microwave Pyrolysis Process. Processes 2023, 11, 3119. https://doi.org/10.3390/pr11113119
Kuo L-A, Tsai W-T, Yang R-Y, Tsai J-H. Production of High-Porosity Biochar from Rice Husk by the Microwave Pyrolysis Process. Processes. 2023; 11(11):3119. https://doi.org/10.3390/pr11113119
Chicago/Turabian StyleKuo, Li-An, Wen-Tien Tsai, Ru-Yuan Yang, and Jen-Hsiung Tsai. 2023. "Production of High-Porosity Biochar from Rice Husk by the Microwave Pyrolysis Process" Processes 11, no. 11: 3119. https://doi.org/10.3390/pr11113119







