Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Model Systems
2.3. Quantification of Proteins
2.4. Simulation of Malaxation Process
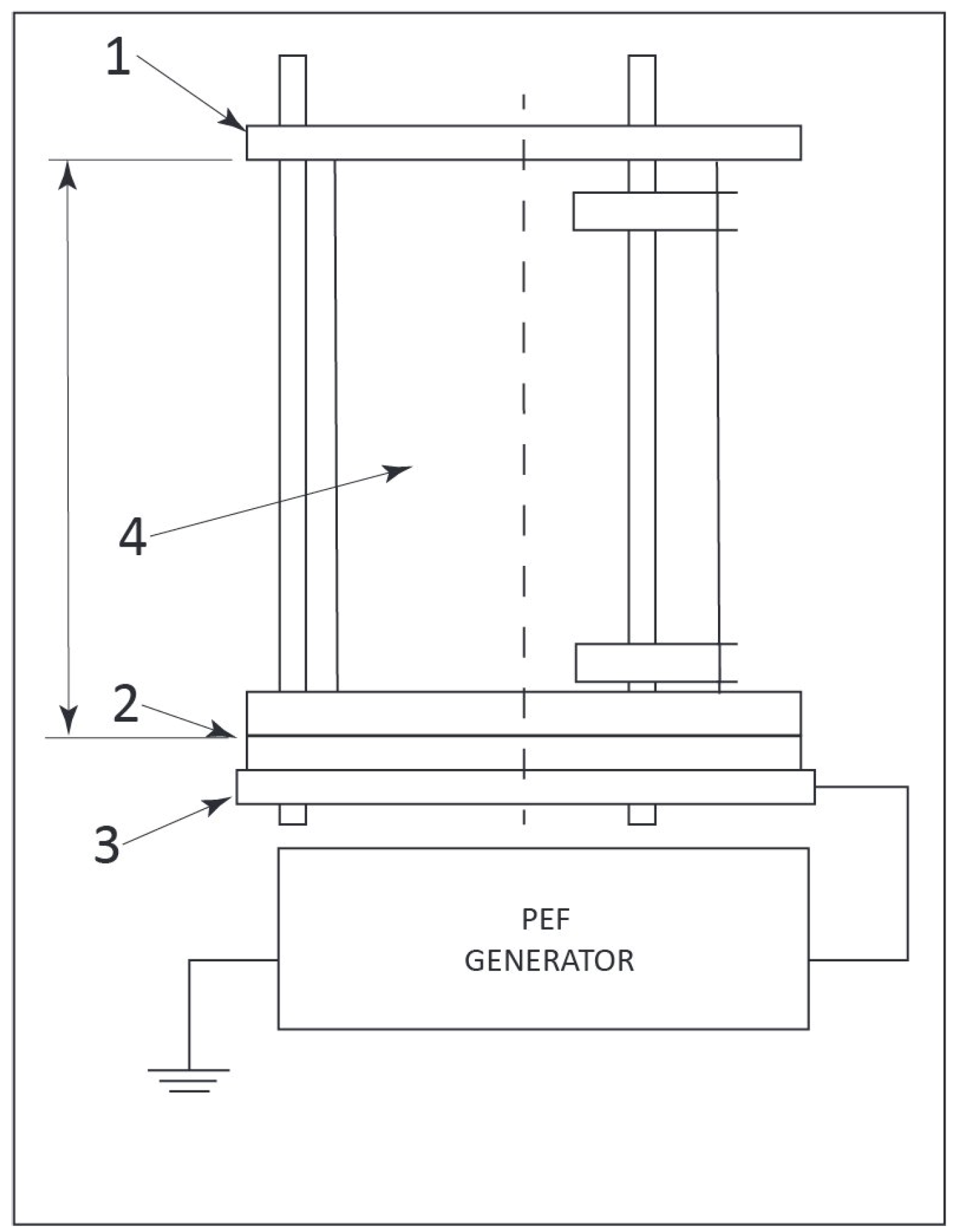
2.5. PEF Treatment and Determination of Enzyme Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Simulation of Malaxation Process
3.2. Influence of PEF Treatment on Enzyme Activity
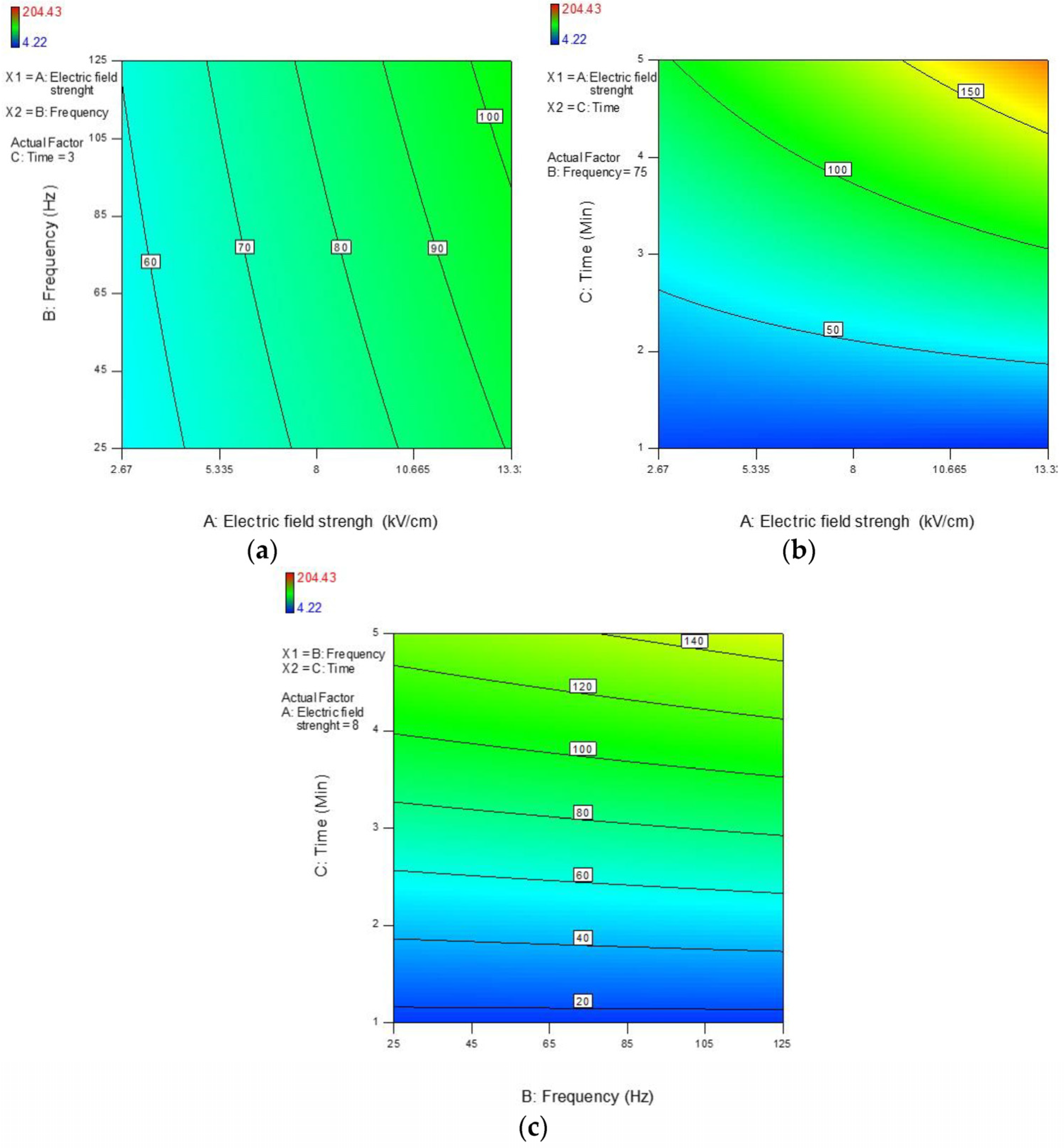
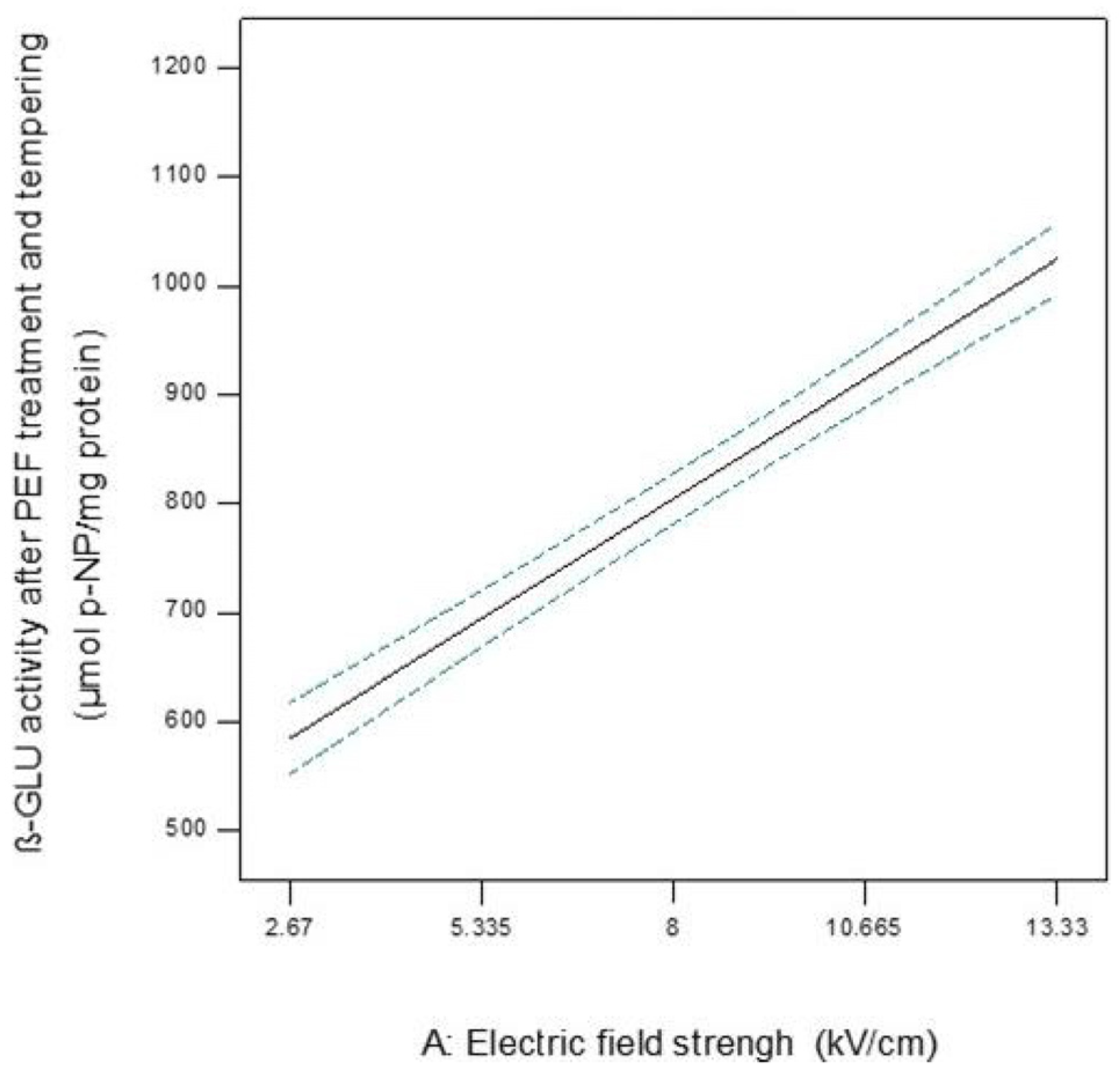
3.2.1. β-Glucosidase
3.2.2. Lipoxygenase
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Tang, J.; Adhikari, B.; Cao, P. Innovative Technologies for Producing and Preserving Intermediate Moisture Foods: A Review. Food Res. Int. 2019, 116, 90–102. [Google Scholar] [CrossRef]
- García-Parra, J.; González-Cebrino, F.; Delgado-Adámez, J.; Cava, R.; Martín-Belloso, O.; Elez-Martínez, P.; Ramírez, R. Application of Innovative Technologies, Moderate-Intensity Pulsed Electric Fields and High-Pressure Thermal Treatment, to Preserve and/or Improve the Bioactive Compounds Content of Pumpkin. Innov. Food Sci. Emerg. Technol. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-Thermal Technologies and Its Current and Future Application in the Food Industry: A Review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Sant’Ana, A.S.; Greiner, R. Mechanisms of Microbial Inactivation by Emerging Technologies. In Innovative Technologies for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–132. [Google Scholar]
- Abenoza, M.; Benito, M.; Saldaña, G.; Álvarez, I.; Raso, J.; Sánchez-Gimeno, A.C. Effects of Pulsed Electric Field on Yield Extraction and Quality of Olive Oil. Food Bioproc. Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Aguilera, M.P.; Beltran, G.; Sanchez-Villasclaras, S.; Uceda, M.; Jimenez, A. Kneading Olive Paste from Unripe ‘Picual’ Fruits: I. Effect on Oil Process Yield. J. Food Eng. 2010, 97, 533–538. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on Virgin Olive Oil Quality. Past, Present and Future—An Overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Guderjan, M.; Töpfl, S.; Angersbach, A.; Knorr, D. Impact of Pulsed Electric Field Treatment on the Recovery and Quality of Plant Oils. J. Food Eng. 2005, 67, 281–287. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Alexandrakis, Z.; Katsaros, G.; Oikonomou, D.; Toepfl, S.; Heinz, V.; Taoukis, P. Shelf-Life Evaluation of Virgin Olive Oil Extracted from Olives Subjected to Nonthermal Pretreatments for Yield Increase. Innov. Food Sci. Emerg. Technol. 2017, 40, 52–57. [Google Scholar] [CrossRef]
- Puértolas, E.; Martínez de Marañón, I. Olive Oil Pilot-Production Assisted by Pulsed Electric Field: Impact on Extraction Yield, Chemical Parameters and Sensory Properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef]
- Navarro, A.; Ruiz-Méndez, M.-V.; Sanz, C.; Martínez, M.; Rego, D.; Pérez, A.G. Application of Pulsed Electric Fields to Pilot and Industrial Scale Virgin Olive Oil Extraction: Impact on Organoleptic and Functional Quality. Foods 2022, 11, 2022. [Google Scholar] [CrossRef]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the Effects of a Pulsed Electric Field (PEF) Continuous System Implemented in an Industrial Olive Oil Plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef]
- Luo, S.; Schiffbauer, J.; Luo, T. Effect of Electric Field Non-Uniformity on Droplets Coalescence. Phys. Chem. Chem. Phys. 2016, 18, 29786–29796. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Zhang, H.Q. Recent Advances in the Action of Pulsed Electric Fields on Enzymes and Food Component Proteins. Trends Food Sci. Technol. 2012, 27, 83–96. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, R.B.; Wang, L.M.; Chen, J.; Guan, Z.C. Conformation Changes of Polyphenol Oxidase and Lipoxygenase Induced by PEF Treatment. J. Appl. Electrochem. 2010, 40, 295–301. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Sobrino-López, Á.; Soliva-Fortuny, R.; Martín-Belloso, O. Influence of High-Intensity Pulsed Electric Field Processing on Lipoxygenase and β-Glucosidase Activities in Strawberry Juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 455–462. [Google Scholar] [CrossRef]
- Kraljić, K.; Balbino, S.; Filipan, K.; Herceg, Z.; Ivanov, M.; Vukušić Pavičić, T.; Stuparević, I.; Pavlić, K.; Škevin, D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques. Processes 2023, 11, 1194. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical Strategies to Increase Nutritional and Sensory Quality of Virgin Olive Oil by Modulating the Endogenous Enzyme Activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Lorenzi, V.; Maury, J.; Casanova, J.; Berti, L. Purification, Product Characterization and Kinetic Properties of Lipoxygenase from Olive Fruit (Olea Europaea L.). Plant Physiol. Biochem. 2006, 44, 450–454. [Google Scholar] [CrossRef]
- Wecksler, A.T.; Garcia, N.K.; Holman, T.R. Substrate Specificity Effects of Lipoxygenase Products and Inhibitors on Soybean Lipoxygenase-1. Bioorg. Med. Chem. 2009, 17, 6534–6539. [Google Scholar] [CrossRef] [PubMed]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The Application of PEF Technology in Food Processing and Human Nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Martín-Belloso, O.; Marsellés-Fontanet, Á.R.; Elez-Martínez, P. Enzymatic Inactivation by Pulsed Electric Fields. In Emerging Technologies for Food Processing; Academic Press: Cambridge, MA, USA, 2014; pp. 155–168. [Google Scholar] [CrossRef]
- Lu, C.; Li, F.; Yan, X.; Mao, S.; Zhang, T. Effect of Pulsed Electric Field on Soybean Isoflavone Glycosides Hydrolysis by β-Glucosidase: Investigation on Enzyme Characteristics and Assisted Reaction. Food Chem. 2022, 378, 132032. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W. Decomposition of Linoleic Acid Hydroperoxides. Enzymic Reactions Compared with Nonenzymic. J. Agric. Food Chem. 1975, 23, 129–136. [Google Scholar] [CrossRef]


| Electric Field Strength (kV/cm) | Frequency (Hz) | Time (min) | Final Temperature (°C) | β-GLU Activity after PEF Treatment (µmol p-NP/mg protein) * | β-GLU Activity after PEF Treatment and Incubation (µmol p-NP/mg protein) * | LOX Activity after PEF Treatment (µmol HPOD/mg protein) * | LOX Activity after PEF Treatment and Incubation (µmol HPOD/mg protein) * |
|---|---|---|---|---|---|---|---|
| Control | 1 | 25.0 ± 0.2 | 2.74 ± 0.23 i | 522.90 ± 1.63 f | 1.87 ± 0.16 e | 8.51 ± 0.13 b | |
| Control | 2 | 25.0 ± 0.2 | 158.03 ± 0.48 c | 809.23 ± 12.07 de | 3.77 ± 0.17 d | 8.62 ± 0.14 b | |
| Control | 5 | 25.0 ± 0.2 | 254.65 ± 11.78 a | 849.27 ± 18.43 cd | 5.68 ± 0.09 b | 8.40 ± 0.25 b | |
| 2.67 | 25 | 1 | 23.7 ± 0.9 | 21.86 ± 1.63 ghi | 594.76 ± 3.96 f | 3.73 ± 0.20 d | 10.51 ± 0.17 a |
| 2.67 | 125 | 1 | 23.7 ± 0.8 | 28.56 ± 6.14 ghi | 652.45 ± 62.70 ef | 3.89 ± 0.09 d | 10.57 ± 0.08 a |
| 2.67 | 25 | 2 | 22.9 ± 0.8 | 32.90 ± 6.06 fghi | 544.66 ± 16.09 f | 4.81 ± 0.10 c | 9.73 ± 0.38 a |
| 2.67 | 125 | 2 | 23.3 ± 0.4 | 35.21 ± 8.86 fgh | 546.80 ± 15.39 f | 4.57 ± 0.12 c | 10.68 ± 0.43 a |
| 2.67 | 25 | 5 | 22.6 ± 0.2 | 90.42 ± 0.23 de | 574.00 ± 37.06 f | 6.08 ± 0.04 ab | 9.78 ± 0.50 a |
| 2.67 | 125 | 5 | 22.6 ± 0.3 | 105.09 ± 11.19 d | 599.05 ± 30.07 f | 6.27 ± 0.13 a | 9.81 ± 0.05 a |
| 13.33 | 25 | 1 | 22.2 ± 1.1 | 15.55 ± 3.59 ghi | 997.98 ± 81.87 abc | 3.34 ± 0.29 d | 10.35 ± 0.27 a |
| 13.33 | 125 | 1 | 22.2 ± 0.8 | 13.01 ± 2.39 hi | 986.58 ± 97.40 bcd | 3.82 ± 0.27 d | 9.98 ± 0.13 a |
| 13.33 | 25 | 2 | 21.7 ± 0.4 | 46.40 ± 2.99 fg | 1004.75 ± 16.13 abc | 4.73 ± 0.06 c | 9.65 ± 0.18 a |
| 13.33 | 125 | 2 | 22.4 ± 0.7 | 62.24 ± 9.26 ef | 1126.73 ± 2.79 a | 4.66 ± 0.05 c | 10.03 ± 0.25 a |
| 13.33 | 25 | 5 | 22.1 ± 0.1 | 170.20 ± 0.00 bc | 986.37 ± 13.44 bcd | 5.98 ± 0.03 ab | 9.92 ± 0.33 a |
| 13.33 | 125 | 5 | 22.0 ± 0.1 | 193.87 ± 14.94 b | 1044.89 ± 36.45 ab | 5.82 ± 0.01 ab | 9.84 ± 0.43 a |
| Source of Variation | β-GLU Activity after PEF Treatment | β-GLU Activity after PEF Treatment and Incubation | LOX Activity after PEF Treatment | LOX Activity after PEF Treatment and Incubation |
|---|---|---|---|---|
| Intercept | 77.42 | 804.36 | 4.99 | 10.03 |
| p-Value | <0.001 | <0.001 | <0.001 | 0.038 |
| A | 20.15 | 219.63 | −0.08 | −0.11 |
| p-Value | <0.001 | <0.001 | 0.157 | 0.155 |
| B | 5.06 | 21.16 | 0.03 | 0.08 |
| p-Value | 0.009 | 0.072 | 0.612 | 0.293 |
| C | 61.90 | −3.36 | 1.11 | −0.23 |
| p-Value | <0.001 | 0.800 | <0.001 | 0.016 |
| AB | 1.94 | - | - | - |
| p-Value | 0.268 | |||
| AC | 22.27 | - | - | - |
| p-Value | <0.001 | |||
| BC | 5.02 | - | - | - |
| p-Value | 0.022 | |||
| Lack of fit | 0.818 | 0.117 | 0.001 | 0.122 |
| R2 | 0.986 | 0.952 | 0.933 | 0.338 |
| R2 adjusted | 0.981 | 0.944 | 0.906 | 0.239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraljić, K.; Balbino, S.; Filipan, K.; Herceg, Z.; Stuparević, I.; Ivanov, M.; Vukušić Pavičić, T.; Jakoliš, N.; Škevin, D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique. Processes 2023, 11, 3283. https://doi.org/10.3390/pr11123283
Kraljić K, Balbino S, Filipan K, Herceg Z, Stuparević I, Ivanov M, Vukušić Pavičić T, Jakoliš N, Škevin D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique. Processes. 2023; 11(12):3283. https://doi.org/10.3390/pr11123283
Chicago/Turabian StyleKraljić, Klara, Sandra Balbino, Katarina Filipan, Zoran Herceg, Igor Stuparević, Mia Ivanov, Tomislava Vukušić Pavičić, Niko Jakoliš, and Dubravka Škevin. 2023. "Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique" Processes 11, no. 12: 3283. https://doi.org/10.3390/pr11123283
APA StyleKraljić, K., Balbino, S., Filipan, K., Herceg, Z., Stuparević, I., Ivanov, M., Vukušić Pavičić, T., Jakoliš, N., & Škevin, D. (2023). Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part II—Non-Thermal Technique. Processes, 11(12), 3283. https://doi.org/10.3390/pr11123283








