Abstract
The extraction of silica particles from rice husks has been extensively studied. This review aims to present the most efficient approach to harnessing rice husk biomass and converting silica into high-value-added materials for direct applications to address current challenges like water purification. Rice husks, as a residue from agriculture, had been largely used as a source of power through direct incineration in major rice-producing countries. However, rice husks present an intriguing opportunity as a renewable source of SiO2, offering a low-cost adsorbent with a high surface area and ease of functionalization that can be transformed into diverse mesoporous silica structures or composites, enabling applications in catalysis, drug delivery, water treatment, etc. This dual potential of rice husks can be harnessed by combining bio-oil and syngas production through pyrolysis with the efficient extraction of SiO2, ensuring the comprehensive utilization of the biomass. This review not only highlights the immense potential of silica nanoparticles but also serves as a roadmap for future investigations, with the ultimate aim of harnessing the full capabilities of this renewable and sustainable resource, contributing to the circular economy by yielding valuable by-products.
1. Introduction
Accessibility to sustainable, low-carbon bio-feedstock is one of the key drivers to achieve a European Union (EU) low-carbon economy by 2050. In this context, the development of biomass conversion technologies is being promoted [1].
Agricultural waste is in the spotlight of sustainable biomass production since an important amount of waste is produced yearly and is usually not fully harnessed. The source of this biomass includes primary agricultural resources produced directly on the field after crop harvesting and secondary plant resources obtained during industrial processing of agricultural crops. The potential estimated for the latter in 2030 in the EU report ranges from 56 to 81 million tons, with France, Austria, Germany and Spain being the countries with the largest contributions [1].
Among secondary plant resources, several residues are included, such as olive pits, fruit pulp, husks, shells, etc. Among the largest residues generated in agriculture are those coming from rice plants (Oryza sativa), especially their husks. Rice husk (RH) is the major by-product in the rice milling industry, which is estimated to be 1.2 billion tons annually worldwide [2,3,4].
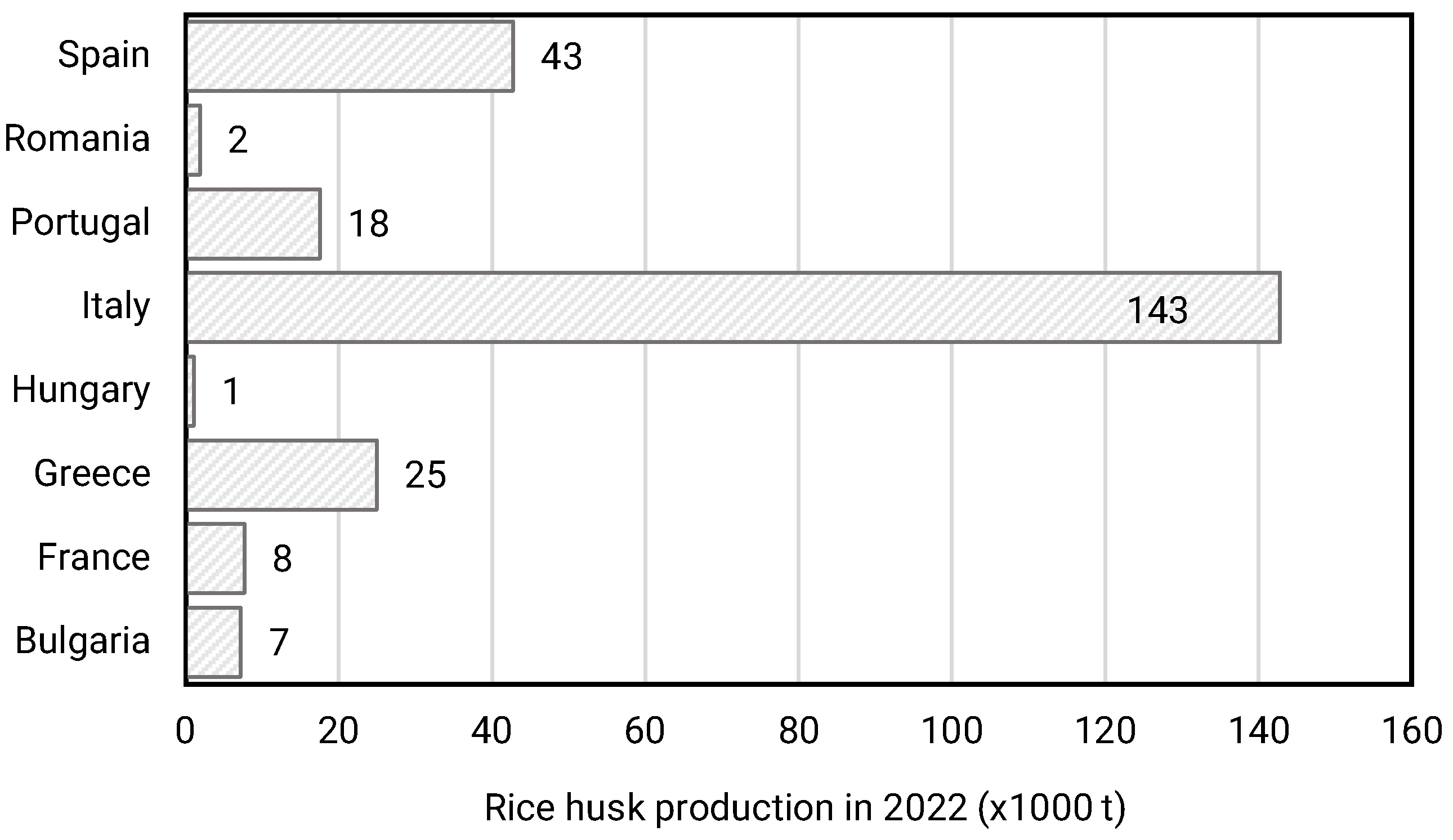
In 2022, the EU produced approximately 1.2 million tons of paddy rice, with rice husk (dry) representing 20 wt% of the processed rice at mills [5]; so it is calculated that at least 250 thousand tons of agricultural waste in Europe correspond to rice husk (more than half are produced by Italy (Figure 1)), representing 10 wt% of the total of secondary agroalimentary residues [6].
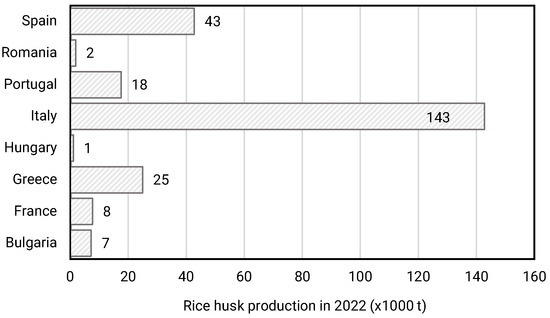
Figure 1.
Rice husk production in Europe (EU-27) by countries (modified data from European Commission report on rice production accessed in August 2023), with RH estimated to be 20% of the weight of the rice processed at mills.
Rice Husk as a Source of Bio-Energy and SiO2
Dried RHs contain about 15–28 wt% silica, depending on the origin and type, and 72–85 wt% lignocellulose (15–22 wt% lignin, 12–29 wt% hemicellulose, 29–36 wt% cellulose and 3–13 wt% traces of metals). As it does not have sufficient protein content (~4 wt% proteins, oils) for agricultural uses, most RHs are either burned or buried in landfills [7]. RH is the biomass waste containing the largest amount of silica (~210 g/kg of husk) [8]. Even when compared to other husks, such as wheat husk, or other parts of the rice plant, such as the straw, its silica content is the highest (Table 1).

Table 1.
Physico-chemical properties of agroalimentary residues with high content in silica.
Therefore, as RHs are not easily decomposed by bacteria due to their highly inorganic content, their accumulation in landfills is not a sustainable option. For this reason, RH incineration has been the most common technique for removal since the heating value is around 15 MJ/kg, and so this energy can be harnessed [9]. Nevertheless, this method produces a high amount of rice husk ash (RHA) that needs to be disposed of as well, with its most common use currently being as a filler, additive, etc., in the building and construction industry. If RH is not properly pretreated before the incineration, the composition of the ashes results in a low-purity silica composite due to the metal and carbon content present in the husk. Rice plants collect silica from the soil, which is stored as silicic acid inside the cellulose micro-compartments of the plant. The metal content varies according to the soil and manures used, but traces of potassium, calcium, magnesium, iron, sodium, manganese, aluminum, phosphorous and sulfur are found [14]. K is the main metal of RH (~2.7 mg/g), followed by Ca (~1.5 mg/g) and Mg and Mn (0.36 mg/g). The presence of K promotes the crystallization of silica into tridymite and cristobalite, a carcinogenic compound featuring a very low surface area (10 m2/g), and leads to the formation of unburnt carbon residues. Then, the silica powder is not white, and the specific surface area is low: from 145 m2/g for calcination at 600 °C to less than 20 m2/g for higher temperatures due to silica crystallization [15].
The low economic efficiency of incineration of RH has led to a search for alternative methods to harness this agricultural waste. For instance, pyrolysis of biomass is a hot topic currently since it leads to the production of pyrolysis gas and bio-oil from the decomposition of the lignocellulosic materials, and in the specific case of rice husk, char rich in silica is obtained as a third by-product. So, for these aforementioned reasons, it is considered to be a more efficient way to thermally degrade organic matter [16,17]. However, it has to be considered that pyrolysis bio-oil has a heating value of around 13–25 MJ/kg, lower than diesel and gasoline [9].
Silicon is the second most abundant element in the Earth’s crust after oxygen, which is brought to the soil by chemical and biological processes. Plants are highly involved in such processes as they transform the silicon present in water into water-soluble silicic acid [H4SiO4, or Si(OH)4], which is accumulated in the form of phytoliths, amorphous hydrated silica (SiO2 with 5–10% H2O). In rice plants, these phytoliths accumulate primarily in the husk. The deposition of silicon in this form offers the plant resistance against pathogens, stresses and pests, enhancing growth and development, thus Si availability in the soil where the rice grows is highly important [18].
Silica is one of the most important inorganic materials in the industry since it has a wide variety of applications, such as in catalyst production, catalyst supports, drug carriers, construction, glass and ceramics and water filtration, among others. Specifically, nanosized silica is of great interest due to its large surface area, thermal conductivity (insulation), good dispersion performance, ease of functionalization, chemical stability and biocompatibility [19]. These properties show an interesting prospect in the development of techniques to harness silica from agricultural waste. Many reviews have been made on the general preparation of silica nanoparticles from organic chemicals or from different biomasses with various techniques [20,21,22,23,24]. Here, we present the general methods for classic chemical synthesis in contraposition to its sustainable production. We focus on the best approach to harnessing rice husk, emphasizing the efficient extraction of silica with a focus on circular economy and the subsequent production of value-added silica structures tailored to specific applications.
2. Overview of General Methods for SiO2 NPs Classical Chemical Synthesis
Numerous reviews report on the synthesis of non-porous and porous SiO2 NPs [25,26,27,28,29]. Industrially, SiO2 NPs are produced as precipitated silica from sodium silicate solution in an acidic medium and as fumed (or pyrogenic) silica from quartz sand or SiCl4 put in a flame. For example, Rhodia uses Fontainebleau quartz sand first mixed with Na2CO3 and heated at 900 °C. The resulting glassy silicate is put in water to obtain silica sols, which are then heated at 85 °C with H2SO4 to precipitate as silica NPs, which are then filtered, water-washed and dried. The precipitated silica is formed by SiO2 NPs of 15 nm, and NPs are aggregated and non-porous. The surface area is around 120–150 m2/g. Fumed silica as Aerosil 200 (Degussa) is produced by reacting SiCl4 with oxygen and hydrogen in a flame. Non-porous SiO2 NPs of 50 nm particle size are obtained, and most of them are aggregated. The surface area is around 200 m2/g. This process is the one majorly used in industry to produce silica NPs powder due to its high production rates at relatively low cost [29].
To obtain independent SiO2 NPs, the most common silica precursor has been tetraethyl orthosilicate (TEOS), a silicon alkoxide usually synthesized from raw silica present in the sand [6]. The main chemical synthesis to produce non-aggregated silica NPs is the Stöber method [30]. It consists of adding TEOS (0.1–0.5 M) in ethanol in the presence of NH4OH (0.5–3 M) and H2O (0.5–17 M) at 25 °C. Well-homogeneous, highly dispersed SiO2 NPs are obtained, whose size depends on the mixture composition. For instance, 80 nm SiO2 NPs are obtained for mixtures with a molar ratio of 1 TEOS:5 NH4OH:10 H2O:85 EtOH, whilst 390 nm SiO2 NPs are obtained for mixtures with a molar ratio of 1 TEOS:2 NH4OH:30 H2O:68 EtOH. The size of the SiO2 NPs ranges between 50 and 800 nm; it increases by decreasing the NH4OH amount (or pH). The particles are obtained in suspension. The particles are non-porous. The specific surface areas range between 20 and 80 m2/g.
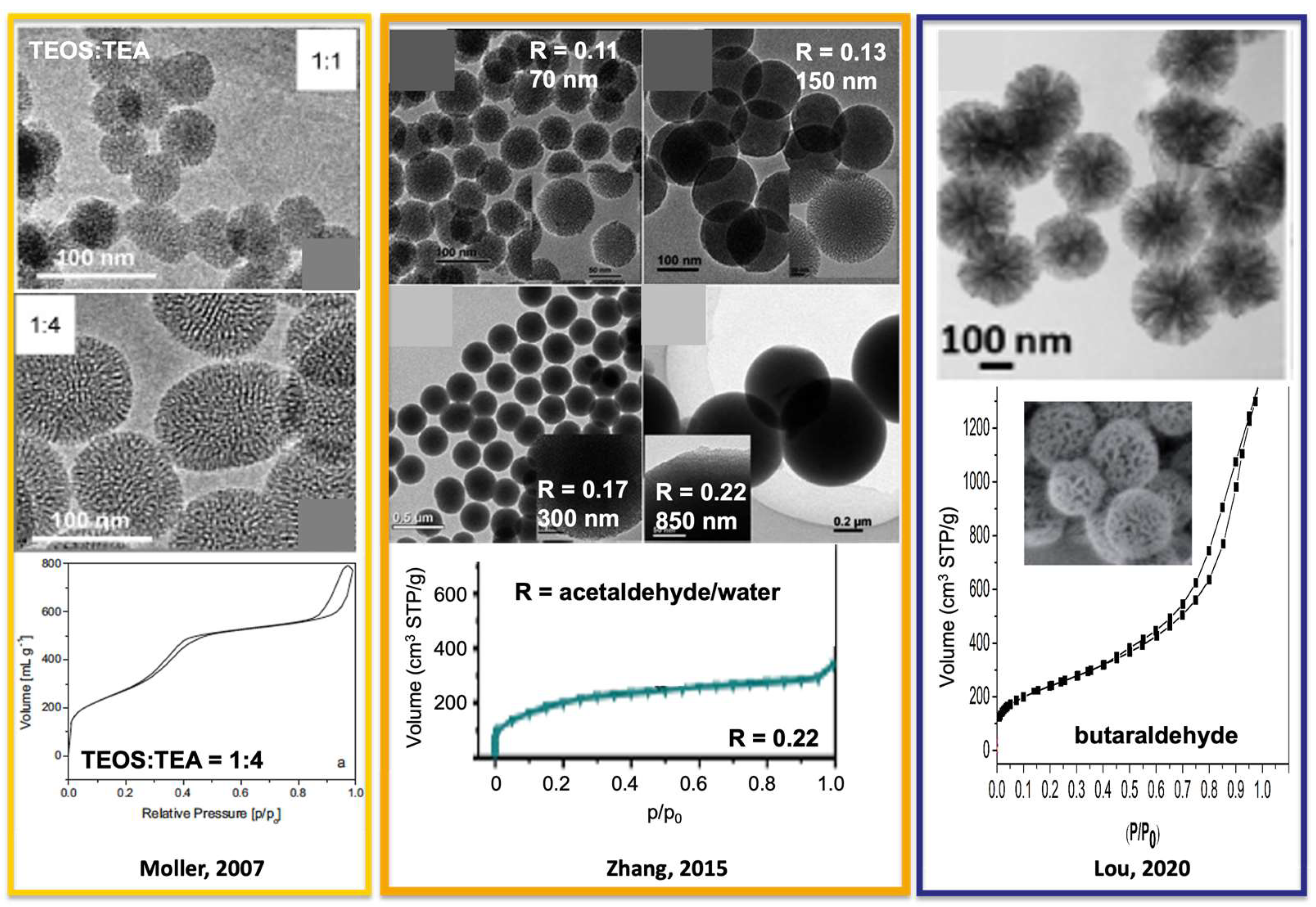
To synthesize highly dispersed mesoporous SiO2 NPs, Stöber synthesis is modified by adding surfactants (such as cetyltrimethylammonium bromide or chloride (CTAB or CTAC)) to direct the mesopores formation by micelle-templating. The water content is also increased. For example, one of the best homogeneous, highly dispersed mesoporous SiO2 NPs was achieved by Möller et al. [31] by replacing NH4OH with triethanolamine (TEA) in mixtures with a molar composition of 1 TEOS: 0.27 CTAC: 1–4 TEA: 137 H2O: 6.2 EtOH. Mesoporous SiO2 NPs of 50 and 100 nm were obtained at 60 °C for TEA/TEOS = 1 and 4, respectively (Figure 2). Surfactant removal was achieved by a twofold extraction in acidified EtOH or by adding NH4NO3 at 60 °C for 2 h. The most convenient method was acidified EtOH at 25 °C with 30 min ultrasounds. The mesoporous SiO2 NPs feature ordered cylindrical homogenous mesopore of 3.5 nm diameter, specific surface areas of 1000 m2/g and mesopore volume of 0.77 mL/g as MCM-41 materials. Another interesting modification of the Stöber synthesis using CTAB is realized by replacing alcohols with aldehydes [32,33]. Mixtures with a molar ratio of 1 TEOS: 0.14 CTAB: 1.6–3.4 NH4OH: 100 H2O: 11–22 acetaldehyde at 27 °C give rise to MCM-41-like mesoporous NPs with particle sizes ranging from 40 to 850 nm. The particle size decreases by increasing the pH (adding more NH4OH) or by decreasing the amount of acetaldehyde. A calcination was performed to remove the surfactants, leading to a slight collapsing on the mesopores in nitrogen adsorption (no abrupt step of capillary condensation was observed). A surfactant removal by extraction should be preferred. By replacing acetaldehyde with propionaldehyde or butaraldehyde, NPs of flower-like morphology made of inverted cone-shaped pores of 20 nm average diameter were synthesized. Both the depth and opening diameter of the cone-like cavities can be finely tuned from 2 to 40 nm. Particles of 100 to 300 nm have been obtained. All these mesoporous SiO2 NPs feature specific surface areas of 600–800 m2/g (Figure 2).
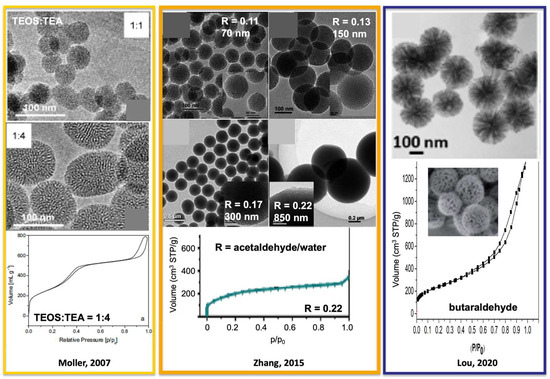
Figure 2.
Mesoporous SiO2 NPs found in the literature synthesized from modified Stöber method with TEOS and CTAB. Adapted from references [31,32,33].
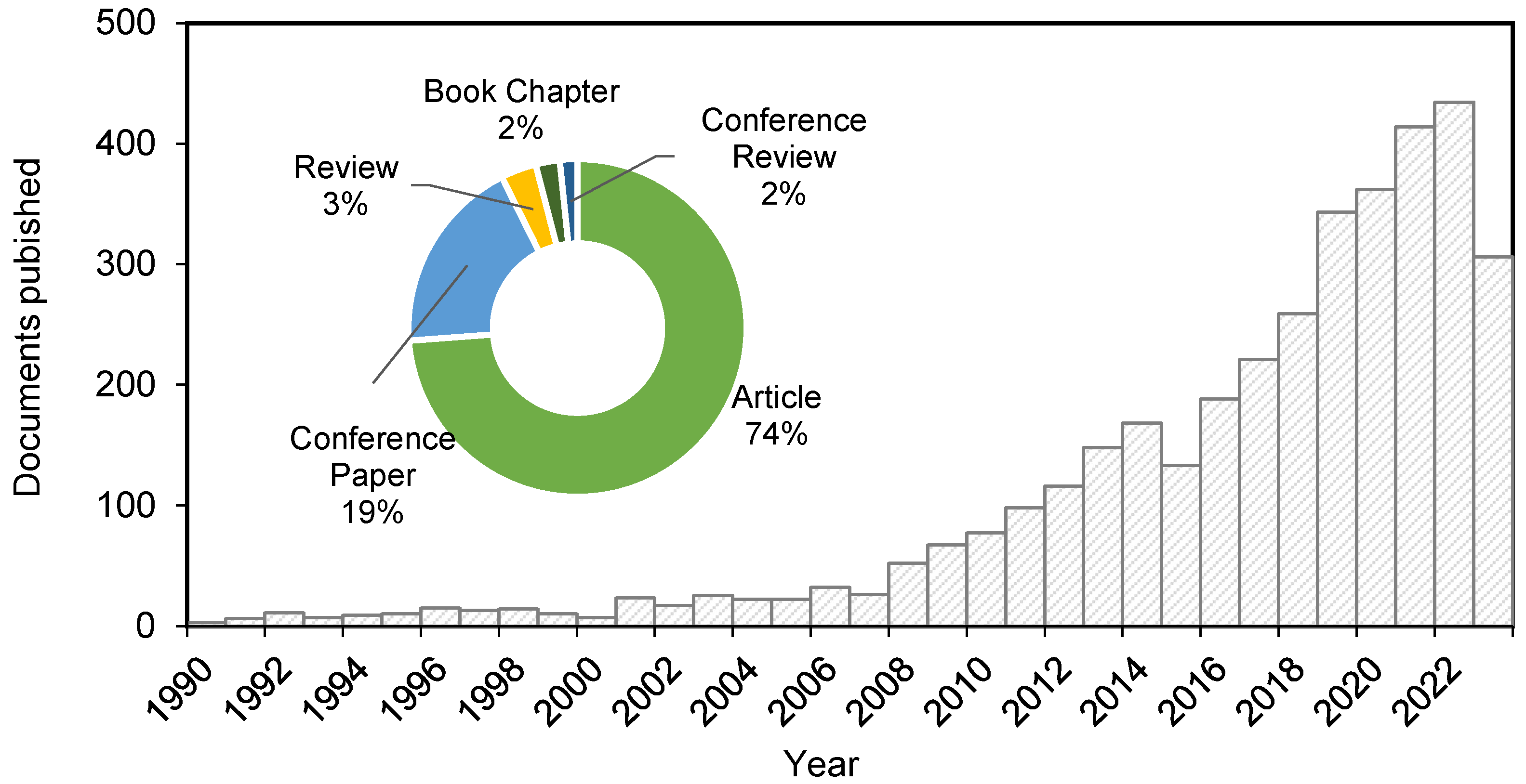
Although great results have been achieved in producing porous silica NPs, the mentioned chemical methods imply the use of hazardous chemicals harmful to the environment, such as CTAB, or costly techniques (by the use of TEOS, specifically). For this reason, the interest in developing green processes for producing silica NPs from biomass and agricultural waste is becoming of great interest [34], as evidenced by the publication of more than 400 research articles on the topic since 2019 (Figure 3).
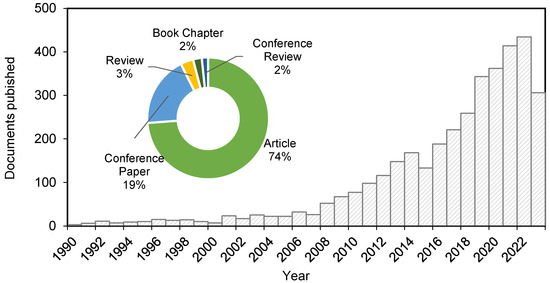
Figure 3.
Documents published on silica nanoparticles from rice husk from 1990 to 2023 (Scopus, April 2023).
Nonetheless, the number of reviews published is still few, considering the fast evolution of green silica production is going through.
3. Sustainable Harnessing of SiO2 from Rice Husks
3.1. Traditional Thermochemical Methods: Calcination
In the 1990s, direct combustion of rice husk without any chemical pretreatment was the most common method [35,36]. The first controlled combustion of RH, where amorphous silica powder was synthesized, was reported by Kapur in 1984. Combustion of rice husk led to a 20 wt% of ashes composed mainly of silica. As no purification pretreatment was applied, no pure silica was obtained [36]. Some acid leaching occurred on the rice husk ash after calcination [37]. However, the specific surface area of the resulting nanoparticles was not reported since the main objective was to determine the diameter of the nanoparticles and the effects of changing the ball milling parameters [37]. Another study performed some acid leaching on rice husk ash obtained after the open burning of rice husk. Then, additional controlled calcination was performed at 700 °C for 2 h, and silicas with specific surface areas varying from 7 to 250 m2/g were obtained depending on the origin of the rice husk [38].
In the early 2000s, efforts were made to study the effects of acid leaching of rice husk before calcination [39]. Calcination was studied by varying temperatures, heating ramp and sequential calcinations [23,40,41,42], as shown in Table 2. High-purity silicas with specific surface areas as high as 320 m2/g were produced [43]. Similar surface areas (330 m2/g) were obtained with a much lower heating rate and higher calcination temperature [44]. In a study from 2015, a further addition of a pre-pyrolysis step before calcination led to an improvement of surface area (350 m2/g) and led to homogeneous silica nanoparticles of 5–8 nm diameter [45].

Table 2.
Influence of rice husk acidic pretreatments and calcination procedures on specific surface area of biogenic silica.
The type of rice affects the produced nanoparticles; silica nanoparticles synthesized from brown rice husk had the smallest diameter (10 nm) in comparison with sticky (50 nm) or red (20 nm) rice [38]; thus, the origin of the RH also determines its final structure. The soil quality also affects the elemental composition of the RH. Benassi L. et al. (2015) [51] found a difference in the metal composition of Italian and Indian rice husks, which affected the availability of amorphous silica at the same calcination temperatures. Indian RH from different regions was found to have different metallic content, which also affected the formation of amorphous silica and its purity, pointing out the relevance of K2O content in this matter [52].
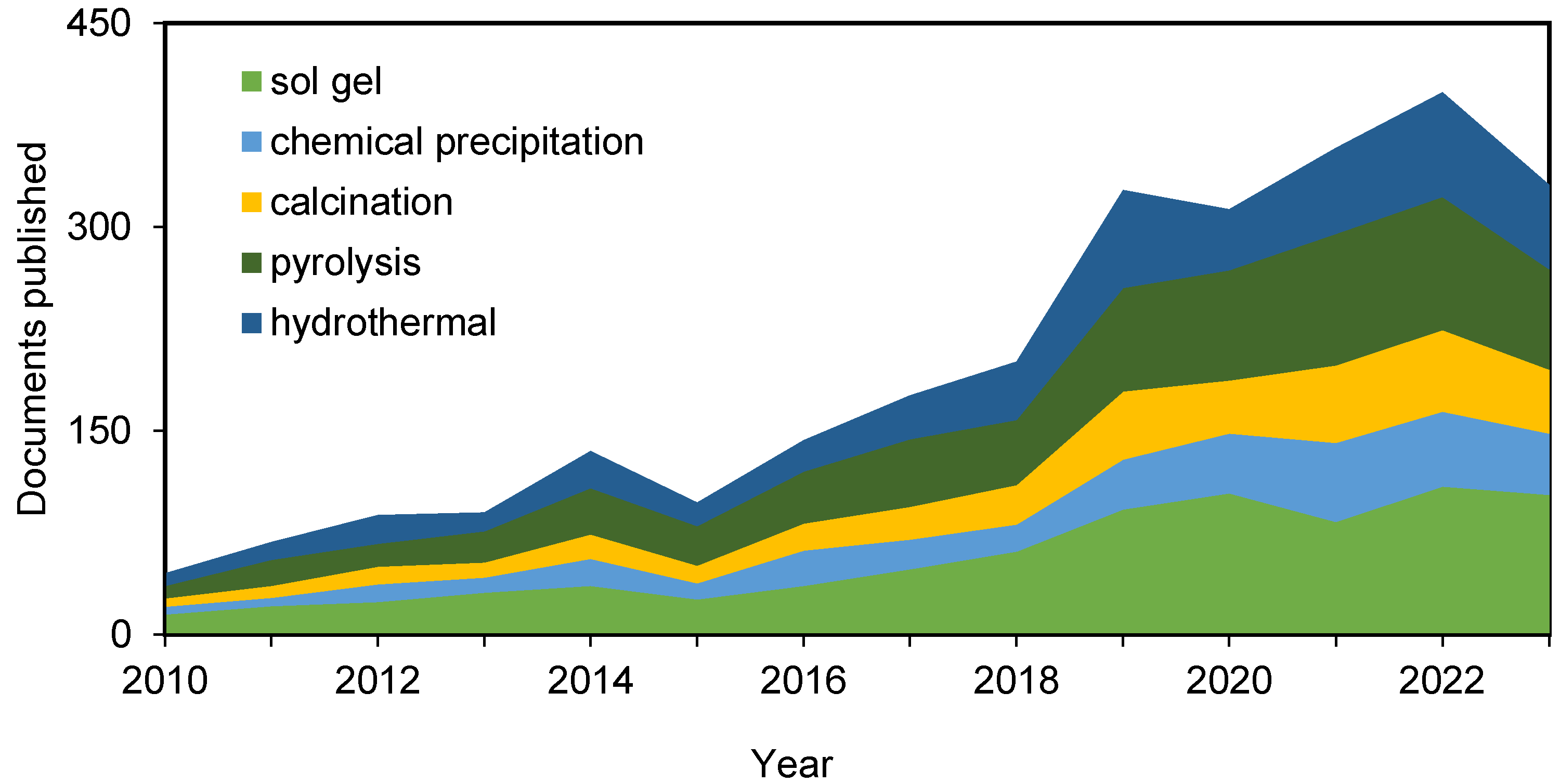
The early development of technologies to harness rice husks has not involved the use of pyrolysis. However, in recent years, the pyrolysis of biomass in order to obtain bio-oil and syngas as by-products, apart from the solid biochar, has been gaining a presence in the field. In fact, the number of articles citing the pyrolysis method has doubled in the last decade (Figure 4).
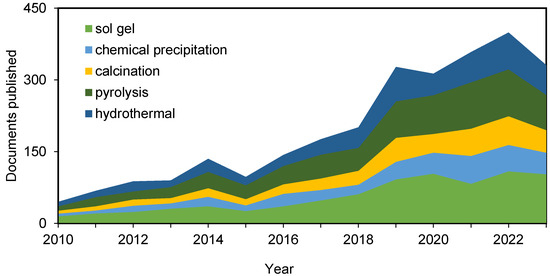
Figure 4.
Evolution and distribution of published papers on silica extraction techniques involving rice husk during the last decade (Scopus).
3.2. Pyrolysis of Rice Husk for Obtaining Bio-Oil and Bio-Silica in the Literature
3.2.1. Bio-Silica Production
The recent advances in pyrolysis of rice husk are shown in Table 3. Pyrolysis of acid-leached rice husk in an N2 atmosphere was described by Liou et al. (2004) [23], obtaining carbon/silica powders with a surface area of 261 m2/g at 900 °C. No bio-oil is recovered from this process. In the same study, the acid-leached rice husks were calcined with air, and the surface area obtained was 235 m2/g.
To our knowledge, the first pyrolysis performed, with the aim of obtaining pure silica nanoparticles, was reported in 2011 by Wang et al. [6]. However, although it is referred to as pyrolysis, it seems more like combustion since heating in the absence of oxygen does not achieve the obtention of pure silica but a composite of carbon and silica [6].
Liou et al. (2011) performed pyrolysis on acid-leached (hot HCl) RH at 700 °C for 1 h and extracted SiO2 by NaOH 1.5 M at 100 °C for 1 h to produce sodium silicate [53]. The addition of 1 M HCl to this sodium silicate solution to reach pH 7 at 50 °C allowed for the precipitation of SiO2 NPs of 6–8 nm diameter (350 m2/g, mesopore diameter 10.4 nm, V = 0.79 mL/g). These NPs are non-porous and agglomerated, and Na content (1.31 mg/g) is superior to the parent RH.
S. Gu et al. (2015) [45] combined pyrolysis with calcination to obtain pure silica (95.85–99.62%), thus removing the carbon from the C/SiO2 composite obtained from pyrolysis. The rice husk was washed with water, dried at 110 °C and pulverized. The RH was then submitted to acid leaching (1 g in 10 mL HCl 2.2 M) at 120 °C for 4 h. According to the same author in a previous work, hydrolysis temperature and acid concentration had the most pronounced effect on purity, increasing silica content from 85% at 40 °C hydrolysis temperature to 98% at 120 °C, while the hydrolysis time had little or no effect [54]. Then, pyrolysis was performed with 5 g of acid-leached RH in a tubular furnace with N2 flow at 1 L/min heated at 20 °C/min and maintained for 30 min at 300–800 °C. Calcination was then applied at a rate of 10 °C/min for 2–3 h at 610 °C. The highest BET surface area (SBET = 353 m2/g and pore volume of 0.48 mL/g) was obtained thanks to this intermediate pyrolysis step at 300 °C.
V. R. Madduluri et al. (2020) [55] studied the production of biochar during pyrolysis of risk husk. They obtained 228 m2/g of biochar with a N2 flow of 30 mL/min and a ramp of 10 °C/min for staying in a plateau of 2 h at 600 °C and then posterior grinding. A comparable result in terms of surface area was obtained by Claoston N. et al. (2014) [56], and again, no side recovery of bio-oil is contemplated.
In order to extract silica nanoparticles from the biochar obtained after pyrolysis of the rice husk, several researchers have applied chemical extraction techniques. Both Su et al. and Gautam et al. treated the biochar with chemical methods, meaning by first an alkali leaching, followed by an acidification to obtain a precipitated or a gel, respectively [57,58].

Table 3.
Rice husk pyrolysis for the extraction of silica/biochar in the literature published in the last decade. (SBET = Brunauer–Emmett–Teller (BET) surface area).
Table 3.
Rice husk pyrolysis for the extraction of silica/biochar in the literature published in the last decade. (SBET = Brunauer–Emmett–Teller (BET) surface area).
| Material Obtained | SBET (m2/g) | Chemical Pretreatment | Thermal Treatment | Milling | Precipitation/Sol-Gel | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | 164 | - | 100 °C with 1 M HCl for 2 h | washed with distilled water | “pyrolyzed” @700 °C for 2 h | - | - | [6] | ||
| Silica | 352 | washed with water | drying 110 °C | 5 g of RH in 500 mL with 8%wt HCl (2.2 M) @120 °C for 4 h | washed with distilled water | 5 g of RH in tubular furnace and pyrolysis gas; 1 L/min @800 °C for 30 min @ 20 °C/min | calcinated @10 C/min for 2–3 h @ 610 °C | pulverized | - | [45] |
| Biochar | - | - | pyrolysis N2 flow rate 40 L/min | - | - | [59] | ||||
| Silica | 328 | - | pyrolysis 600 °C (50 g) N2 flow rate 0.5 L/min | gasification at 875 °C for 20 min | - | alkali leaching/acidic treatment after pyrolysis to extract the silica and to get a gel | [57] | |||
| Biochar | 228 | washed with water | drying 100 °C | 3 N at 100 °C for 1 h | washed with hot distilled water | pyrolysis N2 flow rate of 30 mL/minramp by 10 °C/min for 2 h until reaching 600 °C | ground with pistol mortar | - | [55] | |
| Silica | - | sieved, washed with water and dried | 20 g of RH acetic acid solution (400 mL) (3%vol) for 2 h | washed with distilled water | pyrolysis at 500 °C for 45 min | - | alkali leaching/acidic treatment after pyrolysis to extract the silica and to get a precipitate | [58] | ||
| Silica | 238 | washed with water | HCl leaching at 100 °C with 10%w/w for 1 h (1 g:15 mL) | washed with distilled water | pyrolysis @550 °C | calcination @350 °C for 12 h; slope 4.5 °C/min | - | - | [60] | |
| Biochar | 230 | - | pyrolysis at 500 °C | - | - | [56] | ||||
3.2.2. Combined Bio-Silica and Bio-Oil Production
Research has been made on the production of bio-oil from pyrolyzed rice husks, studying its composition and its feasibility as a biofuel.
Lu Q et al. (2008) [16] analyzed the properties of bio-oil extracted from rice husk, which were similar to other bio-oils in terms of chemical composition. Acids, alcohols, ketones, sugars, etc., were compounds found in rice husk bio-oil similarly as for other bio-oils from different biomasses. However, they found high concentrations of nitrogen, inorganic elements and many low-boiling compounds, but significantly less heavy components, as well as high contents of water, solids and ash, and the presence of alkali metals, which is undesirable as well. The rice husks were not previously leached with acid, which explains the presence of these metals. Their main conclusion was that there is a need to upgrade the bio-oil produced from rice husk before it can be used as biofuel. In this case, the rice husk was not previously treated, and the bio-oil yield was 50 wt%.
Hsu et al. (2015) [59] studied the pyrolysis of RH in a fluidized bed pyrolizer with the aim of producing syngas and bio-oil, but it does not focus on the production of biochar or silica. The yields obtained with a N2 gas flow of 40 L/min and feeding rate of 10 g/min were the following: syngas: 38.52%; bio-oil: 29.44%; and char: 32.04%. The composition of the bio-oil was analyzed, which showed that it was mainly composed of aromatic compounds.
Modelization experiments have been carried out to analyze the composition of pyrolysis products of rice husk, demonstrating that bio-oil from pyrolyzed rice husk is a useful feedstock for biofuel production [61].
Except for a few recent studies, the co-production of bio-oil and bio-silica from risk husk using the same pyrolytic process has not been explored.
Yinhai Su et al. (2020) [58] studied the simultaneous production of bio-oil, syngas and biochar and then extracted silica from biochar by alkali etching. Regarding the effect of acid leaching as a pretreatment before pyrolysis for the removal of soluble inorganics, it had a negative effect on the mass yield of biochar, while the bio-oil and syngas yields increased.
As for its impact on bio-oil composition, non-pretreated rice husk pyrolyzed in the absence of a catalyst (RH-N) mainly generated acids (13.16%), ketones (11.38%), furans (11.32%), phenols (42.08%) and sugars (2.97%), while a high proportion of sugars (33.91%) was detected in pretreated rice husk (AWRH-N), which revealed that when biomass was free of alkali metals, it preferred sugars as the main products from cellulose degradation.
Yinhai Su et al. (2020) concluded that catalytic pyrolysis (with activated carbon catalyst) is necessary to obtain high-quality gas and liquid products that are economically feasible since the bio-oil composition, such as high nitrogen content, needs to be refined. By applying catalytic pyrolysis, the bio-oil produced was abundant in phenolic compounds, which are key components for biofuel production from bio-oil due to their high energy content [58].
A similar experiment was carried out by Choudhary et al. (2023) [56], but it used rice straw as the raw material for the extraction of bio-oil and biochar. Then, silica was extracted from biochar by chemical precipitation in the form of sodium silicate. The obtained solution was used to synthesize mesoporous silica MCM-41, used as a catalyst in the pyrolysis to enhance the bio-oil. Its synthesis is addressed in the following paragraph. The calorific values of the bio-oil were increased after implementing the catalyzer. However, no similar work has been found with rice husk as a raw material.
R. Gomez-Vazquez et al. (2002) [60] published a study of pyrolysis and combustion combined to obtain both mesoporous silica materials and bioenergy (bio-oil and syngas) from rice husk. This is the only work in which silica is obtained by calcination of biochar and not by precipitation. The pyrolysis was carried out in auto-thermal downdraft reactors with a mass flow of 15 kg/h. In this study, pyrolysis and calcination were carried out separately, and the biochar was ground and acid-leached just before the calcination. Thus, pyrolysis was carried out with non-acid-leached rice husks. The purity of the silica obtained was >99%, with a surface area of 238 m2/g.
4. Tailoring of SiO2 from Rice Husk to Enhance Porosity
4.1. Mesoporous RH-SiO2 Nanoparticles
One of the main goals of the optimization of the extraction process of silica from rice husk is achieving a large surface area since the surface area is linked to the value of the produced material. Efforts to adapt acid-leaching, grinding and calcination parameters to enhance the porosity of silica from rice husk have been reported (see Table 2).
The effect of calcination temperature was studied by Manasa et al. (2021). Five silica materials were synthesized, with different calcination conditions in the oven after acid leaching. Temperatures of 550, 600 and 650 °C for 3 h combined with air or N2 flow were studied as parameters for the extraction of SiO2. The highest BET surface area (SA) (376 m2/g) was achieved with RH-SIO2-600A calcined at 600 °C. RH-SiO2-600A had a pore size of 4.5 nm and a pore volume of 0.38 mL/g. The same RH subjected to pyrolysis with only N2 at 600 °C was a composite C/SiO2, named RH-SiO2-600N, which presented a lower SA of 267 m2/g, a smaller pore diameter (2.7 nm) and a smaller pore volume (0.17 mL/g) [62]. With higher temperatures than 600 °C, the SA decreases, and the pore size increases, possibly due to nanoparticles sintering.
Values over 200–288 m2/g have been largely reported by many studies without important variations when SA values of commercial precipitated silica are reported to be higher than 320 m2/g [63,64,65].
Franco et al. (2018) [66] studied the effect of grinding before calcination, and acid leaching was performed by microwave-assisted extraction with HCl (0.1 M 20 mL solution added to 2 g of RHs) and then calcinated at 500 °C for 4 h. RH-Silica extracted with SA of 352 m2/g had a mean pore size diameter (calculated via BJH equation) of 8 nm and a pore volume (BJH) of 0.56 mL/g. Similar SA was obtained by S. Gu et al. (2015) [45] with pyrolysis as a prior step of combustion.
This surface area value surpasses the one obtained by Alyosef et al. (2013) [50], which was 313 m2/g, with a mesopore volume of 0.36 mL/g and a large distribution of mesopores from 4 to 25 nm with an average of 7 nm. The use of a microwave with HCl might improve the leaching treatment.
Schneider et al. (2020) [67] studied the chemical differences of silica obtained from rice husks coming from Italy and Cambodia. They showed slight differences in their compositions. Alyosef and Chandrasekhar both found variations of 5–7 wt% of SiO2 content in rice husk, depending on the origin. The surface area obtained for the silica extracted from RH from Cambodia was 297 m2/g, which was slightly higher than the one from Italy (280 m2/g). The N2 adsorption–desorption isotherms exhibit the shape of a type IV isotherm in all cases, characteristic of mesoporous materials. Schneider et al. (2020) suggest that the hysteresis classified as H3 can indicate the existence of additional macropores and that some larger mesopores can only be accessible through small mesopores and cannot be accessible to nitrogen. A mixture of different biomasses containing SiO2 was treated, obtaining an SA of 303 m2/g and pore volume of 0.43 mL/g, showing that the combination of different sources of biogenic silica can enhance the porosity of the product [67]. Positron lifetime distributions obtained by PALS analysis by the same authors show that that these silica exhibit interconnected mesopores [65].
Nitrogen adsorption–desorption isotherms showed the difference between the calcination temperature and the washing pretreatment of the husk. This study shows that at higher temperatures (also confirmed by Alyosef), larger particles are formed (sintering of particles), which also makes the mesoporosity diminish [50,68].
4.2. Ordered Mesoporous RH-SiO2 Nanoparticles
Ordered structures of silica, known as the MCM (Mobil Composition of Matter) family, have the potential for a wide range of processes. These materials combine amorphous characteristics with the ordered structure of homogeneous mesopores with adjustable diameters from 2 to 10 nm, developing a specific surface area as high as 1000 m2/g due to very thin walls (1 nm) in between the pores. Rice husk has been explored as a raw material to produce biogenic MCM-41 by hydrothermal methods.
Most studies use similar methodologies for the production of biogenic MCM. Rice husk is pretreated with acid and then calcinated, then the silica in RHA is extracted by an alkali solution to form a sodium silicate solution. This solution is mixed with a surfactant solution such as CTAB, the pH is increased, and the solution is aged [69,70,71,72]. The solid obtained needs to be treated, usually by calcination, in order to remove the surfactant.
Alyosef et al. (2014) [70] employed a pseudomorphic transformation methodology to produce ordered mesoporous silica, MCM-41 and MCM-48, derived from rice husk ash. This approach effectively preserved the macroscopic morphology inherent in the original silica particles. The original RHA had a specific surface area (BET) of 313 m2/g and a pore volume of 0.38 mL/g and was obtained by a thermochemical treatment, as previously described by the same research group. This treatment did not involve the milling step. The synthesis of MCM-41 from RHA involved utilizing surfactant CTAOH (Cetyltrimethylammonium hydroxide). Hydrothermal synthesis in an autoclave at 393 K was conducted for an appropriate duration, after which the surfactant was removed by calcination. The optimal duration of the hydrothermal treatment was determined to be 6 days, resulting in a BET surface area of 1210 m2/g, compared to 545 m2/g, after one day. MCM-48 was synthesized through the pseudomorphic transformation of MCM-41, which was achieved by extending the reaction time from 1 to 3 days. Notably, no successful direct transformation from RHA to MCM-48 was achieved.
This paper shows the most effective synthesis method for producing ordered mesoporous silica materials using silica derived from rice husks. The cost-efficient nature of this process establishes a trajectory for the development of advanced materials suitable for diverse applications, such as water treatment and catalysis.
Costa J. et al. (2020) [71] reported a specific surface area of 500 m2/g for their MCM-41 and a diameter of the pores of 3.9 nm. Modifications of the synthesis, such as adding an iron salt, result in the synthesis of magnetic MCM-41 [72]. FTIR spectra revealed an increase of the Si-OH groups in MCM-41 when compared to non-ordered silica nanoparticles. The specific surface areas of MCM-41 and magnetic MCM-41 were 972 and 696 m2/g, respectively. The average pore size for both of them was 2.7 nm. The materials showed a hysteresis type of IV in N2 adsorption isotherm, thus indicating their mesoporosity.
A similar procedure was followed by Zadeh R. et al. (2021) [73]; however, the selected surfactant was polystyrene (ps35000). In this case, the pH of the surfactant-silicate solution was lowered, and a precipitate was obtained and calcinated. Magnetic MCM-41 particles were obtained by adding iron chloride to the process. Also, the latter were functionalized with thiol groups (SH) by adding 3-mecaptopropyl siloxane (MPTMS). The specific surface area for SH-magMCM41 was 135 m2/g. No surface area of the non-functionalized MCM-41 structure was reported.
4.3. Shaping RH-SiO2 NPs into Macroporous Structures
The assembly of mesoporous silica into macroporous structures such as monoliths presents great advantages for industrial applications, such as flow processes.
The use of rice husk as a silica precursor for this material has been explored by Bahrami et al. (2017) [74] by freeze-casting a water-based bio-silica solution.
Both amorphous (ARHA) and crystalline (CRHA) (80/20 cristobalite/tridymite) rice husk ash were synthesized with acid-leaching pretreatment only for ARHA and calcination at 600 and 900 °C, respectively. Obtained ARHA had a specific surface area of 151 m2/g and pore volume of 0.23 mL/g, while CRHA particles form agglomerates with a specific surface area of 1.5 m2/g. Before freeze-casting, both RHA were ground with a high-energy planetary ball mill to produce a fine powder (d < 10 μm).
Freeze-cast monoliths with lamellar and cellular pore structures (macropores in the range of 10–50 μm) were obtained from ARHA and CRHA. It was found that the crystallinity of the starting material does not have a significant impact on the macropores’ structure compared to other parameters of the synthesis (freezing rate and solid content).
The synthesis process consisted of a slurry preparation with the RHA silica suspended in water, dextrin was used as a binder, and polyethyleneimines (PEI) were used as a dispersant, which was then freeze cast. Then, the binder was removed with calcination at 650 °C for 2 h. The effect of sintering temperature (at 1200–1400 °C for 2 h) was studied on CRHA monoliths to evaluate its consequence on the strength of the monoliths. Sintering at 1300 and 1400 °C leads to an increase in the compressive strength to 2.2 MPa. The compressive strength of monoliths with cellular pore structure is considerably higher than that with lamellar pores. CRHA-monoliths are mainly macroporous and feature higher mechanical strength than ARHA-monoliths (0.9 MPa).
The formation of ARHA-monoliths by freeze-casting caused a small decrease in mesoporous surface area (from 151 to 108 m2/g) due to particles sintering, but the presence of macropores leads to a suitable device to develop flow processes with mesoporous RH-SiO2 NPs.
Both monoliths show interesting properties that can be useful for different applications, in either ceramic monoliths, where mechanical strength is required (CRHA-monoliths), or where the focus is made in hierarchical monolithic supports (ARHA-monoliths). Either way, this study opens a research path full of unexplored possibilities for agricultural waste-based materials.
5. RH-SiO2 Applications in the Literature
Silica nanoparticles have shown a great number of applications in fields like chemical synthesis, medicine, energy storage, electronics, environmental remediation or wastewater purification.
5.1. RH-SiO2 for Biomedical Applications
The main goals for biomedical applications such as drug delivery are to reduce side effects and treatment costs. The use of biogenic nanomaterials produced from waste resources accomplishes these objectives.
Biogenic silica nanoparticles (NPs) synthesized by acid leaching at 120 °C and calcination at temperatures of 500–700 °C were tested for cell viability for human stem cells revealing high biocompatibility [75]. In the same way, SiO2 NPs were produced and then milled after calcination to an average diameter of <150 nm in order to be used as a carrier of Penicillin-G. Once the drug was added to the silica, in vitro release was studied in simulated body fluid (SBF), concluding that the silica NPs can act as a carrier that delays the release of the drug in the body [37]. In other studies, silica mesoporous nanocarriers were obtained from rice husk via the sol-gel method, and their Dox (anti-cancer drug) loading efficiency and drug delivery potential were analyzed in vitro. Their efficiency was compared to similarly made silica from wheat husk, which resulted in having a higher loading efficiency due to its cylindrical pores, whereas rice husk showed less accessible slit-like pores, even if RH silica NPs had higher surface area [64].
5.2. RH-SiO2 for Energy Storage
Nanosized SiO2 can also be used for photovoltaic and energy storage applications. SiO2 NPs properties, such as high surface area and hydrophilic surface, enhance electrochemically active centers, which favors electrolyte transport through the material, which can be applied in the development of supercapacitors [22]. SiO2/C composites obtained by direct pyrolysis of rice husk were used for Li-ion sulfur batteries, showing high capacities as electrode material [76]. Other researchers doped the silica with tin oxide in order to gain reactivity through the sol-gel method. These showed good performance as electrodes for making electrochemical supercapacitors [77].
Rice husk has also been used to develop CO2 capturers by producing silica supports through alkaline extraction of SiO2 from RHA and then functionalized with polyethyleneimine (PEI). High adsorption capacity (61.6 mg CO2/g) was found due to the hexagonal shape of the mesopores on silica promoting interaction with CO2 [78]. Similar materials have been developed with different functionalizations, such as aminated silica for the adsorption of CO2, CH4, H2 and N2 [49].
5.3. RH-SiO2 as Catalyst Support
Silica nanoparticles have been used as catalyst supports, taking advantage of their large surface area. Silica prepared through microwave-assisted acid leaching and posterior calcination was used as an iron-oxide-containing nanocatalyst for toluene alkylation and oxidation of benzyl alcohol [66].
A SiO2/C-nickel-based catalyst was obtained through rice husk pyrolysis for the selective hydrogenation of furfural derived from biomass [55]. A similar nickel-based catalyst supported on RH silica showed high performance in yielding H2 production during non-oxidative methane cracking [62]. As can be seen, silica’s thermal stability is really attractive for use in catalytic reactions. Also, various studies have proved SiO2 to be a better support for Ni than other traditional materials [79].
5.4. RH-SiO2 as Adsorbent for Water Cleaning
Adsorption is one of the most efficient techniques to remove organic contaminants from waste-, surface-, and drinking water, which also enables reuse of the adsorbate. Moreover, its operative easiness and adaptability to the nature of the contaminants make it a very attractive solution for the removal of micropollutants in, e.g., wastewater [80].
5.4.1. Adsorption of Chemicals of Emerging Concern and Other Micropollutants
The current trends explored by researchers in adsorption are the utilization of agri-waste-based biomass due to its low cost, simple design and the added value of being a method of waste valorization [80].
Activated carbon has been the most traditionally used adsorbent, and it has been demonstrated to be efficient with hydrophobic compounds but highly inefficient with hydrophilic and electrically charged molecules. Moreover, the efficiency of activated carbon is reduced in the presence of organic matter, and the regeneration of adsorbents is questionable [81].
However, the ease of obtention of activated carbon from lignocellulosic residues via pyrolysis at 500–600 °C and posterior activation at 700 °C has always made this material extremely attractive. Moreover, it can be easily functionalized.
Therefore, the most recent advances that are being made in adsorption correspond to the need to develop efficient, reusable and cheaper adsorbents than activated carbon, as well as materials that can retain PMOCs (persistent mobile organic compounds) or PMTs (persistent mobile and toxic). The recent introduction of nanotechnology into wastewater treatment has shown to be a feasible solution to the issue of CECs [80,82].
Inorganic materials like silica are easily regenerated and thermally and chemically stable. They are good adsorbents due to their porosities and high surface areas. Moreover, nanoscale materials present an intrinsic advantage of higher activity. The use of mesoporous silica nanoparticles as adsorbents for some drugs, dye pollutants and volatile organic compounds has been largely studied [19,81,83,84,85,86]. Silica particles, through their hydroxyl groups, can bond easily with polar contaminants but can be non-specific [83].
These silica NPs are not specific to certain contaminants naturally but can be easily doped or functionalized due to their abundant surface silanol groups [86,87]. For instance, magnetic nanoparticles doped with ferrite present the advantage of easy recovery, and to make silica specific towards acidic dyes, it can be functionalized with carboxylic groups. Amino groups can remove both cationic and anionic contaminants from water [88]. As for functionalization with sulfur-containing groups, it is mostly used for the remediation of heavy metals such as mercury or cadmium from water [89,90].
The synthesis of nanoparticles usually implies the use of costly processes and toxic chemicals; therefore, the development of green synthesis in nanotechnology has grown in the last decade. Biogenic silica nanoparticles are synthesized from residues from agroalimetary industry such as sugarcane bagasse, rice husk or rice straw [19].
In terms of remediation, silica nanoparticles were mainly studied as support and stabilization materials for metal-based nanoparticles in the first decade of the 20th century [91,92,93].
Regarding biogenic silica nanoparticles for dye adsorption, in 2019, Ghorbani et al. developed core–shell magnetic nanocomposites of Fe3O4@SiO2 functionalized with amine groups using rice husk as silica precursor. These nanocomposites were applied to the absorbance of methyl red dye from aqueous environment [94]. Other researchers also synthesized silica nanoparticles from rice husk and applied them to the adsorption of methylene blue [95], as shown in Table 4 below.

Table 4.
Rice husk-derived biogenic silica-based adsorbents used in the adsorption of different organic pollutants (te = time to reach equilibrium; qe = experimental adsorption capacity at equilibrium).
Besides the removal of dyes, a few biogenic silica nanoparticles from rice husk used for micropollutant adsorption can be found in the literature. SiO2 NPs extracted through acid-leaching and calcination of rice husk were applied to the removal of triclosan, a bactericidal used in personal care products and caffeine. The highest adsorption capacities were observed after 30 and 60 min of contact time, respectively; however, they were still lower compared with other synthetic silica nanoparticles [97].
Simultaneous removal of a mixture of nine pesticides (50 µg/L each) in water was studied both in batch and column adsorption experiments with acid-pretreated and calcinated rice husk silica. Column experiments showed that 10 L of polluted water could be treated with a removal efficiency of up to 90%, showing interesting results for future scaling up of water treatment with bio-silica for removing multiple pesticides found in surface waters [96].
Regarding mesoporous silica from rice husks such as MCM-41, a couple of examples are found in the literature for the removal of glyphosate and 2,4-D (herbicides) and polycyclic aromatic hydrocarbons (PAHs). As for glyphosate and 2,4-D removal, functionalization of the silica with thiol groups showed excellent removal compared to other adsorbents in reference [73].
MCM-41 obtained from RHA and TEOS were compared for the removal of PAHs. Higher removal rates were achieved for naphthalene, benzo[b]fluoranthene, benzo[k]fluoranthene and benzo[a]pyrene with MCM-41 from RHA Also, regeneration and reuse experiments showed no significant losses [71].
There are several research paths that could be pursued in the field of adsorption and wastewater treatment using biogenic silica nanoparticles, such as optimizing the silica through functionalization to enhance their selectivity as well as exploring its combination with other treatment technologies to achieve comprehensive water treatment.
Regarding the efficient performance that different bio-silica materials have shown with simple water samples, there is a clear path to follow in order to develop adsorbent materials that can be applied to a larger-scale treatment. The next steps comprise a more realistic evaluation of the performance of these materials with real water samples, the development of pilot scale studies, and the evaluation of regeneration methods to ensure their real-world applicability.
5.4.2. Adsorption of Metals in Water
The adsorptive capacities of silica nanoparticles can be exploited for water cleaning by the recovery of toxic metals present in wastewater. As mentioned before, for micropollutants, currently, there is a great interest in developing cost-effective, simple and environmentally friendly techniques to deal with this issue. SiO2 has been applied to heavy metal removal from water [98,99,100,101,102].
Silica derived from rice husk has been less studied as an adsorbent of metals. Silica NPs extracted from RHA via the sol-gel method with a particle size of around 50 nm and a surface area of 70 m2/g were studied for the removal of Fe2+ ions in water. Iron adsorption capacity was found to be 9 mg/g at its maximum at pHs higher than 5 in 20 min [103].
SiO2 NPs were produced from rice husk by incubation in fungal biomass for its biotransformation. These NPs were tested for their adsorption of Pb in water spiked with 88 ppb, the same concentration measured in samples from fish farms, showing maximum adsorption after 120 h of contact time. Then, the goal of this study was to test the adsorption ability of SiO2 NPs in vivo in fishes supplemented with Pb and silica NPs, which showed effective adsorption of Pb at 1 ppm in the fish O. niloticus, hence reducing its mortality rates [104].
The removal of Cr3+ and Cu2+ by milled rice husk was tested. In this case, no extraction of SiO2 was performed, and the husk was used directly in a batch test with 100 ppm of each metal. As previously seen in other studies, the highest adsorption capacity (22.5 mg Cr3+/g and 30 mg Cu2+/g) reaches its peak at pH > 5 [105]. The use of rice husk ash directly as an adsorbent has also been studied in the literature; however, rice husk ash has smaller surface areas compared with amorphous SiO2 NPs [106]. These adsorption abilities can be implemented for the treatment of real wastewater, such as tannery wastewater, which is rich in Cr3+. Silica NPs were obtained from calcinated and leached rice husks using the sol-gel method. At pH 6, the highest adsorption capacity was determined to be 385 mg Cr3+/g, and the chromium complexes were better adsorbed when the size of the pores was increased since their diffusion into the particles seems to be the limiting factor [107].
While rice husks can be used directly for the adsorption of metals, it can be seen from the literature (Table 5) that amorphous silica extracted from RH has a better performance. Regarding adsorption conditions, researchers agree that working at pH > 5 provides higher adsorption capacities due to the increment in the acidity of silanol groups on the silica surface, but with the limitation of metal precipitation at high pHs. Also, Amirhandeh S. et al. (2022) [107] propose the diffusion of the metallic complexes into the pores to be the delimiting step of metal removal, so when working with big complexes such as the ones that chromium forms at basic pHs, a decline in specific surface area may improve the adsorption. Nickel removal from water with silica from rice husks was tested by Russo et al., showing an adsorption capacity of 11.7 mg/g. This performance was achieved by non-acid-leached silica, which had metal impurities and a low specific surface area of 15 m2/g. Surprisingly, acid-leached pure silica, with a surface area of 330 m2/g, had an adsorption capacity of less than 1 mg/g. Their study showed the role of the impurities of silica from untreated RH on their adsorption capacity, which seemed to have a positive impact on metal removal [44].

Table 5.
Rice-husk-derived biogenic silica adsorbents for toxic metals in water. (te = time to reach equilibrium; qe = experimental adsorption capacity at equilibrium).
To summarize, among the different procedures outlined previously, the following references focus on optimal techniques for generating high-surface silica nanoparticles from rice husk, cost-effective methods for shaping silica nanoparticles into monoliths suitable for flow applications, utilizing silica nanoparticles from rice husk for value-added materials like MCM-41, and the economical application of silica materials derived from rice husk for removing pollutants.
Gu et al. developed a comprehensive use of pyrolysis and calcination of acid-leached rice husk, which represents a significant step towards a circular economy. The investigation into the impact of pyrolysis gases, with an emphasis on optimizing their effects, influenced silica purity, specific surface area (SSA) and pore volume, resulting in an SSA range of 204–352 m2/g. The choice of atmosphere, whether N2 or CO2, during pyrolysis had distinct effects, with N2 enhancing nanosilica porosity and achieving a high surface area at lower temperatures, while CO2 influenced silica purity without achieving higher surface areas [45].
Bahrami et al. (2017) [74] introduce novel methodologies like freeze-casting for shaping silica nanoparticles from rice husk into devices for flow applications, allowing the synthesis of porous silica monoliths with tailored properties. Depending on the application requirements, CRHA-derived monoliths may be preferred for applications needing compressive strength (e.g., filters), whereas ARHA-derived monoliths are more suitable for applications requiring high specific surface areas, such as adsorption.
Alyosef et al. (2014) [70] presented a methodology for producing ordered mesoporous silica (MCM-41, MCM-48) from rice husk ash, maintaining the original macroscopic morphology of RH particles and achieving notable BET surface areas, resulting in 1210 m2/g for MCM-41. These materials have a direct application as sorbents or catalyst supports and represent highly added-value biomass products.
As sorbent applications, Saha et al. (2014) [96] explored the application of rice husk ash silica for pesticide removal from water, demonstrating a removal efficiency of 90% in column studies with a breakthrough value of 10 L using 10 g of RHA. The estimated cost of the RHA adsorbent was considerably lower than other carbon-based adsorbents.
These papers collectively highlight the adaptability of RHA-derived silica across diverse applications, emphasizing its potential for cost-effective and efficient solutions in water treatment, among other applications. The synergistic approach of harnessing silica from rice husk through an optimized pathway, followed by the production of value-added silica structures directly applicable in areas like water purification, catalysis or drug delivery, represents a promising strategy for upgrading rice husk biomass waste into a multitude of possibilities.
6. Conclusions and Future Prospects
Silica nanoparticles have great, well-known properties such as a tunable specific surface area and particle shape and diameter. They can be obtained (i) directly after acid-leaching of rice husk and controlled pyrolysis and/or calcination, (ii) by rice husk calcination (leading to classical rice husk ash from industry) followed by silica dissolution by NaOH (or Na2CO3) and silica precipitation in acidic medium, and (iii) by pyrolysis of acid-leached rice husk followed by NaOH dissolution and silica precipitation in acidic medium. Pathways (i) and (iii) are recommended to obtain higher silica purity and high surface area. They can be easily functionalized and transformed into other mesoporous silica featuring very high surface areas, such as MCM-41 (1000 m2/g), or into composites with other metals. These properties provide it with an excellent adsorption capacity, which can be applied to medicine as drug carriers or into catalysis as metal support, among others. When silica nanoparticles are extracted from biomass, they have been proven to have excellent biocompatibility and properties similar to silica produced by traditional methods but with a significant decrease in chemicals and energy consumption. SiO2 derived from secondary agricultural waste such as rice husk is also advantageous since they are considered to be the major contributors in the future for sustainably sourced energy and materials since their growth does not compete with food crops. With the intent of harvesting most of the rice husk, applying circular economy to SiO2 extraction through methods such as combined pyrolysis and calcination can lead to the obtention of valuable by-products such as bio-oil and pyrolysis gas. By varying processing parameters, SiO2 nanoparticles can be tailored for specific applications. However, based on this review, the authors believe there is a need for further research to be carried out to achieve the following:
- Optimize conditions for pyrolysis of rice husk to obtain porous SiO2 while considering the production of valuable bio-oil and pyrolysis gas;
- Investigate the synthesis of hierarchical silica structures such as monoliths derived from biogenic SiO2 as a precursor and the effect on the adsorptive ability [74];
- To study the effects of organic matter on SiO2 adsorbent and the efficiency of cleaning real waste-, surface- and drinking water. SiO2 has shown high performance in the removal of contaminants in batch or continuous adsorption tests of single pollutants or a simple mixture of pollutants in distilled water;
- Regarding the last point, real contaminated water samples exhibit high complexity. This complexity arises not only from organic matter and salts but also from the multitude and diversity of pollutants present. Consequently, a critical aspect of advancing silica as an adsorbent is its capacity to adapt to shifts in water composition. Investigating how silica can be complemented with other techniques to address the whole complexity of polluted waters will be a key point for its development;
- All syntheses of silica NPs use a direct acidic pretreatment of RH before calcination or, in some other cases, a basic extraction of silica to form sodium aluminate solution to be used in more classical synthesis of silica-based materials. Both methods will generate a large amount of waste solution (acid or alkaline). New strategies are needed to solve this non-sustainable issue. The use of ionic liquid (IL) [108] seems promising as silica NPs from RH featuring 240 m2/g have been obtained after pyrolysis. The use of specific enzymes alone or in a cocktail (cellulase, lignin peroxidase, lytic polysaccharide monooxygenase (LPMOs), etc.) [7] that is capable of promoting dissociation and separation of lignocellulosic components by oxidative or hydrolytic depolymerization [108,109,110,111] could be used alone or in combination with IL to produce silica NPs directly from RH, avoiding thermal treatment to create a more economical and sustainable route than acidic pretreatment or basic extraction.
Author Contributions
Writing—original draft, A.R.-O.; writing—review and editing, A.R.-O., V.V., A.G., J.C., J.H.C. and B.B.; supervision, B.B. and J.H.C.; investigation, A.R.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 945416.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imperial College London. Sustainable Biomass Availability in the EU, to 2050. 2021. Available online: https://www.concawe.eu/publication/sustainable-biomass-availability-in-the-eu-to-2050/ (accessed on 27 September 2022).
- An, D.; Guo, Y.; Zhu, Y.; Wang, Z. A green route to preparation of silica powders with rice husk ash and waste gas. Chem. Eng. J. 2010, 162, 509–514. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Tan, K.-H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice husk. Microporous Mesoporous Mater. 2015, 204, 204–209. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, X.; Chen, X.; Ma, Y.; Wang, Z.; Zhao, X. A new method of utilizing rice husk: Consecutively preparing d-xylose, organosolv lignin, ethanol and amorphous superfine silica. J. Hazard. Mater. 2015, 291, 65–73. [Google Scholar] [CrossRef]
- European Comission. European Commission|Agri-Food Data Portal|Agricultural Markets|Rice. 2019. Available online: https://agridata.ec.europa.eu/extensions/DataPortal/rice.html (accessed on 23 November 2022).
- Wang, W.; Martin, J.C.; Zhang, N.; Ma, C.; Han, A.; Sun, L. Harvesting silica nanoparticles from rice husks. J. Nanoparticle Res. 2011, 13, 6981–6990. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Martin, J.C.; Oliphant, A.J.; Doerr, P.A.; Xu, J.F.; DeBorn, K.M.; Chen, C.; Sun, L. Extraction of Lignocellulose and Synthesis of Porous Silica Nanoparticles from Rice Husks: A Comprehensive Utilization of Rice Husk Biomass. ACS Sustain. Chem. Eng. 2013, 1, 254–259. [Google Scholar] [CrossRef]
- Gebretatios, A.G.; Pillantakath, A.R.K.K.; Witoon, T.; Lim, J.-W.; Banat, F.; Cheng, C.K. Rice husk waste into various template-engineered mesoporous silica materials for different applications: A comprehensive review on recent developments. Chemosphere 2023, 310, 136843. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Restiawaty, E.; Bindar, Y. Routes for energy and bio-silica production from rice husk: A comprehensive review and emerging prospect. Renew. Sustain. Energy Rev. 2021, 149, 111329. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Rahman, T.; A Fadhlulloh, M.; Abdullah, A.G.; Hamidah, I.; Mulyanti, B. Synthesis of silica particles from rice straw waste using a simple extraction method. IOP Conf. Ser. Mater. Sci. Eng. 2016, 128, 012040. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. [Google Scholar] [CrossRef]
- Mendes, R.F.; Vilela, A.P.; Farrapo, C.L.; Mendes, J.F.; Tonoli, G.H.D.; Mendes, L.M. Lignocellulosic residues in cement-bonded panels. In Sustainable and Nonconventional Construction Materials Using Inorganic Bonded Fiber Composites; Woodhead Publishing: Sawston, UK, 2017; pp. 3–16. [Google Scholar] [CrossRef]
- Kaniapan, S.; Pasupuleti, J.; Nesan, K.P.; Abubackar, H.N.; Umar, H.A.; Oladosu, T.L.; Bello, S.R.; Rene, E.R. A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment. Int. J. Environ. Res. Public Health 2022, 19, 3427. [Google Scholar] [CrossRef]
- Rao, G.R.; Sastry, A.R.K.; Rohatgi, P.K. Nature and reactivity of silica available in rice husk and its ashes. Bull. Mater. Sci. 1989, 12, 469–479. [Google Scholar] [CrossRef]
- Chen, P.; Gu, W.; Fang, W.; Ji, X.; Bie, R. Removal of metal impurities in rice husk and characterization of rice husk ash under simplified acid pretreatment process. Environ. Prog. Sustain. Energy 2017, 36, 830–837. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.-L.; Zhu, X.-F. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198. [Google Scholar] [CrossRef]
- Nawaz, S.; Jamil, F.; Akhter, P.; Hussain, M.; Jang, H.; Park, Y.-K. Valorization of lignocellulosic rice husk producing biosilica and biofuels—A review. J. Physics Energy 2023, 5, 012003. [Google Scholar] [CrossRef]
- Mahmad-Toher, A.-S.; Govender, N.; Dorairaj, D.; Wong, M.-Y. Comparative evaluation on calcium silicate and rice husk ash amendment for silicon-based fertilization of Malaysian rice (Oryza sativa L.) varieties. J. Plant Nutr. 2021, 45, 1336–1347. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of high quality biogenic silica by combustion of rice husk and rice straw combined with pre- and post-treatment strategies—A review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Prabha, S.; Durgalakshmi, D.; Rajendran, S.; Lichtfouse, E. Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: A review. Environ. Chem. Lett. 2021, 19, 1667–1691. [Google Scholar] [CrossRef]
- Liou, T.-H. Evolution of chemistry and morphology during the carbonization and combustion of rice husk. Carbon 2004, 42, 785–794. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, P.; Shao, Q. Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater. 2014, 188, 46–76. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Lee, J.-H.; Cho, E.-B. Recent Trends in Morphology-Controlled Synthesis and Application of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Moghaddam, S.P.H.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Agrawal, M.; Alexander, A. Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Akhter, F.; Rao, A.A.; Abbasi, M.N.; Wahocho, S.A.; Mallah, M.A.; Anees-Ur-Rehman, H.; Chandio, Z.A. A Comprehensive Review of Synthesis, Applications and Future Prospects for Silica Nanoparticles (SNPs). Silicon 2022, 14, 8295–8310. [Google Scholar] [CrossRef]
- Bogush, G.; Tracy, M.; Zukoski, C. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Crystalline Solids 1988, 104, 95–106. [Google Scholar] [CrossRef]
- Möller, K.; Kobler, J.; Bein, T. Colloidal suspensions of mercapto-functionalized nanosized mesoporous silica. J. Mater. Chem. 2007, 17, 624–631. [Google Scholar] [CrossRef]
- Zhang, A.; Gu, L.; Hou, K.; Dai, C.; Song, C.; Guo, X. Mesostructure-tunable and size-controllable hierarchical porous silica nanospheres synthesized by aldehyde-modified Stöber method. RSC Adv. 2015, 5, 58355–58362. [Google Scholar] [CrossRef]
- Lou, F.; Zhang, A.; Zhang, G.; Ren, L.; Guo, X.; Song, C. Enhanced kinetics for CO2 sorption in amine-functionalized mesoporous silica nanosphere with inverted cone-shaped pore structure. Appl. Energy 2020, 264, 114637. [Google Scholar] [CrossRef]
- Akinjokun, A.I.; Ojumu, T.V.; Ogunfowokan, A.O. Biomass, Abundant Resources for Synthesis of Mesoporous Silica Material. In Microporous and Mesoporous Materials; BoD—Books on Demand: Norderstedt, Germany, 2016; pp. 105–177. [Google Scholar] [CrossRef]
- James, J.; Rao, M. Silica from rice husk through thermal decomposition. Thermochim. Acta 1986, 97, 329–336. [Google Scholar] [CrossRef]
- Kapur, P. Production of reactive bio-silica from the combustion of rice husk in a tube-in-basket (TiB) burner. Powder Technol. 1985, 44, 63–67. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Javidi, J.; Dadkhah, M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb. Chem. High Throughput Screen. 2013, 16, 458–462. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Real, C.; Alcala, M.D.; Criado, J.M. Preparation of Silica from Rice Husks. J. Am. Ceram. Soc. 1996, 79, 2012–2016. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Ding, X.; Lei, H.; Chen, X.; An, D.; Li, Y.; Wang, Z. A study on the consecutive preparation of d-xylose and pure superfine silica from rice husk. Bioresour. Technol. 2010, 101, 1263–1267. [Google Scholar] [CrossRef]
- Della, V.; Kühn, I.; Hotza, D. Rice husk ash as an alternate source for active silica production. Mater. Lett. 2002, 57, 818–821. [Google Scholar] [CrossRef]
- Matori, K.A.; Haslinawati, M.M. Producing Amorphous White Silica from Rice Husk. Masaum J. Basic Appl. Sci. 2009, 1, 512. Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1563650 (accessed on 3 June 2022).
- Yalçin, N.; Sevinç, V. Studies on silica obtained from rice husk. Ceram. Int. 2001, 27, 219–224. [Google Scholar] [CrossRef]
- Russo, B.; Causse, J.; Rey, C.; Lautru, J.; Rebiscoul, D.; Ayral, A. Biosourced adsorbent prepared with rice husk part 1: A complete understanding of the structure of materials, the major role of mineral impurities for metal extraction. Sustain. Mater. Technol. 2023, 36, e00601. [Google Scholar] [CrossRef]
- Gu, S.; Zhou, J.; Yu, C.; Luo, Z.; Wang, Q.; Shi, Z. A novel two-staged thermal synthesis method of generating nanosilica from rice husk via pre-pyrolysis combined with calcination. Ind. Crops Prod. 2015, 65, 1–6. [Google Scholar] [CrossRef]
- Abu Bakar, R.; Yahya, R.; Gan, S.N. Production of High Purity Amorphous Silica from Rice Husk. Procedia Chem. 2016, 19, 189–195. [Google Scholar] [CrossRef]
- Castillo, J.; Vargas, V.; Macero, D.; Le Beulze, A.; Ruiz, W.; Bouyssiere, B. One-step synthesis of SiO2 α−Fe2O3/Fe3O4 composite nanoparticles with magnetic properties from rice husks. Phys. B Condens. Matter 2021, 605, 412799. [Google Scholar] [CrossRef]
- Xu, W.T.; Wei, J.; Chen, J.; Zhang, B.; Xu, P.; Ren, J.; Yu, Q. Comparative Study of Water-Leaching and Acid-Leaching Pretreatment on the Thermal Stability and Reactivity of Biomass Silica for Viability as a Pozzolanic Additive in Cement. Materials 2018, 11, 1697. [Google Scholar] [CrossRef]
- Bakdash, R.S.; Aljundi, I.H.; Basheer, C.; Abdulazeez, I. Rice husk derived Aminated Silica for the efficient adsorption of different gases. Sci. Rep. 2020, 10, 19526. [Google Scholar] [CrossRef] [PubMed]
- Alyosef, H.A.; Eilert, A.; Welscher, J.; Ibrahim, S.S.; Denecke, R.; Schwieger, W.; Enke, D. Characterization of biogenic silica generated by thermo chemical treatment of rice husk. Part. Sci. Technol. 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Benassi, L.; Bosio, A.; Dalipi, R.; Borgese, L.; Rodella, N.; Pasquali, M.; Depero, L.E.; Bergese, P.; Bontempi, E. Comparison between rice husk ash grown in different regions for stabilizing fly ash from a solid waste incinerator. J. Environ. Manag. 2015, 159, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, S.; Pramada, P.N.; Praveen, L. Effect of organic acid treatment on the properties of rice husk silica. J. Mater. Sci. 2005, 40, 6535–6544. [Google Scholar] [CrossRef]
- Liou, T.-H.; Yang, C.-C. Synthesis and surface characteristics of nanosilica produced from alkali-extracted rice husk ash. Mater. Sci. Eng. B 2011, 176, 521–529. [Google Scholar] [CrossRef]
- Gu, S.; Zhou, J.; Luo, Z.; Wang, Q.; Ni, M. A detailed study of the effects of pyrolysis temperature and feedstock particle size on the preparation of nanosilica from rice husk. Ind. Crops Prod. 2013, 50, 540–549. [Google Scholar] [CrossRef]
- Madduluri, V.R.; Mandari, K.K.; Velpula, V.; Varkolu, M.; Kamaraju, S.R.R.; Kang, M. Rice husk-derived carbon-silica supported Ni catalysts for selective hydrogenation of biomass-derived furfural and levulinic acid. Fuel 2020, 261, 116339. [Google Scholar] [CrossRef]
- Claoston, N.; Samsuri, A.; Husni, M.A.; Amran, M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Gautam, N.; Athira Merlin Rose, K.V.; Chaurasia, A. Study on chemical kinetics and characterization of nanosilica from rice husk and rice straw in the fixed-bed pyrolysis process. Biomass Convers. Biorefinery 2020, 12, 1435–1448. [Google Scholar] [CrossRef]
- Su, Y.; Liu, L.; Zhang, S.; Xu, D.; Du, H.; Cheng, Y.; Wang, Z.; Xiong, Y. A green route for pyrolysis poly-generation of typical high ash biomass, rice husk: Effects on simultaneous production of carbonic oxide-rich syngas, phenol-abundant bio-oil, high-adsorption porous carbon and amorphous silicon dioxide. Bioresour. Technol. 2020, 295, 122243. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-P.; Huang, A.-N.; Kuo, H.-P. Analysis of the Rice Husk Pyrolysis Products from a Fluidized Bed Reactor. Procedia Eng. 2015, 102, 1183–1186. [Google Scholar] [CrossRef]
- Gómez-Vásquez, R.; Fernández-Ballesteros, E.; Camargo-Trillos, D. Biogenic nanoporous oxides recovery from by-products of bioenergy production: Rice husks and corncob biochars. Biomass Bioenergy 2022, 161, 106455. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Odetoye, T.E.; Titiloye, J.; Ighalo, J.O. A Thermodynamic Study of Rice Husk (Oryza sativa) Pyrolysis. Eur. J. Sustain. Dev. Res. 2019, 3, em0094. [Google Scholar] [CrossRef]
- Manasa, K.; Naresh, G.; Kalpana, M.; Sasikumar, B.; Velisoju, V.K.; Chary, K.V.; Michalkiewicz, B.; Venugopal, A. Improved H2 yields over rice husk derived SiO2 nanoparticles supported Ni catalyst during non-oxidative methane cracking. J. Energy Inst. 2021, 99, 73–81. [Google Scholar] [CrossRef]
- Rajan, R.; Zakaria, Y.; Shamsuddin, S.; Hassan, N.F.N. Robust synthesis of mono-dispersed spherical silica nanoparticle from rice husk for high definition latent fingermark development. Arab. J. Chem. 2020, 13, 8119–8132. [Google Scholar] [CrossRef]
- Porrang, S.; Rahemi, N.; Davaran, S.; Mahdavi, M.; Hassanzadeh, B. Preparation and in-vitro evaluation of mesoporous biogenic silica nanoparticles obtained from rice and wheat husk as a biocompatible carrier for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2021, 163, 105866. [Google Scholar] [CrossRef]
- Schneider, D.; Attallah, A.G.; Wassersleben, S.; Wenzel, M.; Matysik, J.; Krause-Rehberg, R.; Enke, D. Advanced textural characterization of biogenic silica by nitrogen physisorption, positron annihilation lifetime spectroscopy and hyperpolarized 129Xe NMR spectroscopy. Microporous Mesoporous Mater. 2020, 307, 110515. [Google Scholar] [CrossRef]
- Franco, A.; De, S.; Balu, A.M.; Romero, A.A.; Luque, R. Integrated Mechanochemical/Microwave-Assisted Approach for the Synthesis of Biogenic Silica-Based Catalysts from Rice Husk Waste. ACS Sustain. Chem. Eng. 2018, 6, 11555–11562. [Google Scholar] [CrossRef]
- Schneider, D.; Wassersleben, S.; Weiß, M.; Denecke, R.; Stark, A.; Enke, D. A Generalized Procedure for the Production of High-Grade, Porous Biogenic Silica. Waste Biomass Valorization 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Zareihassangheshlaghi, A.; Dizaji, H.B.; Zeng, T.; Huth, P.; Ruf, T.; Denecke, R.; Enke, D. Behavior of Metal Impurities on Surface and Bulk of Biogenic Silica from Rice Husk Combustion and the Impact on Ash-Melting Tendency. ACS Sustain. Chem. Eng. 2020, 8, 10369–10379. [Google Scholar] [CrossRef]
- Choudhary, P.; Sharma, R.; Kumar, V.; Singh, A.; Sharma, N. Synthesis, Characterization and Catalytic Activity of Bio-MCM-41 for Production of Bio Crude Oil via Pyrolysis of Rice Straw. Waste Biomass Valorization 2023, 14, 4173–4186. [Google Scholar] [CrossRef]
- Alyosef, H.A.; Uhlig, H.; Münster, T.; Kloess, G.; Einicke, W.-D.; Gläser, R.; Enke, D. Biogenic silica from rice husk ash-Sustainable sources for the synthesis of value-added silica. Chem. Eng. Trans. 2014, 37, 667–672. [Google Scholar]
- Costa, J.A.S.; Sarmento, V.H.V.; Romão, L.P.C.; Paranhos, C.M. Adsorption of organic compounds on mesoporous material from rice husk ash (RHA). Biomass Convers. Biorefinery 2020, 10, 1105–1120. [Google Scholar] [CrossRef]
- Kamari, S.; Ghorbani, F. Extraction of highly pure silica from rice husk as an agricultural by-product and its application in the production of magnetic mesoporous silica MCM–41. Biomass Convers. Biorefinery 2021, 11, 3001–3009. [Google Scholar] [CrossRef]
- Zadeh, R.J.; Sayadi, M.H.; Rezaei, M.R. Synthesis of Thiol modified magMCM-41 nanoparticles with rice husk ash as a robust, high effective, and recycling magnetic sorbent for the removal of herbicides. J. Environ. Chem. Eng. 2021, 9, 104804. [Google Scholar] [CrossRef]
- Bahrami, A.; Simon, U.; Soltani, N.; Zavareh, S.; Schmidt, J.; Pech-Canul, M.I.; Gurlo, A. Eco-fabrication of hierarchical porous silica monoliths by ice-templating of rice husk ash. Green Chem. 2017, 19, 188–195. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alhazmi, M.; Alatiah, K.A.; Alshatwi, A.A. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram. Int. 2015, 41, 275–281. [Google Scholar] [CrossRef]
- Huang, S.-S.; Tung, M.T.; Huynh, C.D.; Hwang, B.-J.; Bieker, P.M.; Fang, C.-C.; Wu, N.-L. Engineering Rice Husk into a High-Performance Electrode Material through an Ecofriendly Process and Assessing Its Application for Lithium-Ion Sulfur Batteries. ACS Sustain. Chem. Eng. 2019, 7, 7851–7861. [Google Scholar] [CrossRef]
- Vijayan, R.; Kumar, G.S.; Karunakaran, G.; Surumbarkuzhali, N.; Prabhu, S.; Ramesh, R. Microwave combustion synthesis of tin oxide-decorated silica nanostructure using rice husk template for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2020, 31, 5738–5745. [Google Scholar] [CrossRef]
- Henao, W.; Jaramillo, L.Y.; López, D.; Romero-Sáez, M.; Buitrago-Sierra, W.A.H. Insights into the CO2 capture over amine-functionalized mesoporous silica adsorbents derived from rice husk ash. J. Environ. Chem. Eng. 2020, 8, 104362. Available online: https://www.sciencedirect.com/science/article/pii/S2213343720307119 (accessed on 2 December 2022). [CrossRef]
- An, D.; Guo, Y.; Zou, B.; Zhu, Y.; Wang, Z. A study on the consecutive preparation of silica powders and active carbon from rice husk ash. Biomass Bioenergy 2011, 35, 1227–1234. [Google Scholar] [CrossRef]
- Kumar, P.S.; Joshiba, G.J.; Femina, C.C.; Varshini, P.; Priyadharshini, S.; Karthick, M.A.; Jothirani, R. A critical review on recent developments in the low-cost adsorption of dyes from wastewater. Desalination Water Treat. 2019, 172, 395–416. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Kollarahithlu, S.C.; Balakrishnan, R.M. Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. J. Clean. Prod. 2021, 294, 126155. [Google Scholar] [CrossRef]
- Zeb, S.; Ali, N.; Ali, Z.; Bilal, M.; Adalat, B.; Hussain, S.; Gul, S.; Ali, F.; Ahmad, R.; Khan, S.; et al. Silica-based nanomaterials as designer adsorbents to mitigate emerging organic contaminants from water matrices. J. Water Process. Eng. 2020, 38, 101675. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Microscale scavenging of pentachlorophenol in water using amine and tripolyphosphate-grafted SBA-15 silica: Batch and modeling studies. J. Environ. Manag. 2014, 146, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, F.; Huang, D.; Hou, S.; Wang, H.; Wang, M.; Chi, Y.; Zhao, Z. A facile route to magnetic mesoporous core–shell structured silicas containing covalently bound cyclodextrins for the removal of the antibiotic doxycycline from water. RSC Adv. 2018, 8, 31348–31357. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Liu, J.; Xu, Z. Tannic acid adsorption on amino-functionalized magnetic mesoporous silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Vargas, V.; Castillo, J.; Ocampo-Torres, R.; Lienemann, C.-P.; Bouyssiere, B. Surface modification of SiO2 nanoparticles to increase asphaltene adsorption. Pet. Sci. Technol. 2018, 36, 618–624. [Google Scholar] [CrossRef]
- Mudhoo, A.; Sillanpää, M. Magnetic nanoadsorbents for micropollutant removal in real water treatment: A review. Environ. Chem. Lett. 2021, 19, 4393–4413. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kumar, A.; Zhang, Y. A novel approach for the removal of Pb2+ and Cd2+ from wastewater by sulfur-ferromagnetic nanoparticles (SFMNs). Chemosphere 2022, 287, 132156. [Google Scholar] [CrossRef]
- Mohmood, I.; Lopes, C.B.; Lopes, I.; Tavares, D.S.; Soares, A.M.; Duarte, A.C.; Trindade, T.; Ahmad, I.; Pereira, E. Remediation of mercury contaminated saltwater with functionalized silica coated magnetite nanoparticles. Sci. Total Environ. 2016, 557–558, 712–721. [Google Scholar] [CrossRef]
- Qiu, X.; Fang, Z.; Liang, B.; Gu, F.; Xu, Z. Degradation of decabromodiphenyl ether by nano zero-valent iron immobilized in mesoporous silica microspheres. J. Hazard. Mater. 2011, 193, 70–81. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Z.; Li, T.; Li, S. Removal of hexavalent chromium in soil and groundwater by supported nano zero-valent iron on silica fume. Water Sci. Technol. 2011, 63, 2781–2787. [Google Scholar] [CrossRef]
- Zheng, T.; Zhan, J.; He, J.; Sunkara, B.; Lu, Y.; McPherson, G.L.; Piringer, G.; Kolesnichenko, V.; John, V.T. Nanostructured Multifunctional Materials for Environmental Remediation of Chlorinated Hydrocarbons. In Environmental Applications of Nanoscale and Microscale Reactive Metal Particles; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 163–179. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell magnetic nanocomposite of Fe3O4@SiO2@NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments. Environ. Technol. Innov. 2019, 14, 100333. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini–Bandegharaei, A.; Dotto, G.L. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Saha, A.; Gajbhiye, V.T.; Gupta, S.; Kumar, R.; Ghosh, R.K. Simultaneous Removal of Pesticides from Water by Rice Husk Ash: Batch and Column Studies. Water Environ. Res. 2014, 86, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, J.; Guerrero, V.H.; Vizuete, K.; Debut, A. Environmentally friendly synthesis of silicon dioxide nanoparticles and their application for the removal of emerging contaminants in aqueous media. J. Physics Conf. Ser. 2022, 2238, 012005. [Google Scholar] [CrossRef]
- Peralta, M.E.; Mártire, D.O.; Moreno, M.S.; Parolo, M.E.; Carlos, L. Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal. J. Environ. Chem. Eng. 2021, 9, 104841. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Khorramabadi, G.S.; Khataee, A.; Jorfi, S. Silica nanopowders/alginate composite for adsorption of lead (II) ions in aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 973–980. [Google Scholar] [CrossRef]
- Knight, A.W.; Tigges, A.B.; Ilgen, A.G. Adsorption of copper (II) on mesoporous silica: The effect of nano-scale confinement. Geochem. Trans. 2018, 19, 13. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Liang, Y.; Ding, H.; Sun, S. Adsorption Properties and Mechanism of Cd2+ in Water by Zr-containing Silica Residue Purification. Front. Chem. 2018, 6, 556. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A.; Navratil, J.D. Adsorption of Cesium, Strontium, and Cobalt Ions on Magnetite and a Magnetite−Silica Composite. Ind. Eng. Chem. Res. 2001, 40, 1615–1623. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ma, H.T.; Avti, P.; Bashir, M.J.K.; Ng, C.A.; Wong, L.Y.; Jun, H.K.; Ngo, Q.M.; Tran, N.Q. Adsorptive removal of iron using SiO2 nanoparticles extracted from rice husk ash. J. Anal. Methods Chem. 2019, 2019, 6210240. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.; Rashwan, A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021, 28, 5119–5130. Available online: https://www.sciencedirect.com/science/article/pii/S1319562X21003958 (accessed on 6 December 2022). [CrossRef]
- Sobhanardakani, S.; Parvizimosaed, H.; Olyaie, E. Heavy metals removal from wastewaters using organic solid waste—Rice husk. Environ. Sci. Pollut. Res. 2013, 20, 5265–5271. [Google Scholar] [CrossRef]
- Priya, A.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef]
- Amirhandeh, S.Z.H.; Salem, A.; Salem, S. Treatment of tannery wastewater by silica nanoparticles produced from rice husk ash via a green route. Environ. Sci. Pollut. Res. 2022, 30, 13039–13047. [Google Scholar] [CrossRef] [PubMed]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.-S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef]
- Østby, H.; Hansen, L.D.; Horn, S.J.; Eijsink, V.G.H.; Várnai, A. Enzymatic processing of lignocellulosic biomass: Principles, recent advances and perspectives. J. Ind. Microbiol. Biotechnol. 2020, 47, 623–657. [Google Scholar] [CrossRef] [PubMed]
- Wattanasiriwech, S.; Wattanasiriwech, D.; Svasti, J. Production of amorphous silica nanoparticles from rice straw with microbial hydrolysis pretreatment. J. Non-Crystalline Solids 2010, 356, 1228–1232. [Google Scholar] [CrossRef]
- Zemnukhova, L.A.; Skiba, E.A.; Budaeva, V.V.; Panasenko, A.E.; Polyakova, N.V. Composition of inorganic components of oat husks and products of their chemical and enzymatic transformation. Russ. J. Appl. Chem. 2018, 91, 230–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).